Abstract
Objective
This prospective study was designed to determine whether variation in angiogenic (placental growth factor [PlGF]) and/or anti-angiogenic (soluble fms-like tyrosine kinase [sFlt-1]) factors contribute to the protective effect of highland ancestry (Andean) from altitude-associated reductions in fetal growth.
Study design
Plasma sFlt-1 and PlGF levels, uterine artery (UA) blood flow, and fetal biometry were determined in low-altitude (400 m; Andean n = 27, European n = 28) and high-altitude (3600 m; Andean n = 51, European n = 44) residents during pregnancy (20 and 36 weeks) and 4 months postpartum.
Results
High-altitude decreased sFlt-1 levels in both groups, Andeans had lower sFlt-1, comparable PlGF, lower sFlt-1/PlGF ratios, and higher UA blood flow throughout pregnancy relative to Europeans. Altitude decreased birth weight in Europeans but not Andeans. In high-altitude Europeans sFlt-1/PlGF and sFlt-1 levels were negatively associated with UA diameter and birth weight, respectively.
Conclusions
Lower sFlt-1 and sFlt-1/PLGF ratio may contribute to or result from variations in maternal vascular adaptation to pregnancy between Andean and Europeans at high altitude. Subsequently, these effects could potentially influence ancestry-associated differences in birth weight.
Keywords: Fetal growth restriction, high-altitude adaptation, placenta growth factor, pregnancy, soluble fms-like tyrosine kinase 1
Introduction
The uterine artery (UA) undergoes profound dilation, growth, and remodeling during pregnancy that, collectively, permit a progressive rise in maternal UA blood flow and hence delivery of oxygen and other nutrients to the fetoplacental circulation.1-4 The chronic hypoxia of residence at high altitude (≥2500 m) impairs maternal vascular adaptation to pregnancy, reducing the normal pregnancy-associated increase in UA diameter and rise in UA blood flow by about one third.5,6 Such reduction in UA blood flow likely contributes to diminished fetal growth and the increased frequency of preeclampsia at high compared to low altitude.7-11
Notably, populations with many generations of residence at high altitude, such as Andeans or Tibetans, are relatively protected against this altitude-associated reduction in fetal growth.9,11,12 Such protection in Andeans appears to be due, in part, to the preservation of a normal pregnancy-associated increase in UA diameter and blood flow; this is in contrast to Europeans, whose UA diameters and blood flow do not increase to the same extent.13-15 The mechanisms responsible for these ancestry-associated differences in UA diameter, blood flow, and fetal growth are unclear.
Because angiogenesis (and angiogenic factors) are known to play an important role in the uterine vascular response to pregnancy,3,16-19 we hypothesized that higher levels of angiogenic compared to anti-angiogenic factors in the maternal circulation may contribute to the higher UA blood flows and birth weights in Andeans relative to Europeans at high altitude. Placental growth factor (PlGF), and the anti-angiogenic factor soluble fms-like tyrosine kinase (sFlt-1) and their ratio are frequently used to describe angiogenic processes during pregnancy as levels of these factors are altered in both preeclampsia20,21 and fetal growth restriction.22,23 Chronic hypoxia downregulates PlGF and upregulates sFlt-1 expression via the hypoxia-inducible factor (HIF) pathway;24,25 we, therefore, asked whether Andeans had higher PlGF, lower sFlt-1, and/or lower sFlt-1/PlGF ratio levels than Europeans and, if so, whether such differences were associated with UA blood flow or fetal growth at high altitude.
Material and Methods
Participants
Participants were 150 women, including 51 Andeans and 44 Europeans living at high altitude in La Paz or El Alto (3600-4100 m), as well as 27 Andean and 28 European low-altitude residents of Santa Cruz (400 m), Bolivia. Women were recruited through their prenatal care physician and were required to be of good health, no more than 20 weeks pregnant, receiving prenatal care, and at no known risk of developing pregnancy complications (eg, diabetes, chronic hypertension). Patients were excluded if they had lived at low altitude for more than 3 months, smoked, consumed more than 2 alcoholic beverages weekly before pregnancy, had any known risk factor for developing PE (ie, a current multiple-gestation pregnancy, chronic hypertension, gestational diabetes, or any cardiovascular or renal disease) or had evidence of infection; implementing these criteria minimized potentially confounding effects on fetal or gestational outcome. All women gave written informed consent to study procedures that had been approved by the human participant review boards of the University of Colorado–Denver and its Bolivian counterpart, Colegio Médico. Patients were excluded if they had preeclampsia (>2 blood pressures ≥ 140/90 mm Hg at least 6 hours apart and >1 + proteinuria by dipstick or 300 mg/L in a 24-hour collection). Women who delivered babies with neonatal complications (eg, respiratory distress) or were missing birth or other essential data were also excluded.
