Abstract
It has long been known that angiotensin type-1 receptors (AT1R) play a critical role in sympathetic regulation, cardiovascular activity, and hormone secretion under physiological and pathological states. On the other hand, the functional significance of angiotensin type-2 receptors (AT2R) is poorly understood. In a recent study we demonstrated that, in rats with chronic heart failure, AT1R protein expression was increased but AT2R expression was decreased in the rostral ventrolateral medulla (RVLM). This imbalance of angiotensin receptors contributed to sympatho-excitation in the heart failure state. In the current experiment, we measured AT1R and AT2R protein expressions in the brainstem, kidney and liver from male foetuses (3 days before birth), male neonates (3 days after birth), male and female adults (8 weeks) and male aged (28 months) rats by Western blot analysis. In the brainstem, we found that the foetuses and neonates exhibited a significantly lower AT2R protein expression compared with adult rats (foetus 0.08 ± 0.01, neonate 0.12 ± 0.01, male adult 0.25 ± 0.01, female adult 0.22 ± 0.02; n = 4 per group, p < 0.001 foetus and neonate compared with male or female adults). In contrast, the foetuses and neonates expressed significantly higher AT1R protein than that of the adults (foetus 0.64 ± 0.09, neonate 0.56 ± 0.01, male adult 0.13 ± 0.02, female adult 0.08 ± 0.02; n = 4 each group, p < 0.001 foetus and neonate compared with male and female adults). In the liver, the AT2R protein was also higher in foetus and neonate, than in adult rats. Interestingly, the foetal liver expressed higher AT1R protein compared with that of the neonate. In the kidney, AT2R expression was significantly increased with age (foetus 0.08 ± 0.01, neonate 0.19 ± 0.02, male adult 0.49 ± 0.04, female adult 0.90 ± 0.10; n = 4 per group, p < 0.01–0.001). AT1R expression, on the other hand, was higher in the foetuses than that in both neonate and male adults. This study provides data contrary to existing dogma that AT2R expression is higher in foetal life and low in adults, suggesting an involvement of a potentially important functional role for AT2R in adult animals and AT1R in foetal development and/or physiology.
Keywords: angiotensin II receptors, brainstem, development, kidney, liver, protein expression
Introduction
The renin–angiotensin system (RAS) has been identified and investigated for more than 100 years.1 This system, however, is becoming more complex due to the continual discovery of newer functional components.2,3 Angiotensin II (Ang II) is the primary peptide in this system. This octapeptide activates two well-known G protein-coupled receptors: the Ang II type 1 receptor (AT1R) and the Ang II type 2 receptor (AT2R).4,5 The AT1R mediates the majority of classical biological functions of Ang II6,7 and plays a critical role in the control of sympathetic nerve activity, the regulation of blood pressure, water and electrolyte balance, thirst, hormone secretion and renal function.4 The AT1R has been implicated in a variety of pathologic conditions primarily because its effects are so widespread. Indeed, the AT1R has been regarded as a primary target for cardiovascular and renal pharmacotherapy for common diseases such as hypertension, chronic heart failure and diabetic nephropathy. Antagonists to AT1R and inhibitors of angiotensin-converting enzyme (ACE) have been routinely used to treat patients with these diseases.8–10
The AT2R on the other hand, has been viewed, for some time, as being involved only in development and growth, due to the observation of its ubiquitous expression at very high levels in the foetus and its rapid regression to low levels or disappearance after birth.4,5,11 This assertion, however, is primarily derived from studies using autoradiography,12–14 ligand binding15–17 and in situ hybridisation techniques.18–20 Notably lacking from the literature are reports of AT2R protein expression at various stages of animal development and growth. Therefore, in the present experiment, we employed Western blot analysis to measure AT1R and AT2R protein expression in brainstem, kidney and liver from Sprague–Dawley or Fisher 344 rats at various stages of development.
