Abstract
This review highlighted the following: (i) pathogenic mechanism of the thermostable direct hemolysin produced by Vibrio parahaemolyticus, especially on its cardiotoxicity, (ii) heat-labile and heat-stable enterotoxins produced by enterotoxigenic Escherichia coli, especially structure–activity relationship of heat-stable enterotoxin, (iii) RNA N-glycosidase activity of Vero toxins (VT1 and VT2) produced by enterohemorrhagic Escherichia coli O157:H7, (iv) discovery of Vibrio cholerae O139, (v) isolation of new variant of Vibrio cholerae O1 El Tor that carries classical ctxB, and production of high concentration of cholera toxin by these strains, and (vi) conversion of viable but nonculturable (VBNC) Vibrio cholerae to culturable state by co-culture with eukaryotic cells.
Keywords: thermostable direct hemolysin, heat-labile and heat-stable enterotoxins, RNA N-glycosidase activity of Vero toxins, Vibriocholerae O139, Viable but nonculturable (VBNC) Vibriocholerae
Introduction
My scientific career was initiated in 1961 as a student of Dr. Tsunesaburo Fujino, Professor of Bacteriology and Serology, Research Institute for Microbial Diseases, Osaka University, who discovered Vibrio parahaemolyticus. During last 50 years, I have worked on enteric pathogens such as Vibrio parahaemolyticus, enterotoxigenic Escherichia coli, enterohemorrhagic E. coli and Vibrio cholerae. In this article I will summarize what my colleagues and myself have accomplished on these enteric pathogens. Some background to understand why I have worked on the specific topics will also be discussed.
Vibrio parahaemolyticus
In 1950, Fujino et al.1) discovered Vibrio parahaemolyticus as a causative bacterium of a case of food poisoning occurred in Osaka, Japan. It is one of the most important causative agents of bacterial food poisoning. In epidemiological studies on V. parahaemolyticus, Kato et al.2) found that strains isolated from patients caused hemolysis on special blood agar. The agent to cause the hemolysis was identified to be thermostable direct hemolysin (TDH).3)
To study the pathogenesis of TDH, we first purified TDH from a strain isolated from a patient.4) The purified TDH is composed of two subunits of 21K dalton5) and is not inactivated by heating at 100 ℃ for 10 minutes. It shows various biological activities such as hemolytic activity, cytotoxic activity on various cultured cells, enterotoxicity in rabbit ileal loops and lethal toxicity in mice and rats.
We demonstrated that the lethal toxicity of the purified TDH was due to its cardiotoxicity.6) This was first suggested by a rapid death of mice and rats after intravenous injection of TDH. As shown in Table 1 , more than 5 µg of TDH killed mice within 1 minute after intravenous injection. Even 1 µg of TDH killed mice within 10 minutes. Several bacterial toxins, such as streptolysin O, tetanolysin, hemolysin of Listeria monocytogenes, caused rapid death of animals on intravenous injection and it was demonstrated that they showed cardiotoxicity. Thus, we attempted to demonstrate the cardiotoxicity of TDH.
Table 1.
Lethal activity of TDH on intravenous injection into mice (from Ref. 6)
| Amount of TDH injected (µg of protein per mouse) |
Survival time after injection (mean ± S.D.) |
| 10.0 | 35.5 ± 4.8 sec. |
| 5.0 | 49.0 ± 8.4 |
| 2.5 | 561.2 ± 368.8 |
| 1.0 | 1121.5 ± 291.0 |
| 0.5 | no death |
When ventricular tissue from the hearts of 14–16 days old mouse fetuses were cultured in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum at 36 ℃ under an atmosphere of 5% CO2 and 95% air, the cells beat spontaneously and regularly. When 1–1.5 × 106 cells were seeded into dishes, a large cluster of 2–4 mm in diameter containing more than 105 cells was obtained after cultivation for 1 day. All the myocardial cells in cell cluster beat synchronously and regularly at 100–180 beats/minute and this rate was maintained for at least 24 hours.
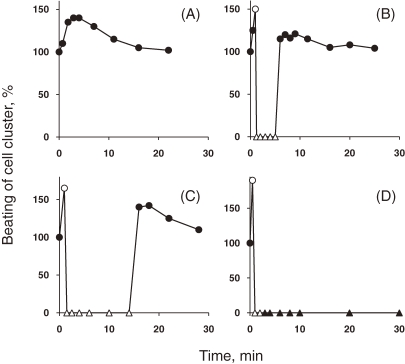
Figure 1 shows the effect of TDH on the beating of a cell cluster of cultured myocardial cells.6) On addition of 0.05 µg/ml of TDH to the medium the beating increased slightly, but it returned to normal within 10 minutes (Fig. 1A). On addition of 0.1 µg of TDH, the beating was first rapidly stimulated and then stopped suddenly within one minute. Then, six minutes after the addition of TDH, the beating suddenly started again at the normal rate and remained unchanged during further observation (Fig. 1B). Similarly on addition of 0.2 µg of TDH, the beating at first increased, then stopped and then started again (Fig. 1C), but interval between the time of stopping and of starting again was longer than that on addition of 0.1 µg of TDH. On addition of 1 µg or more of TDH per ml of medium, the beating also first increased and then stopped abruptly, and then almost all the cells rapidly disintegrated (Fig. 1D).
Figure 1.
Effect of TDH on the beating of a cell cluster of cultured myocardial cells (from Ref. 6). Mouse heart cells were cultured and the amounts of TDH indicated below were added to the medium at zero time. The beating of the cell clusters was expressed as a percentage of that observed in the absence of TDH. The concentrations of TDH were: (A) 0.05 µg/ml, (B) 0.1 µg/ml, (C) 0.2 µg/ml and (D) 1 µg/ml. (●) normal beating, (○) weaker beating, (△) no beating but no cell disintegration, (▲) cell disintegration.