Ancestry was determined by self-identification as being either Andean (ie, Aymara or Quechua) or European and confirmed by examining surnames (parental and grandparental) and genetic markers. We used a panel of 100 ancestry informative markers (AIMs) to quantify the proportion of each woman's genetic that could be ascribed to West African, European, or Indigenous American origin using the maximum likelihood method as described in our previous studies.13,15,26 Data for the percentage AIMs in each ancestry group are reported in Table 1. Final classification as Andean required confirmation by having at least 3 of their 4 parental surnames being of Aymara or Quechua origin and >60% Indigenous American genetic ancestry. Women were classified as European if they self-identified as being of European or of other low-altitude origin and had <50% Indigenous American genetic ancestry. Women who did not fit these criteria were excluded from the analyses.
Table 1.
Maternal and Newborn Characteristicsa
| Altitude |
|||||
|---|---|---|---|---|---|
| Variables | Ancestry | Low | High | P Altitude | |
| A. Maternal characteristics | |||||
| Ancestry, % | European | European | 50.9 ± 2.9 (28) | 76.2 ± 3.1 (44) | P < .001 |
| Andean | 15.2 ± 1.7 (27) | 4.6 ± 1.1 (51) | P < .001 | ||
| P ancestry | P < .001 | P < .001 | |||
| Amer-indian | European | 39.6 ± 2.3 (28) | 17.6 ± 2.7 (44) | P < .001 | |
| Andean | 75.3 ± 1.7 (27) | 93.1 ± 1.3 (51) | P < .001 | ||
| P ancestry | P < .001 | P < .001 | |||
| West African | European | 9.4 ± 1.1 (28) | 6.2 ± 0.9 (44) | P < .05 | |
| Andean | 9.6 ± 0.7 (27) | 2.2 ± 0.5 (51) | P < .001 | ||
| P ancestry | NS | P < .001 | |||
| Age | years | European | 27.1 ± 1.1 (28) | 31.8 ± 0.7 (43) | P < .001 |
| Andean | 24.7 ± 1.1 (27) | 26.9 ± 0.9 (49) | NS | ||
| P ancestry | NS | P < .001 | |||
| Parity | no. live births | European | 2 ± 0 (28) | 2 ± 0 (43) | NS |
| Andean | 2 ± 0 (27) | 3 ± 0 (49) | P < .001 | ||
| P ancestry | NS | P < .01 | |||
| Height | cm | European | 159.5 ± 1.1 (28) | 162.4 ± 1.1 (44) | NSc |
| Andean | 155.2 ± 0.8 (25) | 150.0 ± 0.7 (49) | P < .001 | ||
| P ancestry | P < 0.01 | P < 0.001 | |||
| Weight at 36 wks | kg | European | 77.0 ± 2.5 (24) | 71.8 ± 1.5 (38) | NSc |
| Andean | 67.4 ± 2.3 (19) | 66.0 ± 1.3 (45) | NS | ||
| P ancestry | P <.01 | P < .01 | |||
| Monthly income | US dollars | European | 320 ± 56 (22) | 1793 ± 322 (28) | P < .001 |
| Andean | 204 ± 32 (18) | 131 ± 20 (51) | P < .01 | ||
| P ancestry | NSc | P < .001 | |||
| B. Newborn Characteristics | |||||
| Birth weight | g | European | 3365 ± 91 (26) | 3172 ± 57 (43) | NSc |
| Andean | 3267 ± 100 (21) | 3119 ± 64 (38) | NS | ||
| P ancestry | NS | NS | |||
| Birth weight, adjustedb | g | European | 3369 ± 89 (25) | 3094 ± 69 (36) | P < .05 |
| Andean | 3278 ± 123 (19) | 3247 ± 83 (27) | NS | ||
| P ancestry | NS | NS | |||
| Infant length | cm | European | 49 ± 0.2 (26) | 50 ± 0.4 (39) | NS |
| Andean | 50 ± 0.3 (21) | 49 ± 0.3 (34) | NS | ||
| P ancestry | NS | NS | |||
| Ponderal index | kg/m3 | European | 28 ± 0.6 (26) | 26 ± 0.6 (39) | NSc |
| Andean | 27 ± 0.7 (21) | 27 ± 0.6 (34) | NS | ||
| P ancestry | NS | NS | |||
| Ponderal index adjustedb | kg/m3 | European | 28 ± 0.6 (26) | 26 ± 0.6 (39) | NSc |
| Andean | 27 ± 0.7 (21) | 27 ± 0.7 (32) | NS | ||
| P ancestry | NS | NS | |||
| Gestational age | wks | European | 38 ± 0.3 (26) | 39 ± 0.2 (43) | P < .01 |
| Andean | 39 ± 0.5 (21) | 39 ± 0.3 (36) | NS | ||
| P ancestry | P < .05 | NS | |||
Values are shown as mean ± SEM or 95% confidence intervals for proportions with sample sizes parentheses.