Methods
Animals
A total of 24 rats were used in this experiment. Sixteen Sprague–Dawley rats were purchased from SASCO (Madison, WI): four male foetuses (3 days before birth), four male neonates (3 days after birth), four male adults (8 weeks) and four female adults (8 weeks); eight Fisher 344 rats were obtained from the National Institute of Ageing: four male adults (8 weeks) and four male aged (28 months). The four foetuses were taken from four pregnant female rats, and the four neonates were taken from four litters. The sex of the foetuses and neonates was identified by sex determining region Y (SRY) expression employing reverse transcriptase polymerase chain reaction (RT-PCR; NCBI Reference Sequence: NM_001109181.1; left primer AGGGCTGGGAGAAAGAAGAG and right primer TTGCTGATCTCCGAGTTGTG). All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health, Guide for the Care and Use of Laboratory Animals.
Preparation of tissues
All rats were euthanised by CO2. The whole brain, kidney and liver were removed and immediately frozen on dry ice, then stored at −80°C. The samples were stored for a maximum of 2 weeks. For the brainstem samples, a rostral coronal section 0.5 mm from the obex was taken. For the kidney sample from the foetuses, the whole right kidney was used. For the kidney sample from the neonates and adults, a 0.5 mm horizontal section from the middle of the right kidney was taken, which included renal cortex, medulla, calyx and vessels. The hepatic anterior lobe was used as the liver sample.
Western Blot analysis
The tissues were homogenised in RIPA buffer (50 mM TrisHCl pH7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) and total protein was extracted from this homogenate. The protein concentration in each sample extract was measured using a protein assay kit (Pierce; Rockford, IL) and then was adjusted to the same value in all samples with 2X 4% SDS sample buffer. The samples were boiled for 5 min followed by loading on a 7.5% SDS-PAGE gel (30 μg protein/10 μl per well) for electrophoresis using a Bio-Rad mini gel apparatus at 40 mA/gel for 45 min. The fractionated protein on the gel was transferred onto a PVDF membrane (Millipore) and electrophoresed at 300 mA for 90 min. The membrane was first probed with AT2R primary antibody (AT2R rabbit polyclonal IgG, sc-9040, Santa Cruz Biotechnology Inc, 1:500) and secondary antibody (goat anti-rabbit IgG-HRP, Santa Cruz, 1:2500), and then treated with enhanced chemiluminescence substrate (Pierce; Rockford, IL) for 5 min at room temperature. The bands in the membrane were visualised and analysed using UVP BioImaging Systems. After obtaining the AT2R blot density, the membrane was then treated using Restore Western Blot Stripping Buffer (Thermo Scientific) to remove the AT2R signal, followed by probing with an AT1R primary antibody (AT1R rabbit polyclonal IgG, sc-1173, Santa Cruz Biotechnology Inc, 1:500) and finally with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibodies (GAPDH mouse monoclonal IgG, sc-32233, Santa Cruz Biotechnology Inc, 1:1000) using the same process as the AT2R antibody to get the AT1R and GAPDH blot densities. The final reported data are the normalised AT2R and AT1R band densities by GAPDH.
Statistical analyses
All data are reported as the mean ± SEM. A one-way analysis of variance (ANOVA) was used followed by the Student–Newman–Keuls post hoc analysis, where appropriate. Statistical analysis was performed with the aid of SigmaStat software. p < 0.05 was considered statistically significant.