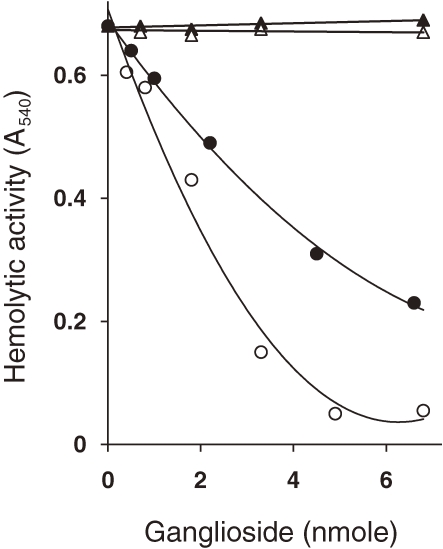
To study further the various kinds of biological activities of TDH on various kinds of cells, such as hemolysis on erythrocytes and cardiotoxicity on myocardial cells, attempts to determine the membrane receptor for TDH was carried out. Figure 2 shows the result of such an experiment.7) When TDH was preincubated with GT1 and GD1a gangliosides, its hemolytic activity was lost, whereas when it was preincubated with GM1 and GM2 gangliosides its hemolytic activity did not decrease. Treatment of ganglioside GT1 and GD1a with neuraminidase abolished their inhibitory effects on the hemolytic activity of TDH. It is known that TDH caused hemolysis of human erythrocytes, but not of horse erythrocytes. The absence of GT1 and GD1a gangliosides on horse erythrocytes well explained the above results.
Figure 2.
Effect of various gangliosides on the hemolytic activity of TDH (from Ref. 7). The indicated amounts of various gangliosides were mixed with 5 µg (∼0.12 nmol) of TDH in 0.01 M Tris–HCl buffer (pH 7.2) in a volume of 0.125 ml and incubated at 37 ℃ for 30 min. Hemolytic activity was assayed after further incubation at 37 ℃ for 30 min by measuring the absorbance at 540 nm. (○) GT1, (●) GD1, (△) GM1, (▲) GM2.
Diarheagenic Escherichia coli
Escherichia coli is a commensal bacterium in human large intestine. However, several kinds of diarheagenic E. coli that cause diarrhea in humans have been reported. They are enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (EHEC) and enteroaggregative E. coli (EAgEC). Each of them causes diarrhea in human by different mechanisms. Among these five different kinds of diarrheagenic E. coli, diseases caused by ETEC and EHEC are known to be toxin-mediated. In this section, I will discuss on the toxins produced by ETEC and EHEC and the diseases caused by these diarrheagenic E. coli.
(i). ETEC.
ETEC was first reported in 1956 by De in India.8) He found that E. coli strain isolated from stool of cholera-like patient gave a similar positive reaction in the ileal loop test as V. cholerae did. Later in 1971, Sack et al.9) demonstrated a protein toxin in the culture supernatant of ETEC, which is currently known as heat-labile enterotoxin (LT).
The importance of ETEC is highlighted in 1976 when Merson et al.10) reported that ETEC is a major enteropathogen that cause traveller’s diarrhea. In our country, we reported that ETEC was isolated from about 40% of diarrheal patients who just came back to Osaka (Itami) International Airport from South-East Asian countries.11)
Smith and Gyles12) reported that ETEC produces heat-stable enterotoxin (ST) in addition to LT already reported by Sack et al.9) LT is a protein toxin inactivated by heating at 60 ℃ for 10 minutes while ST is a peptide toxin not inactivated by heating even 100 ℃ for 10 minutes.
Among many reports that attempted to purify LT, the most successful one is that reported by Clements and Finkelstein.13) Their method is quite unique: they found that LT was absorbed by Agarose A5m gel and was eluted out by a buffer containing 0.2 M galactose. Applying their method to purify LT we found that there are two immunologically distinct LTs.14) One is LT from ETEC isolated from human patient (LTh) and the other is that from porcine stool (LTp). At that time, it was known that LT is cross-reactive with cholera toxin (CT) produced by V. cholerae. We examined the immunological relationship among these three enterotoxins, LTh, LTp and CT, and found that they are immunologically related but not identical.15) Several other investigators confirmed our finding by nucleotide sequence analysis of the genes encoding LTh, LTp and CT.
Regarding ST, we purified ST in collaboration with Dr. Yasutsugu Shimonishi and his colleagues of Protein Institute of Osaka University. Like LT, we found that there are two different kinds of ST, that is one from human patient (STh) and the other from porcine stool (STp). It was found that STh consisted of 19 amino acids,16) while STp consisted of 18 amino acids.17) As shown in Table 2 , primary structure of both STs is quite similar but is differ in some amino acids.
Table 2.
| STh: Asn-Ser-Ser-Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr |
| STp: Asn-Thr-Phe-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr |
To study a structure–activity relationship of STs, we tried chemical synthesis of mature STs as well as their short analogues.18,19) It was found that chemically synthesized STh and STp showed the similar biological activity to those produced by ETEC in suckling mouse assay (accumulation of fluid in intestine). Moreover, as shown in Table 3 , short analogues of both STh and STp, which have less numbers of amino acids at their N-terminus as well as C-terminus, showed positive reaction in the suckling mouse assay, thus was concluded that a peptide consist of 13 amino acids was a core to show the biological activity.20)
Table 3.
Biological activity of chemically synthesized STh, STp and their analogues (from Ref. 20)
| Amino acid sequence | Minimum effective dose (ng/100 µl) |
|
| Asn-Ser-Ser-Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(1–19) | 0.8 |
| Asn-Ser-Ser-Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys | STh(1–18) | 0.4 |
| Ser-Ser-Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(2–19) | 0.8 |
| Ser-Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(3–19) | 1.3 |
| Asn-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(4–19) | 1.1 |
| Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(5–19) | 0.8 |
| Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys | STh(5–18) | 0.5 |
| Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys-Tyr | STh(6–19) | 0.6 |
| Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Thr-Gly-Cys | STh(6–18) | 0.6 |
| Asn-Thr-Phe-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr | STp(1–18) | 1.0 |
| Asn-Thr-Phe-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys | STp(1–17) | 1.3 |
| Thr-Phe-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr | STp(2–18) | 0.5–2.0 |
| Phe-Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr | STp(3–18) | 1.5–2.0 |
| Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr | STp(4–18) | 0.8–1.0 |
| Tyr-Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys | STp(4–17) | 1.2 |
| Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys-Tyr | STp(5–18) | 0.8–1.0 |
| Cys-Cys-Glu-Leu-Cys-Cys-Asn-Pro-Ala-Cys-Ala-Gly-Cys | STp(5–17) | 0.7 |
(ii). EHEC.