Values are corrected for gestational age and maternal height, and displayed for comparisons made between the ancestry groups within altitude.
05 < P < 0.10 and NS = not significant.
Protocol, Variables, and Instrumentation
Women were studied prospectively at 20 and 36 weeks of pregnancy and 4 months postpartum for a measurement in the nonpregnant state. Actual times of study were 21.7 ± 0.24 and 36.0 ± 0.17 weeks of pregnancy and 4.25 ± 0.43 months postpartum.
All samples were collected at or close to the participant's altitude of residence using the laboratory facilities at the Instituto Boliviano de Biología de Altura in La Paz or Clínica Siraní in Santa Cruz, Bolivia. At the first visit, each woman filled out a questionnaire to provide information regarding her ancestry, altitude of birth, age, body weight before pregnancy, education, and residential history. A general clinical examination including the measurement of height by stadiometer (at the first visit only), body weight by balance scale, and proteinuria by dipstick (Albustix, Bayer, Canada) was conducted at each visit. Venous blood was drawn by venipuncture into collection tubes containing EDTA; all samples were stored in 1-mL aliquots at –80°C and transported to our Denver-based laboratory for the measurement of sFlt-1 (specificity: 3.5 pg/mL, inter- and intra-assay precision of 5.5% and 3.2%, respectively) and PlGF (specificity: 7.0 pg/mL, inter- and intra-assay precision of 10.0% and 5.6%, respectively) by enzyme-linked immunosorbet assay ([ELISA]; R&D systems, Minneapolis, Minnesota). All nonpregnant PlGF levels were below the assay's lower limit of detection and zero values were assigned as previously described.27
Uterine artery diameter, blood flow velocity, and fetal biometry (cm) were measured percutaneously using an ATL3000 transabdominal ultrasound unit configured for obstetric use with color imaging and Doppler. Uterine artery diameter and blood flow velocity were used to calculate UA blood flow (mL/min). Briefly, measurements were taken with a 4-MHz curved linear array probe by the same operator using the same machine at the same location within each altitude study site to minimize inter-instrument and inter-operator variability. The angle of insonation was required to be ≤45° for all flow variables to avoid considerable error.13 The UA was measured at its crossover with the external iliac as previously described.13 Fetal abdominal and head circumference and femur length were performed after the maternal blood flow velocity and diameter measurements. Gestational age was calculated using ultrasound at week 20, based on our previous observations that these values are within 2 weeks of that calculated from last menstrual period.13,15 Birth weight, length, gestational age, and infant sex were collected from medical records completed by hospital personnel at the time of delivery.
Statistics
Data are reported as the mean ± standard error of the mean (SEM) or as a percentage and 95% confidence interval (CI) for proportions. Comparisons between ancestry and altitude groups at single points were made using t tests for continuous variables or chi-square (χ2) tests for nominal or ordinal variables. The effects of pregnancy, ancestry, or altitude were examined using 1-, 2-, or 3-way analysis of variance (ANOVA) and differences over time determined with Scheffé post hoc tests. Factors known to influence birth weight (income, gestational age, infant sex, maternal weight, and height) were included in the calculation of adjusted birth weight but were only retained in the model if they had significant effects thus, birth weight and ponderal index were corrected for maternal height and actual gestational age as a covariate as estimated from the 20-week ultrasound examination; these adjusted values are displayed in grams and gr/ht. Relationships between pro- or anti-angiogenic factors, UA blood flow and or diameter, fetal biometry, and birth weight were assessed in the pregnant group, using linear regression, with the actual gestational age of study included as a covariate. All analyses were conducted using SPSS (v. 12.0; Chicago, Illinois). Significance was reported when 2-tailed P values were <.05 unless otherwise specified. A trend was defined when .05 < P < .10.