Results
AT2R and AT1R protein expression in the brainstem
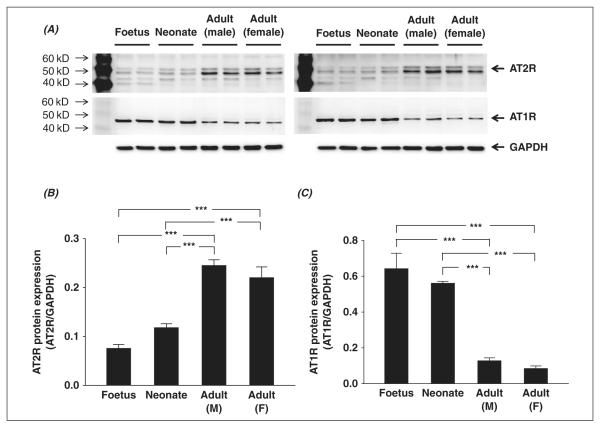
Figure 1 shows the AT2R and AT1R expressions in brainstem tissues from foetus, neonate, male adult, and female adult Sprague–Dawley rats. The top panels show the original blots from all samples (four samples for each group) and the bottom depicts the mean data. AT2R expression in the brainstem was significantly increased with age. Foetuses exhibited the lowest AT2R protein levels (0.08 ± 0.01, n = 4) and male adult rats expressed the highest AT2R protein (0.25 ± 0.01, n = 4). There were no significant differences in AT2R expression between foetus and neonate or between male and female adult tissues. Panel C shows AT1R expression in each group of rats. Mean AT1R expression in foetus (0.64 ± 0.09, n = 4) and neonate (0.56 ± 0.01, n = 4) were higher than that in male (0.13 ± 0.02, n = 4; p < 0.001 versus foetus and neonate) and female (0.08 ± 0.02, n = 4; p < 0.001 versus foetus and neonate) adult tissues. However, there were no significant differences between foetus and neonate or between male and female tissues.
Figure 1.
Western blot showing AT1R and AT2R protein expression in brainstem samples of male foetus, male neonate, male adult and female adult Sprague-Dawley rats. Panel A shows the original Western blot images (the upper bands are AT2R and the middle bands show AT1R) from all samples (four rats per group). Panel B shows the mean data for AT2R protein expression. Panel C shows the mean data for AT1R protein expression. ***p < .001.
We also examined the AT2R and AT1R expression in the brainstem of aged rats (28 months), which is shown in figure 2. We did not find any significant difference in AT2R and AT1R protein expression between adult (8 weeks) (AT2R: 0.81 ± 0.09, AT1R: 1.60 ± 0.41, n = 4) and aged (AT2R: 1.00 ± 0.08, AT1R: 1.32 ± 0.12, n = 4) Fisher 344 rats.
Figure 2.
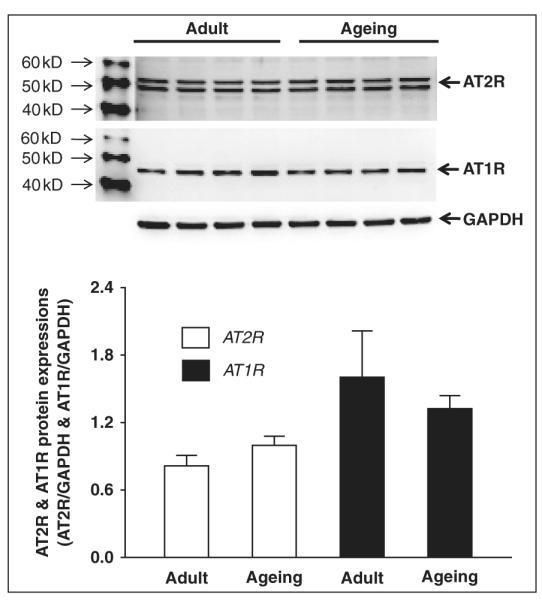
Western blot showing AT1R and AT2R protein expression in brainstem samples of adult and aged Fisher 344 rats. Panel A shows the original Western blot images (the upper bands are AT2R and the middle bands are AT1R) from all samples (four rats per group). Panel B shows the mean data for AT2R protein expression. Panel C shows the mean data for AT1R protein expression.