EHEC is defined by its ability to produce Vero toxin (VT) that is cytotoxic to Vero cells. The first outbreak of food poisoning caused by EHEC was reported in 1982 in the US. In this outbreak, Riley et al.21) isolated an E. coli O157:H7 strain as a new kind of bacteria to cause diarrhea. Symptoms associated with this organism were quite severe with abdominal cramps and bloody diarrhea, which was named as hemorrhagic colitis. O’Brien et al.22) found that E. coli O157:H7 reported by Riley et al.21) produced VT and the cytotoxic activity was neutralized by an antitoxin to Shiga toxin produced by Shigella dysenteriae type 1. This finding was quite unique at that time as the toxins produced by two different bacterial species were immunologically related each other. It is because of this related characteristic that EHEC is also called Shiga-toxin producing E. coli (STEC).
There are two types of VT, namely VT1 and VT2. VT1 was first reported by Konowalchuk23) in 1977 and several years later confirmed by others.24,25) On the other hand, VT2 that was immunological related but different to VT1 was isolated for the first time in 1986 from a patient admitted to an Infectious Disease Hospital in Tokyo.26) Almost the same time, Scotland et al.27) and Strockbine et al.28) reported the existence of VT2.
Several investigators including our group purified both VT129) and VT2.30) Although both VTs have the similar biological activities, VT2 was more potent than VT1. Both VT1 and VT2 consist of two subunits: one molecule of A subunit and five molecules of B subunit. The A subunit has an enzymatic activity as described below and the B subunit binds to the receptor molecule of cell surface. Many investigators reported the primary structure of both VTs. We determined by both amino acid and nucleotide sequencing that the primary structure of VT1 is exactly the same to that of Shiga toxin.31)
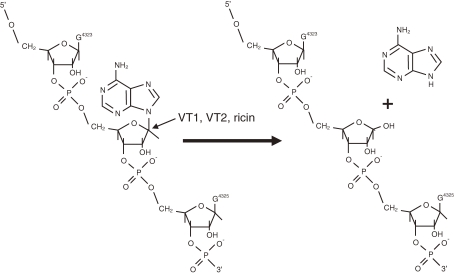
Regarding the molecular mode of action of Shiga toxin, it has been known that Shiga toxin inhibits protein synthesis in eukaryotic cells by inhibiting elongation factor 1 (EF-1)-dependent aminoacyl-tRNA binding to 60S ribosomal subunits.32) We demonstrated that not only VT1 but also VT2 inactivated 60S ribosomal subunits and inhibited EF-1-dependent aminoacyl-tRNA binding in eukaryotic cells.33,34) To elucidate further the mode of action of VTs, we studied in conjunction with the results obtained on the mode of action of a plant rectin, ricin. Endo and his coworkers35,36) demonstrated that ricin showed RNA N-glycosidase activity and cleaved N-glycosidic bond of an adenosine at position 4,324 from the 5′ terminus of the 28S ribosomal RNA of 60S ribosomal subunits of eukaryotic cells, which caused the inhibition of protein synthesis. Having recognized that the mode of action of Shiga toxin was similar to that of ricin, we demonstrated that Shiga toxin and VT2 exhibited an RNA N-glycodidase activity and cleaved the N-glycosidic bond of the adenosine residue, as shown in Fig. 3 , at position 4,324 from the 5′ terminus of the 28S ribosomal RNA of 60S ribosomal subunit of rabbit reticulocytes (Fig. 3).37)
Figure 3.
RNA N-glycosidase activity of VT1 (Shiga toxin), VT2 and ricin (from Ref. 37).
Since the molecular modes of action of Shiga toxin and VTs were found to be exactly the same as that of ricin, the amino acid sequences of the A subunit of VT1, VT2 and ricin were compared to determine sequence homology(s). Three regions of the homology containing conserved (identical or chemically similar) amino acid residues were identified. By site-directed mutagenesis of the targeted gene to encode a specific amino acid, we constructed several mutant genes that encoded mutant VT1 with a single amino acid replacement in each of the three regions described above. It was found that among many mutant VT1s, two mutants that had the replacement of Glu 167 by glutamine (E167Q) and of Arg 170 by leucine (R170L) showed significantly reduced toxicity.38) The mutant VT1s were purified and their activities were examined. As shown in Table 4 , both cytotoxic activity to Vero cells and inhibitory activity of protein synthesis in rabbit reticulocyte lysate were markedly decreased in the purified E167Q.39) It was demonstrated that the mutant VT1, E167Q, possessed similar antigenicities to that of wild type VT1, suggesting that the mutant VT1, E167Q, may be used as a candidate toxoid to protect VT1-mediated disease.
Table 4.
Comparison of biological activities of the purified mutant VT1s and wild-type VT1 (from Ref. 38)
| Toxin | Cytotoxic activity CD50 (µg) |
Mouse lethality LD50 (µg) |
Inhibition of protein synthesis ED50 (µg) |
| E167Q | 30 | 80 | >100 |
| R170L | 9 | 10 | 2 |
| Wild-type | 0.0001 | 0.04 | 0.008 |
Vibrio cholerae
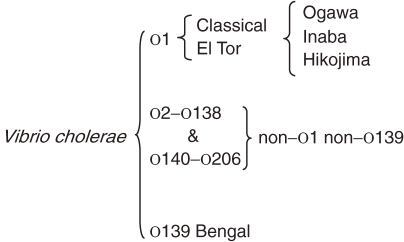
V. cholerae is classified into two biotypes, namely classical and El Tor. The classification is based on several phenotypes, such as susceptibility to polymixin B, chicken erythrocytes agglutination, hemolysis of sheep erythrocytes and Voges–Proskauer test which measures the production of acetylmethylcarbinol, and phage susceptibilities. The organisms of each biotype are further classified into serogroups on the basis of variations in the cell surface lipopolysaccharide (O antigen) More than 200 serogroups are so far idenitified. Moreover, both classical and El Tor biotypes show three different serotypes, namely Ogawa, Inaba and Hikojima. A summary of the classification is as shown in Fig. 4 .