In order to confirm the specificity of the AT2R antibody used in the above experiment, we employed another AT2R antibody, sc-48452 (AT2R goat polyclonal IgG, Santa Cruz Biotechnology), to repeat this experiment. We obtained similar results (Panel A of figure 3). Panel B of figure 3 shows the negative control using AT2R antibody blocking peptide, sc-48452-P (Santa Cruz Biotechnology).
Figure 3.
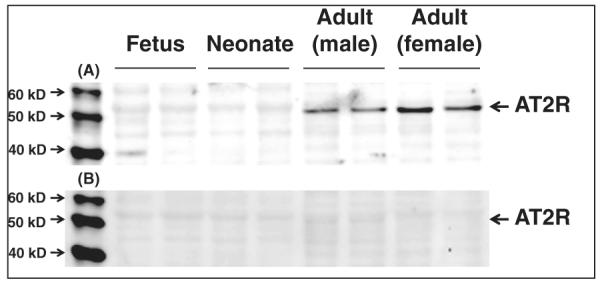
A re-evaluation of AT2R protein expression in the brainstem samples of male foetus, male neonate, male adult, and female adult Sprague-Dawley rats using a second AT2R antibody, sc-48452 (panel A) and its negative control by the blocking peptide sc48452-P (panel B).
AT2R and AT1R protein expressions in liver
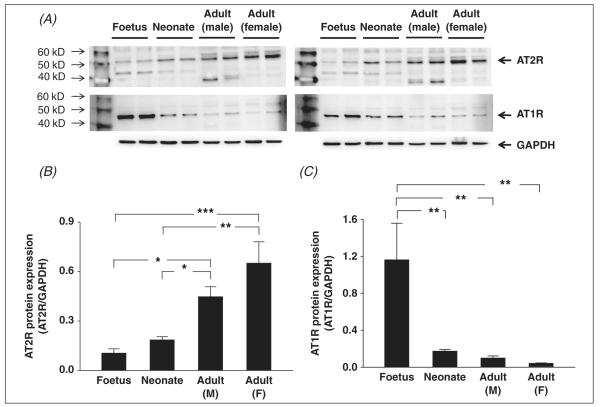
In the liver, AT2R expression in male (0.45 ± 0.06, n = 4) and female (0.65 ± 0.13, n = 4) adults was significantly higher than that in foetal rats (0.11 ± 0.03, n = 4) and neonates (0.19 ± 0.02, n = 4), however, there were no significant differences between foetal and neonate livers or between male and female adult livers (panel B in figure 4). In contrast, we found that foetal rats exhibited a significantly higher AT1R expression in the liver (1.16 ± 0.40, n = 4) compared with the other groups (neonate: 0.17 ± 0.02, male adult: 0.10 ± 0.02, female adult: 0.04 ± 0.01, n = 4 each group). AT1R expression in foetal liver was 6.7 times that of the neonate, 11.7 times that of male adults and 28.1 times that of female adults. However, there were no differences in AT1R expression between neonate, male adult and female adult rats (Panel C in figure 4).
Figure 4.
Western blot showing AT1R and AT2R protein expression in the liver of male foetus, male neonate, male adult and female adult Sprague-Dawley rats. Panel A show the original Western blot images (the upper panels are AT2R and the middle panels are AT1R) from all samples (four rats each group). Panel B show the mean data for AT2R protein expression. Panel C shows the mean data for AT1R protein expression. *p < .05, **p < .01, ***p < .001.
AT2R and AT1R protein expression in kidney
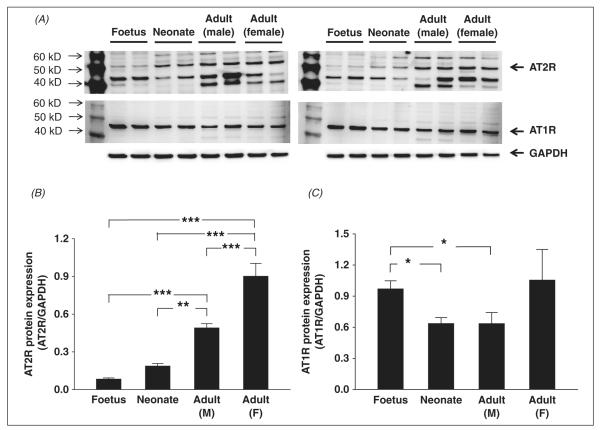
As in brainstem and liver, AT2R protein expression in the kidney also significantly increased with age. The lowest expression was in foetal rats (0.08 ± 0.01, n = 4) and the highest expression was in female adult rats (0.90 ± 0.10, n = 4) (panel B, figure 5). In renal tissue, foetal rats also had higher AT1R expression (0.97 ± 0.08, n = 4) compared with neonates (0.64 ± 0.06, n = 4) and male adult rats (0.64 ± 0.11, n = 4). There were no significant differences in AT2R expression between foetal and female adult or neonates and male kidneys (panel C, figure 5). Even though there was a tendency for female adult rats to express the highest AT1R protein level (1.05 ± 0.30, n = 4), the difference did not reach statistical significance.
Figure 5.
Western blot showing AT1R and AT2R protein expression in the kidney of male foetus, male neonate, male adult and female adult Sprague-Dawley rats. Panel A shows the original western blot images (the upper panel shows AT2R and the middle panel shows are AT1R) from all samples (four rats each group). Panel B shows the mean data for AT2R protein expression. Panel C shows the mean data for AT1R protein expression. *p < .05, **p < .01, ***p < .001.
Discussion
The major new finding from this study is that AT2R protein expression in the brainstem, liver, and kidney of rats increases progressively from foetal life to adulthood. It has been accepted generally that AT2R expression is high in foetal tissue, and then dramatically decreases or even disappears after birth.6,11,21–29 The current data clearly challenges that concept. At least in brainstem, liver and kidney tissue, adult rats exhibited a higher AT2R protein level compared with foetus or neonates.
The conventional concept on AT2R expression profiles before and after birth was derived from autoradiographical data,12–14 ligand binding experiments15–17 and in situ hybridisation.18–20 Autoradiography and ligand binding provide information on the binding and activity of a ligand to its receptor. The accuracy of these techniques depends largely on the specificity of the agonists and antagonists used. On the other hand, in situ hybridisation detects mRNA, and is a good method to locate target mRNA, however it is not a reliable technique to quantify gene expression. To the best of the authors’ knowledge the data presented here are the first to directly determine AT2R protein expression in rats at various developmental stages. More importantly, our findings are in direct opposition to the previously reported results using other techniques.12–20
The above discrepancy is not clear, but at least two potential reasons should be considered. First, we used Western blot to measure total AT2R protein, which included both cytoplasmic receptor protein (immature receptor) and plasma membrane receptor protein (mature receptor). However, autoradiography and ligand binding primarily determine mature receptors only on the cell surface. Our data might reflect differences between the total AT2R protein and the mature AT2R protein. Second, different regions, even in the same organ, may exhibit different AT2R expression level. Using competition binding Gwathmey et al.30 demonstrated that AT2R was the predominant receptor subtype in the renal cortex but that AT1R was predominant in the renal medulla of both adult and foetal sheep. However, they did not find significant differences in AT2R or AT1R binding activity between adult and foetal kidneys. In the foetal ovine brain, on the other hand, Hu et al.31 documented a predominance of AT2R in the cerebellum but AT1R in the hypothalamus. In a recent review Mao et al.32 summarised mRNA expression levels for angiotensin receptors in different regions of the foetal rat brain: AT2R mRNA predominated in some brain regions but AT1R in others.