Figure 4.
Classification of Vibrio cholerae with special reference to O serogroup.
(i). Discovery of V. cholerae O139.
Until 1992, it was known that only O1 serogroup of V. cholerae strain was associated with epidemic and pandemic cholera, and that strains which did not agglutinate with the O1 antiserum (collectively called non-O1 V. cholerae) were widely distributed in the aquatic environment and were responsible for sporadic cases of gastroenteritis.
In November 1992, non-O1 V. cholerae strains were isolated from patients of cholera-like disease in Chennai (then Madras), India where a large explosive outbreak of the disease occurred. Almost concurrently, an unexplained shift from the previously dominant O1 serogroup to the non-O1 sergroup occurred in the isolation rates of V. cholerae from cholera patients admitted to the Infectious Diseases Hospital in Kolkata (then Calcutta). This was followed by a large outbreak of clinical cholera due to the non-O1 strains of V. cholerae in the southern coastal belt of Bangladesh between December 1992 and January 1993.40,41)
We carried out an extensive characterization of the isolated non-O1 strains and found that all the non-O1 strains of V. cholerae having the following unusual properties: (i) all the strains did not agglutinate with polyvalent O1 antiserum or with monoclonal antibodies against factors A, B and C of the O1 serogroup that are the determinant factors of Ogawa, Inaba and Hikojima serotypes; (ii) all the strains did not agglutinate with antisera against any of the existing 137 serogroups of V. cholerae non-O1 recognized at that time; (iii) all the strains produced cholera toxin, which is unusual for the strains of the non-O1 serogroups.
Serological studies revealed that the non-O1 outbreak strains from India and Bangladesh were similar and distinct from the existing 138 serogroups of V. cholerae; consequently these strains were assigned to a new serogroup, namely O139 and given a synonym “Bengal” to symbolize the first isolation of these strains from coastal areas located on the Bay of Bengal.42)
On investigation, the clinical features of the disease caused by the O139 V. cholerae were found to be indistinguishable from those of cholera caused by the O1 V. cholerae.43) Cholera working group, International Centre for Diarrhoeal Diseases Research, Bangladesh44) reported similar findings with patients in Bangladesh. Based on these findings we designated the disease caused by V. cholerae O139 as cholera.43) WHO promptly responded to these reports and designated the disease caused by V. cholerae O139 as cholera.45)
V. cholerae O139 spreads rapidly in India46) and Bangladesh, and to several Asian countries; first isolated in Thailand47) and then in Nepal, Pakistan, Malaysia and China. Imported cases were also reported from several countries worldwide including Japan.
Initially it was predicted that V. cholerae O139 might spread all over the world and the eighth pandemic of cholera might be recoded, but the spread was restricted in the Indian subcontinent. Moreover, the isolation of O139 strains from cholera patients was so limited that the isolation rate in Kolkata these days has been less than 1%.48)
(ii). Emergence of V. cholerae El Tor variant and its cholera toxin production.
In two biotypes of Vibrio cholerae O1, the classical biotype has been responsible for the fifth and sixth cholera pandemics, which were recorded during 1881–1896 and 1899–1923, respectively, while the El Tor biotype is responsible to the seventh pandemic which started in 1961 from Celebes (currently Sulawesi), Indonesia and is still ongoing. One of the characteristics of these two biotypes is that each biotype has unique gene sequences for cholera toxin B subunit (CTB), that is, classical ctxB and El Tor ctxB.
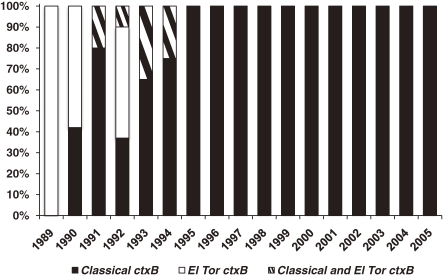
Nair et al.49) in 2006 in Bangladesh isolated strains that possess phenotypic El Tor biotype with classical ctxB. For this new type of strains of V. cholerae O1, we have recently proposed the designation of El Tor variants.50) Subsequent to the isolation of El Tor variant in Bangladesh,49) El Tor variant strains were isolated from several countries and areas in Asia and Africa.51–54) In Kolkata, India, we also found that all V. cholerae O1 isolated were El Tor variant.55) Moreover, as shown in Fig. 5 , El Tor variant appeared in 1990 and a complete replacement of prototype El Tor strains by El Tor variant strains occurred since 1995.55)
Figure 5.
Rate of classical ctxB and El Tor ctxB in Vibrio cholerae O1 strains isolated in Kolkata, India during 1989–2005 (from Ref. 55).
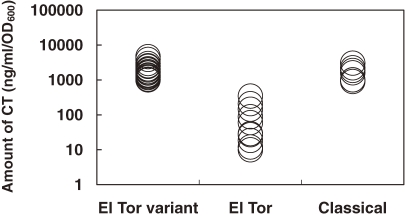
It has been known that clinical manifestation of cholera caused by classical strains is more severe than that caused by prototype El Tor strains. This phenomenon has been hypothetically explained due to a significant difference between the amounts of CT produced by these two biotype strains, that is, classical strains produce much more CT than prototype El Tor strains. Recently World Health Organization56) reported that V. cholerae El Tor variant causes more severe episodes of cholera with higher case fatality rates. This report prompted us to examine whether El Tor variant strains produce more CT than prototype El Tor strains. As shown in Fig. 6 , it was found that the amount of CT produced by El Tor variant strains was more or less similar to that produced by classical strains and was much higher than that produced by prototype El Tor strains.57) From these data we hypothesize that severe symptoms of cholera caused by El Tor variant strains of V. cholerae might be due to high CT production of the strains.
Figure 6.
Amount of CT produced by various biotypes of Vibrio cholerae O1. The amount of CT was measured by the bead-ELISA reported by Oku et al.75) Each circle represents the amount produced by 1 strain (from Ref. 57).