Our present finding of lower AT2R protein expression in the foetus and neonate compared with adults suggests a re-examination of the functional significance of this receptor. Even though AT2R has been conventionally thought of as an important modulator of foetal development and growth due to the currently accepted concept that this receptor is expressed transiently and abundantly only during embryonic/foetal development, there is little evidence to support this notion. For example, AT2R knockout mice demonstrate apparently normal embryogenesis.33,34 These data therefore fail to support the hypothesis of an essential role of this receptor in growth and development. In contrast, the AT2R has been often documented as a predominantly negative regulator of vascular smooth-muscle-cell proliferation both in vivo and in vitro experiments.35,36 These anti-proliferative and anti-growth effects of AT2R were also observed in neurons,37 pheochromocytoma,38 fibroblasts,39 renal mesangial cells,40 coronary endothelial cells41 and in vivo on microvascular growth.42 These data are consistent with our current findings that AT2R expression is lower in foetal and neonatal tissue. In contrast, compatible with the well-known growth and proliferative function of AT1R,4,7 our findings that foetal and neonatal tissue expresses a higher AT1R protein implies a potential role for this angiotensin receptor subtype in organism growth and development. Interestingly, functional data also appear to support our current finding that, in the foetal brain, the AT1R but not AT2R is the dominant angiotensin receptor subtype. For example, in the ovine foetus, intracerebroventricular (icv) injection of Ang II evoked a significant increase in mean arterial blood pressure,43,44 which typifies a central AT1R effect, not that of AT2R stimulation. Indeed, Shi et al.45 further demonstrated that the pressor response to Ang II in the ovine foetal brain was completely abolished by the AT1R antagonist, Losartan, but not by the AT2R blocker, PD123319. These functional data strongly suggest that, at least in the ovine foetal brain, AT1R mediates the responses to central Ang II.
It is of interest that the pattern of reciprocal changes in AT1R and AT2R during maturation occurred in all tissues examined. This phenomenon may imply a possible reciprocal inhibition of protein expression between these two angiotensin receptors during development. The underlying mechanisms are not clear, but the interaction between AT1R and AT2R at the transcriptional level has been well documented. AT1R activation enhances AT2R mRNA degradation46 and decreases AT2R mRNA accumulation.47 In addition, AT1R mRNA and protein are increased in AT2R knockout mice.48 Transfection of vascular smooth muscle cells with the AT2R gene results in a decreased expression of AT1R mRNA and protein.49 This possible reciprocal inhibition in gene expression between AT1R and AT2R has also been observed in pathologic conditions. Employing Western blot analysis, we found that in the rostral ventrolateral medulla (RVLM) of rats with chronic heart failure, AT1R protein expression was up regulated while AT2R protein expression was down regulated.50 Using RT-PCR and autoradiography, Peng and Phillips51 demonstrated an increased AT1R mRNA expression and receptor binding, but decreased AT2R mRNA and receptor binding in the hypothalamus and brainstem of rats with cold-induced hypertension.
We recognise that Western blotting is the only technique we used in this experiment, and therefore some limitations of our data should be discussed. Owing to the lack of mRNA data, we do not know whether this change in AT2R/AT1R protein occurs at the transcriptional or translation level. Because we did not determine ligand-receptor binding activity, we are not sure if the observed AT2R/AT1R protein expression profile represents similar changes in receptor function. However, the current data raise some critical questions concerning angiotensin receptors: does the AT2R really decline after birth? What is the role of AT2R in the adult? Does the AT1R have any functional significance in foetal development and growth?
Acknowledgments
Funding This study was supported by a Scientist Development Grant from the American Heart Association (grant number 0635007N) and the NIH (grant numbers RO1HL093028, PO1HL62222 and RO1HL038690).