(iii). Viable but nonculturable V. cholerae.
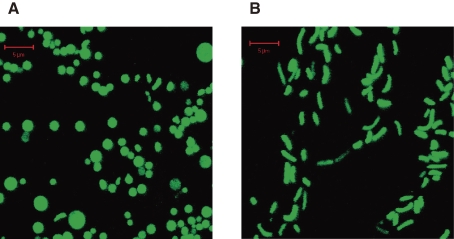
The viable but nonculturable (VBNC) state in bacteria is defined as the bacteria remain viable but the cells do not grow or divide on, or in, routinely used bacteriological media. VBNC state of Vibrio cholerae O1 was first reported by Colwell and her colleagues58) and this was subsequently confirmed by many groups.59–64) In V. cholerae O1 or O139, fresh culture of the bacteria was inoculated in a liquid of little nutrient like buffers and kept at low temperature in dark, cells became VBNC state. VBNC state of V. cholerae can be easily identified morphologically, as it became a round form as shown in Fig. 7 .65)
Figure 7.
Morphology of VBNC and culturable Vibrio cholerae O139. The cells were labeled by GFP. (A) VBNC state, (B) culturable state. A bar represents 5 µm (from Ref. 65).
VBNC states of more than 60 species of pathogenic and non-pathogenic bacteria have so far been described.66) However, there has been some skepticism on this phenomenon as a current gold standard of bacteriology is based on culturability of bacteria on, or in, appropriate media.
Several conditions to convert VBNC to culturable state have so far been reported. These are temperature upshift,60,67) incubation in phosphate buffer,68) supplementation with H2O2-degrading compounds, such as catalase or sodium pyruvate,69) addition of heat-stable autoinducer of growth,70) addition of resuscitation promoting factor,71) and presence of Acanthamoeba castellanii.72) However, there is no consensus to any of the above conditions as the stable condition to convert VBNC to culturable state.
Quite recently, we discovered that VBNC Vibrio cholerae O1 and O139 were converted to the culturable state when co-cultured with eukaryotic cells, such as HT-29, Caco-2, T84, HeLa, and Intestine 407 and CHO cells (Table 5 ).65) In early works by Colwell’s group,73) it was demonstrated that inoculation of VBNC V. cholerae O1 into rabbit ileal loops resulted in fluid accumulation from which culturable V. cholerae O1 could be isolated. Moreover, it was shown that VBNC V. cholerae O1 converted to the culturable state after ingestion during a human volunteer study.74) Our results that showed the cultured eukaryotic cells converted VBNC V. cholrae strains to culturable state might have reproduced the phenomenon that Colwell and her colleagues73,74) have demonstrated in rabbit and human volunteers.
Table 5.
Conversion of VBNC V. cholerae O139 VC-280 to the culturable state by co-culture with various eukaryotic cells (from Ref. 65)
| HT-29 | Caco-2 | T84 | HeLa | Intestine 407 |
CHO | |
| With eukaryotic cells in MEM-FBS | 1:32 | 1:16 | 1:32 | 1:16 | 1:16 | 1:32 |
| With MEM-FBS alone | — | — | — | — | — | — |
Profile
Yoshifumi Takeda graduated from School of Medicine, Osaka University in 1960 and initiated his scientific career in 1961 as a student of Dr. Tsunesaburo Fujino, the discoverer of Vibrio parahaemolyticus, at the Research Institute for Microbial Diseases, Osaka University. During last 50 years, he has been working on the pathogenesis of several enteric bacteria, such as Vibrio parahaemolyticus, diarrheagenic Escherichia coli, and Vibrio cholerae. Especially the role of toxins produced by enteric bacteria is the focus of his research interest. Among many contributions he made, several outstanding ones are: demonstration of the cardiotoxicity of the thermostable direct hemolysin produced by Vibrio parahaemolyticus, study on the structure–activity relationship of heat-stable enterotoxins produced by enterotoxigenic E. coli, demonstration of the RNA N-glycosidase activity of Shiga and Shiga-like toxins produced by Shigella dysenteriae and enterohemorrhagic E. coli and discovery of V. cholerae O139.
He had worked as Professor and Chairman of Department of Bacterial Infections at the Institute of Medical Sciences, the University of Tokyo (1983–1989), Professor and Chairman of Department of Microbiology, Faculty of Medicine of Kyoto University (1987–1995), Director General of the Research Institute of International Medical Center of Japan (1994–1999), and Director General of the National Institute of Infectious Diseases, Japan (1999–2001). Since June 2007, he is working as the Director of Collaborating Research Center of Okayama University for Infectious Diseases in India, which is located at the National Institute of Cholera and Enteric Diseases, Kolkata, India. His current research interest is study on Viable But Nonculturable V. cholerae and on a newly emerged V. cholerae El Tor variant.
References
- 1).Fujino T., Okuno Y., Nakada D., Aoyama A., Fukai K., Mukai T., Ueho T. (1953) On the bacteriological examination of shirasu food poisoning. Med. J. Osaka Univ. 4, 299–304 [Google Scholar]
- 2).Kato T., Obara Y., Ichinohe H., Yamai S., Nagashima K., Sakazaki R. (1966) Hemolytic activity and toxicity of Vibrio parahaemolyticus. Nippon Saikingaku Zasshi 21, 442–443(in Japanese) [Google Scholar]
- 3).Sakurai J., Matsuzaki A., Miwatani T. (1973) Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect. Immun. 8, 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Honda T., Taga S., Takeda T., Hasibuan M.A., Takeda Y., Miwatani T. (1976) Identification of lethal toxin with thermostable direct hemolysin produced by Vibrio parahaemolyticus and some physicochemical properties of the purified toxin. Infect. Immun. 13, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Takeda Y., Taga S., Miwatani T. (1978) Evidence that thermostable direct hemolysin of Vibrio parahaemolyticus is composed of two subunits. FEMS Microbiol. Lett. 4, 271–274 [Google Scholar]
- 6).Honda T., Goshima K., Takeda Y., Sugino Y., Miwatani T. (1976) Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect. Immun. 13, 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Takeda Y., Takeda T., Honda T., Sakurai J., Ohtomo N., Miwatani T. (1975) Inhibition of hemolytic activity of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside. Infect. Immun. 12, 931–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).De S.N., Bhattacharya K., Sarkar J.K. (1956) A study of the pathogenicity of strains of Bacterium coli from acute and chronic enteritis. J. Pathol. Bacteriol. 71, 201–209 [DOI] [PubMed] [Google Scholar]
- 9).Sack R.B., Gorbach S.L., Banwell J.G., Jacobs B., Chatterjee B.D., Mitra R.C. (1971) Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J. Infect. Dis. 123, 378–385 [DOI] [PubMed] [Google Scholar]
- 10).Merson M.H., Morris G.K., Sack D.A., Wells J.G., Feeley J.C., Sack R.B., Creech W.B., Kapikian A.Z., Gangarosa E.J. (1976) Travelers’ diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N. Engl. J. Med. 294, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 11).Abe H., Ichiki S., Hashimoto H., Nakano K., Sato T., Kanda Y., Yanai T., Tsukamoto T., Kinoshita M., Arita M., Honda T., Takeda Y., Miwatani T. (1984) Isolation and characterization of enterotoxigenic Escherichia coli from patients with traveller’s diarrhea in Osaka. J. Diarrhoeal Dis. Res. 2, 83–87 [PubMed] [Google Scholar]
- 12).Smith H.W., Gyles C.L. (1970) The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J. Med. Microbiol. 3, 387–401 [DOI] [PubMed] [Google Scholar]
- 13).Clements J.D., Finkelstein R.A. (1979) Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli culture. Infect. Immun. 24, 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Honda T., Tsuji T., Takeda Y., Miwatani T. (1981) Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect. Immun. 34, 337–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Honda T., Takeda Y., Miwatani T. (1981) Isolation of special antibodies which react only with homologous enterotoxins from Vibrio cholerae and enterotoxigenic Escherichia coli. Infect. Immun. 34, 333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Aimoto S., Takao T., Shimonishi S., Hara S., Takeda T., Takeda Y., Miwatani T. (1982) Amino acid sequence of a heat-stable enterotoxin produced by human enterotoxigenic Escherichia coli. Eur. J. Biochem. 129, 257–263 [DOI] [PubMed] [Google Scholar]
- 17).Takao T., Hitouji T., Aimoto S., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. (1983) Amino acid sequence of a heat-stable enterotoxin isolated from enterotoxigenic Escherichia coli 18D. FEBS Lett. 152, 1–5 [DOI] [PubMed] [Google Scholar]
- 18).Aimoto S., Watanabe H., Ikemura H., Shimonishi Y., Takeda T., Takeda Y., Miwatani T. (1983) Chemical synthesis of a highly potent and heat-stable analog of an enterotoxin produced by a human strain of enterotoxigenic Escherichia coli. Biochem. Biophys. Res. Commun. 112, 320–326 [DOI] [PubMed] [Google Scholar]
- 19).Yoshimura S., Takao T., Ikemura H., Aimoto S., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. (1984) Chemical synthesis of fully active and heat-stable fragments of heat-stable enterotoxin of enterotoxigenic Escherichia coli strain 18D. Bull. Chem. Soc. Jpn. 57, 1381–1387 [Google Scholar]
- 20).Yoshimura S., Ikemura H., Watanabe H., Aimoto S., Shimonishi S., Hara S., Takeda T., Miwatani T., Takeda Y. (1985) Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 181, 138–142 [DOI] [PubMed] [Google Scholar]
- 21).Riley L.W., Remis R.S., Helgerson S.D., McGee H.R., Wells J.G., Davis B.R., Hebert R.J., Olcott E.S., Jonson L.M., Hargrett N.T., Blake P.A., Choen M.I. (1983) Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308, 681–685 [DOI] [PubMed] [Google Scholar]
- 22).O’Brien A.D., Lively T.A., Chen M.E., Rothman S.W., Formal S.B. (1983) Escherichia coli O157:H7 strains associated with hemorrhagic clitis in the United States produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet i, 702. [DOI] [PubMed] [Google Scholar]
- 23).Konowalchuk J., Speirs J.I., Stavric S. (1977) Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18, 775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Wade W.G., Thom B.T., Evans N. (1979) Cytotoxic enteropathogenic Escherichia coli. Lancet ii, 1235–1236 [DOI] [PubMed] [Google Scholar]
- 25).Scotlan S.M., Day N.P., Row B. (1980) Production of a cytotoxin affecting Vero cells by strains of Escherichia coli belonging to traditional enteropathogenic serogroups. FEMS Microbiol. Lett. 7, 15–17 [Google Scholar]
- 26).Yutsudo T., Nakabayashi N., Saku K., Noda M., Hirayama T., Takeda Y. (1986) Purification and some properties of a Vero toxin produced by Escherichia coli O157:H7 that is immunologicaly distinct from Shiga toxin. Nippon Saikingaku Zasshi 41, 328.(in Japanese) [Google Scholar]