References
- 1.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 2.Santos RA, Ferreira AJ, Simoes E, Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 3.Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–137. [PMC free article] [PubMed] [Google Scholar]
- 4.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 5.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 6.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35:1001–1015. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 7.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 8.Basile J, Toth PP. Angiotensin receptor blockers: role in hypertension management, cardiovascular risk reduction, and nephropathy. South Med J. 2009;102(10 Suppl):S1–S12. doi: 10.1097/SMJ.0b013e3181ba0d8a. [DOI] [PubMed] [Google Scholar]
- 9.Neutel JM. Choosing among renin-angiotensin system blockers for the management of hypertension: from pharmacology to clinical efficacy. Curr Med Res Opin. 2010;26:213–223. doi: 10.1185/03007990903444434. [DOI] [PubMed] [Google Scholar]
- 10.Berl T. Review: renal protection by inhibition of the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2009;10:1–8. doi: 10.1177/1470320309102747. [DOI] [PubMed] [Google Scholar]
- 11.Carey RM. Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens. 2005;14:67–71. doi: 10.1097/00041552-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88:921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci U S A. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciuffo GM, Viswanathan M, Seltzer AM, Tsutsumi K, Saavedra JM. Glomerular angiotensin II receptor subtypes during development of rat kidney. Am J Physiol. 1993;265:F264–F271. doi: 10.1152/ajprenal.1993.265.2.F264. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi K, Saavedra JM. Characterization of AT2 angiotensin II receptors in rat anterior cerebral arteries. Am J Physiol. 1991;261:H667–H670. doi: 10.1152/ajpheart.1991.261.3.H667. [DOI] [PubMed] [Google Scholar]
- 17.Sechi LA, Griffin CA, Grady EF, Kalinyak JE, Schambelan M. Characterization of angiotensin II receptor subtypes in rat heart. Circ Res. 1992;71:1482–1489. doi: 10.1161/01.res.71.6.1482. [DOI] [PubMed] [Google Scholar]
- 18.Aguilera G, Kapur S, Feuillan P, Sunar-Akbasak B, Bathia AJ. Developmental changes in angiotensin II receptor subtypes and AT1 receptor mRNA in rat kidney. Kidney Int. 1994;46:973–979. doi: 10.1038/ki.1994.356. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugam S, Llorens-Cortes C, Clauser E, Corvol P, Gasc JM. Expression of angiotensin II AT2 receptor mRNA during development of rat kidney and adrenal gland. Am J Physiol. 1995;268:F922–F930. doi: 10.1152/ajprenal.1995.268.5.F922. [DOI] [PubMed] [Google Scholar]
- 20.Shanmugam S, Corvol P, Gasc JM. Angiotensin II type 2 receptor mRNA expression in the developing cardiopulmonary system of the rat. Hypertension. 1996;28:91–97. doi: 10.1161/01.hyp.28.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Stoll M, Unger T. Angiotensin and its AT2 receptor: new insights into an old system. Regul Pept. 2001;99:175–182. doi: 10.1016/s0167-0115(01)00246-4. [DOI] [PubMed] [Google Scholar]
- 22.Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 23.Widdop RE, Vinh A, Henrion D, Jones ES. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008;35:386–390. doi: 10.1111/j.1440-1681.2008.04883.x. [DOI] [PubMed] [Google Scholar]
- 24.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 25.Steckelings UM, Kaschina E, Unger T. The AT2 receptor—a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Wolf G. ‘The road not taken’: role of angiotensin II type 2 receptor in pathophysiology. Nephrol Dial Transplant. 2002;7:195–198. doi: 10.1093/ndt/17.2.195. [DOI] [PubMed] [Google Scholar]
- 27.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Carey RM. Angiotensin type-2 receptors and cardiovascular function: are angiotensin type-2 receptors protective? Curr Opin Cardiol. 2005;20:264–269. doi: 10.1097/01.hco.0000166596.44711.b4. [DOI] [PubMed] [Google Scholar]