- 27).Scotland S.M., Smith H.R., Rowe B. (1985) Two distinct toxins active on Vero cells from Escherichia coli O157. Lancet ii, 885–886 [DOI] [PubMed] [Google Scholar]
- 28).Strockbine N.A., Marques L.R.M., Newland J.W., Smith H.W., Holmes R.K., O’Brien A.D. (1986) Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53, 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Noda M., Yutsudo T., Nakabayashi N., Hirayama T., Takeda Y. (1987) Purification and some properties of Shiga-like toxin from Escherichia coli O157:H7 that is immunologically identical to Shiga toxin. Microb. Pathog. 2, 339–349 [DOI] [PubMed] [Google Scholar]
- 30).Yutsudo T., Nakabayashi N., Hirayama T., Takeda Y. (1987) Purification and some properties of a Vero toxin from Escherichia coli O157:H7 that is immunologically unrelated to Shiga toxin. Microb. Pathog. 3, 21–30 [DOI] [PubMed] [Google Scholar]
- 31).Takao T., Tanabe T., Hong Y.M., Shimonishi Y., Kurazono H., Yutsudo T., Sasakawa C., Yoshikawa M., Takeda Y. (1988) Identity of molecular structure of Shiga-like toxin I (VT1) from Escherichia coli O157:H7 with that of Shiga toxin. Microb. Pathog. 5, 357–369 [DOI] [PubMed] [Google Scholar]
- 32).Brown J.E., Obrig T.G., Ussery M.A., Moran T.P. (1986) Shiga toxin from Shigella dysenteriae 1 inhibits protein synthesis in reticulocytes lysates by inactivation of aminoacyl-tRNA binding. Microb. Pathog. 1, 325–334 [DOI] [PubMed] [Google Scholar]
- 33).Igarashi K., Ogasawara T., Ito K., Yutsudo T., Takeda Y. (1987) Inhibition of elongation factor 1-dependent aminoacyl-tRNA binding to ribosomes by Shiga-like toxin I (VT1) from Escherichia coli O157:H7 and by Shiga toxin. FEMS Microbiol. Lett. 44, 91–94 [DOI] [PubMed] [Google Scholar]
- 34).Ogasawara T., Ito K., Igarashi K., Yutsudo T., Nakabayashi N., Takeda Y. (1988) Inhibition of protein synthesis by a Vero toxin (VT2 or Shiga-like toxin II) produced by Escherichia coli O157:H7 at the level of elongation factor 1-dependent aminoacyl-tRNA binding. Microb. Pathog. 4, 127–135 [DOI] [PubMed] [Google Scholar]
- 35).Endo Y., Mitsui K., Mochizuki M., Tsurugi K. (1987) The mechanism of action of ricin and related toxin lectins on eukaryotic ribosomes: The site and the characteristics of the modification in 28S ribosomal RNA caused by the toxins. J. Biol. Chem. 262, 5908–5912 [PubMed] [Google Scholar]
- 36).Endo Y., Tsurugi K. (1987) RNA N-glycosidase activity of ricin A-chain. J. Biol. Chem. 262, 8128–8130 [PubMed] [Google Scholar]
- 37).Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. (1988) Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171, 45–50 [DOI] [PubMed] [Google Scholar]
- 38).Yamasaki S., Furutani M., Ito K., Igarashi K., Nishibuchi M., Takeda Y. (1991) Importance of arginine at position 170 of the A subunit of Vero toxin 1 produced by enterohemorrhagic Escherichia coli for toxin activity. Microb. Pathog. 11, 1–9 [DOI] [PubMed] [Google Scholar]
- 39).Ohmura M., Yamasaki S., Kurazono H., Igarashi K., Takeda Y. (1993) Characterization of non-toxic mutant toxins of Vero toxin 1 that are constructed by replacing amino acids in A subunit. Microb. Pathog. 15, 169–176 [DOI] [PubMed] [Google Scholar]
- 40).Ramamurthy T., Garg S., Sharma R., Bhattacharya S.K., Nair G.B., Shimada T., Takeda T., Karasawa T., Kurazono H., Pal A., Takeda Y. (1993) Emergence of a novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341, 703–704 [DOI] [PubMed] [Google Scholar]
- 41).Albert M.J., Siddique A.K., Islam M.S., Farque A.S.G., Ansaruzzama M., Faruque S.M., Sack R.B. (1993) A large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341, 704. [DOI] [PubMed] [Google Scholar]
- 42).Shimada T., Nair G.B., Deb B.C., Albert M.J., Sack R.B., Takeda Y. (1993) Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet 341, 1347. [DOI] [PubMed] [Google Scholar]
- 43).Bhattacharya S.K., Bhattachary M.K., Nair G.B., Dutta D., Deb A., Ramamurthy T., Garg S., Saha P.K., Dutta P., Moitra A., Mandal B.K., Shimada T., Takeda Y., Deb B.C. (1993) Clinical profile of acute diarrhoea cases infected with new epidemic strain of Vibrio cholerae O139: Designation of the disease as cholera. J. Infect. 27, 11–15 [DOI] [PubMed] [Google Scholar]
- 44).Cholera Working Group, International Center for Diarrhoeal Diseases Research, Bangladesh (1993) Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet 342, 387–390 [PubMed] [Google Scholar]
- 45).World Health Organization (1993) Epidemic diarrhoeae due to Vibrio cholerae non-O1. Wkly. Epidemiol. Rec. 68, 141–142 [PubMed] [Google Scholar]
- 46).Nair G.B., Ramamurthy T., Bhattacharya S.K., Mukhopadhyay S., Garg S., Bhattacarya M.K., Takeda T., Shimada T., Takeda Y., Deb B.C. (1994) Spread of Vibrio cholerae O139 Bengal in India. J. Infect. Dis. 169, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 47).Chongsa-nguan M., Chaicumpa W., Moolasart P., Kandhasingha P., Shimada T., Kurazono H., Takeda Y. (1993) Vibrio cholerae O139 Bengal in Bangkok. Lancet 342, 430–431 [DOI] [PubMed] [Google Scholar]
- 48).Nair G.B., Ramamurthy T., Bhattacharya M.K., Krishnan T., Ganguly S., Saha D.R., Rajendran K., Manna B., Ghosh M., Okamoto K., Takeda Y. (2010) Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Nair G.B., Qadri F., Holmgren J., Svennerholm A.M., Safa A., Bhuiyan N.A., Ahmad Q.S., Faruque S.M., Faruque A.S., Takeda Y., Sack D.A. (2006) Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J. Clin. Microbiol. 44, 4211–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Raychoudhuri A., Mukhopadhyay A.K., Nandy R.K., Ramamurthy T., Nandy R.K., Takeda Y., Nair G.B. (2008) Biotyping of Vibrio cholerae O1: Time to redefine the scheme. Indian J. Med. Res. 128, 695–698 [PubMed] [Google Scholar]
- 51).Safa A., Sultana J., Cam P.D., Mwansa J.C., Kong R.Y.C. (2008) Vibrio cholerae O1 hybrid El Tor strains, Asia and Africa. Emerg. Infect. Dis. 14, 987–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Sithivong N., Izumiya H., Munnalath K., Phouthavane T., Chomlasak K., Sisavath L., Vongdouangchanh A., Vongprachanh P., Watanabe H., Ohnishi M. (2010) Cholera outbreak, Laos, 2007. Emerg. Infect. Dis. 16, 745–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Taneja N., Mishra A., Sangar G., Singh G., Sharma M. (2009) Outbreaks caused by new variants of Vibrio cholerae O1 El Tor, India. Emerg. Infect. Dis. 15, 352–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Nguyen B.M., Lee J.H., Cuong N.T., Choi S.Y., Hien N.T., Anh D.D., Lee H.R., Ansaruzzaman M., Endtz H.P., Chun J., Lopez A.L., Czerkinsky C., Clemens J.D., Kim D.W. (2009) Cholera outbreaks caused by an altered Vibrio cholerae O1 El Tor biotype strain producing classical cholera toxin B in Vietnam in 2007 to 2008. J. Clin. Microbiol. 47, 1568–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Raychoudhuri A., Patra T., Ghosh K., Ramamurthy T., Nandy R.K., Takeda Y., Nair G.B., Mukhopadhyay A.K. (2009) Classical ctxB in Vibrio cholerae O1, Kolkata, India. Emerg. Infect. Dis. 15, 131–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).World Health Organization (2007) Cholera 2007. Wkly. Epidemiol. Rec. 83, 269–284 [Google Scholar]
- 57).Ghosh-Banerjee J., Senoh M., Takahashi T., Hamabata T., Barman S., Koley H., Mukhopadhyay A.K., Ramamurthy T., Chatterjee S., Asakura M., Yamasaki S., Nair G.B., Takeda Y. (2010) Cholera toxin production by El Tor variant of Vibrio cholerae O1 as compared to prototype El Tor and classical biotypes. J. Clin. Microbiol. 48, 4283–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Xu H.S., Roberts N., Singleton F.L., Attwell R.W., Grimes D., Colwell R.R. (1982) Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8, 313–323 [DOI] [PubMed] [Google Scholar]
- 59).Kondo K., Takade A., Amako K. (1994) Morphology of the viable but nonculturable Vibrio cholerae as determined by the freeze fixation technique. FEMS Microbiol. Lett. 123, 179–184 [DOI] [PubMed] [Google Scholar]
- 60).Wai S.N., Moriya T., Kondo K., Misumi H., Amako K. (1996) Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat shock. FEMS Microbiol. Lett. 136, 187–191 [DOI] [PubMed] [Google Scholar]
- 61).Binsztein N., Costagliola M.C., Pichel M., Jurquiza V., Ramirez F.C., Akselman R., Vacchino M., Huq A., Colwell R.R. (2004) Viable but nonculturable Vibrio cholerae O1 in the aquatic environment of Argentina. Appl. Environ. Microbiol. 70, 7481–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Asakura H., Ishiwa A., Arakawa E., Makino S., Okada Y., Yamamoto S., Igimi S. (2007) Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ. Microbiol. 9, 869–879 [DOI] [PubMed] [Google Scholar]
- 63).Alam M., Sultana M., Nair G.B., Siddique A.K., Hasan N.A., Sack R.B., Sack D.A., Ahmed K.U., Sadique A., Watanabe H., Grim C.J., Huq A., Colwell R.R. (2007) Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc. Natl. Acad. Sci. USA 104, 17801–17806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Halpern M., Landsber O., Raats D., Rosenberg E. (2007) Culturable and VBNC Vibrio cholerae: interactions with chironomid egg masses and their bacterial population. Microb. Ecol. 53, 285–293 [DOI] [PubMed] [Google Scholar]
- 65).Senoh M., Ghosh-Banerjee J., Ramamurthy T., Hamabata T., Kurakawa T., Takeda M., Colwell R.R., Nair G.B., Takeda Y. (2010) Conversion of viable but nonculturable Vibrio cholerae to the culturable state by co-culture with eukaryotic cells. Microbiol. Immunol. 54, 502–507 [DOI] [PubMed] [Google Scholar]
- 66).Oliver J.D. (2005) The viable but nonculturable state in bacteria. J. Microbiol. 43, 91–100 [PubMed] [Google Scholar]
- 67).Gupte A.R., de Rexende C.L., Josepha S.W. (2003) Induction and resuscitation of viable but nonculturable Salmonella enteritica serovar Typhimurium DT 104. Appl. Environ. Microbiol. 68, 4788–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Dukan S., Levi Y., Touati D. (1997) Recovery of culturability of an HOCl-stressed population of Escherichia coli after incubation in phosphate buffer: resuscitation or regrowth? Appl. Environ. Microbiol. 63, 4204–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Mizunoe Y., Wai S.N., Ishikawa T., Takade A., Yoshida S. (2000) Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol. Lett. 186, 115–120 [DOI] [PubMed] [Google Scholar]
- 70).Reissbrodt R., Rienaecker I., Romanova J.M., Freestone P.P.E., Haigh R.D., Lyte M., Tschape H., Williams P.H. (2002) Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl. Environ. Microbiol. 68, 4788–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Panutdaporn N., Kawamoto K., Asakura H., Makino S. (2006) Resuscitation of the viable but non-culturable state of Salmonella enteritica serovar Oranienburg by recombinant resuscitation-promoting factor derived from Salmonella Typhimurium strain LT2. Int. J. Food Microbiol. 106, 241–247 [DOI] [PubMed] [Google Scholar]
- 72).Steinert M., Emody L., Amann R., Hacker J. (1997) Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63, 2047–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Colwell R.R., Brayton P.R., Grimes D.J., Roszak D.R., Huq S.A., Palmer L.M. (1985) Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implication for release of genetically engineered microorganisms. Bio/Technology 3, 817–820 [Google Scholar]
- 74).Colwell R.R., Brayton P.R., Heerington D., Tall B.D., Huq A., Levine M.M. (1996) Viable but nonculturable Vibrio cholerae O1 revert to a culturable state in human intestine. World J. Microbiol. Biotechnol. 12, 28–31 [DOI] [PubMed] [Google Scholar]
- 75).Oku Y., Uesaka Y., Hirayama T., Takeda Y. (1988) Development of a highly sensitive bead-ELISA to detect bacterial protien toxins. Microbiol. Immunol. 32, 807–816 [DOI] [PubMed] [Google Scholar]