- 29.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 30.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, et al. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu F, Morrissey P, Yao J, Xu Z. Development of AT(1) and AT(2) receptors in the ovine fetal brain. Brain Res Dev Brain Res. 2004;150:51–61. doi: 10.1016/j.devbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain renin-angiotensin system and hypertension programmed in fetal origins. Prog Neurobiol. 2009;87:252–263. doi: 10.1016/j.pneurobio.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 34.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–570. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 35.Akishita M, Horiuchi M, Yamada H, Zhang L, Shirakami G, Tamura K, et al. Inflammation influences vascular remodeling through AT2 receptor expression and signaling. Physiol Genomics. 2000;2:13–20. doi: 10.1152/physiolgenomics.2000.2.1.13. [DOI] [PubMed] [Google Scholar]
- 36.Tea BS, Der SS, Touyz RM, Hamet P, deBlois D. Proapoptotic and growth-inhibitory role of angiotensin II type 2 receptor in vascular smooth muscle cells of spontaneously hypertensive rats in vivo. Hypertension. 2000;35:1069–1073. doi: 10.1161/01.hyp.35.5.1069. [DOI] [PubMed] [Google Scholar]
- 37.Laflamme L, Gasparo M, Gallo JM, Payet MD, Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J Biol Chem. 1996;271:22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- 38.Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122:59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- 39.Tsuzuki S, Eguchi S, Inagami T. Inhibition of cell proliferation and activation of protein tyrosine phosphatase mediated by angiotensin II type 2 (AT2) receptor in R3T3 cells. Biochem Biophys Res Commun. 1996;228:825–830. doi: 10.1006/bbrc.1996.1739. [DOI] [PubMed] [Google Scholar]
- 40.Goto M, Mukoyama M, Suga S, Matsumoto T, Nakagawa M, Ishibashi R, et al. Growth-dependent induction of angiotensin II type 2 receptor in rat mesangial cells. Hypertension. 1997;30:358–362. doi: 10.1161/01.hyp.30.3.358. [DOI] [PubMed] [Google Scholar]
- 41.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z, Shi L, Yao J. Central angiotensin II-induced pressor responses and neural activity in utero and hypothalamic angiotensin receptors in preterm ovine fetus. Am J Physiol Heart Circ Physiol. 2004;286:H1507–H1514. doi: 10.1152/ajpheart.00764.2003. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Shi L, Hu F, White R, Stewart L, Yao J. In utero development of central ANG-stimulated pressor response and hypothalamic fos expression. Brain Res Dev Brain Res. 2003;145:169–176. doi: 10.1016/s0165-3806(03)00226-8. [DOI] [PubMed] [Google Scholar]
- 45.Shi L, Mao C, Thornton SN, Sun W, Wu J, Yao J, Xu Z. Effects of intracerebroventricular losartan on angiotensin II-mediated pressor responses and c-fos expression in near-term ovine fetus. J Comp Neurol. 2005;493:571–579. doi: 10.1002/cne.20802. [DOI] [PubMed] [Google Scholar]
- 46.Volpe M, Musumeci B, De Paolis P, Savoia C, Morganti A. Angiotensin II AT2 receptor subtype: an uprising frontier in cardiovascular disease? J Hypertens. 2003;21:1429–1443. doi: 10.1097/00004872-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 47.De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, et al. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens. 1999;17:1873–1877. doi: 10.1097/00004872-199917121-00015. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Tsuchida S, Imai T, Fujii N, Miyazaki H, Ichiki T, et al. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem Biophys Res Commun. 1999;258:194–198. doi: 10.1006/bbrc.1999.0500. [DOI] [PubMed] [Google Scholar]
- 49.Jin XQ, Fukuda N, Su JZ, Lai YM, Suzuki R, Tahira Y, et al. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension. 2002;39:1021–1027. doi: 10.1161/01.hyp.0000016179.52601.b4. [DOI] [PubMed] [Google Scholar]
- 50.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng JF, Phillips MI. Opposite regulation of brain angiotensin type 1 and type 2 receptors in cold-induced hypertension. Regul Pept. 2001;97:91–102. doi: 10.1016/s0167-0115(00)00218-4. [DOI] [PubMed] [Google Scholar]