Results
Maternal Characteristics
High-altitude Andeans were overwhelmingly of Indigenous American genetic ancestry (93.1% ± 1.3%) as assessed by AIMs. Andeans at low altitude had slightly higher European admixture (39.6% ± 2.3%) than those at high altitude (17.6% ± 2.7%), but all were largely of Indigenous American ancestry. High-altitude Europeans had somewhat larger European genetic ancestry proportions (76.2% ± 3.1%) than their low-altitude counterparts (50.9% ± 2.9%), due to greater Indigenous American admixture but questionnaire-derived data indicated that such admixture was of low-altitude, Central American or Caribbean origin (data not shown). All women had relatively low percentages of West African AIMs (Table 1A.).
Low-altitude Europeans were taller, weighed more at 36 weeks of pregnancy, and had higher educational levels than Andeans (Table 1A). At high altitude, the Andeans weighed less at week 36, were younger, of greater parity, shorter, had lower monthly incomes (Table 1A) and educational levels (Andeans: 11.76% [5.06, 22.65] vs Europeans: 73.17% [58.37, 84.83] P < .001), and had lived at high altitude longer than Europeans (20.4 vs 8.4 years, respectively, P < .001). Andeans at high versus low altitude were of greater parity, shorter, and had lower monthly incomes (Table 1A). Europeans at high versus low altitude were older, tended to be taller, weighed less at 36 weeks of pregnancy, and had higher incomes (Table 1A).
Newborn Characteristics
High altitude tended to reduce birth weight in Europeans but not Andeans (Table 1B). Gestational age varied between groups such that low-altitude Europeans delivered earlier (Table 1B), due largely to the high proportion of elective Cesarean sections and the practice of scheduling such deliveries 1 to 2 weeks before the due date. After correcting the birth weight for variation in gestational age and maternal height, high-altitude European babies weighed 275 g less than their low-altitude counterparts. No differences were observed when ancestry groups were compared at both altitudes.
Uterine Artery Characteristics
Pregnancy increased UA diameter and blood flow in all women but these pregnancy-associated changes were greater in Andean than European women at high altitude (Table 2). In both groups, maximal UA diameter was achieved by 20 weeks. High altitude reduced the pregnancy-associated rise in UA diameter in Europeans, but not in Andeans. Uterine artery blood flow was equivalent between ancestry groups at low altitude but was 22% and 26% greater in Andeans than Europeans at high altitude at 20 and 36 weeks, respectively. It should be noted that nonpregnant UA diameter and calculated UA blood flow were greater at high than low altitude in both ancestry groups.
Table 2.
Fetal Biometry and Uterine Artery Characteristicsa
| Week of Pregnancy |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Altitude | Ancestry Group | Nonpregnant | Week 20 | Week 36 | P Time | |
| UA diameter | cm | Low | European | 0.26 ± 0.01 (25)d | 0.49 ± 0.01 (28)c | 0.51 ± 0.02 (24) | P < .001 |
| Andean | 0.20 ± 0.01 (16)e | 0.47 ± 0.01 (26)e | 0.48 ± 0.02 (19)d | P < .001 | |||
| P ancestry | P < .05 | NS | NS | ||||
| High | European | 0.41 ± 0.04 (30)d | 0.54 + 0.01 (26)c | 0.55 ± 0.01 (37) | P < .001 | ||
| Andean | 0.39 ± 0.01 (l8)e | 0.63 ± 0.01 (45)e | 0.64 ± 0.01 (43)d | P < .001 | |||
| P ancestry | NS | P < .001 | P < .001 | ||||
| UA blood flow | m L/min. | Low | European | 25.59 ± 3.70 (25)d | 295.09 ± 25.17 (28) | 400.76 ± 35.87(24) | P < .001 |
| Andean | 12.50 ± 2.78 (16) | 262.87 ± 26.12 (26)e | 349.01 ± 32.41 (19)d | P < .001 | |||
| P ancestry | P < .01 | NS | NS | ||||
| High | European | 73.94 ± 14.01 (19)d | 361.57 ± 39.07 (25) | 418.90 ± 33.91(34) | P < .001 | ||
| Andean | 55.28 ± 22.28 (4) | 564.43 ± 47.40 (22)e | 705.25 ± 75.92 (31)d | P < .01 | |||
| P ancestry | NS | P < .01 | P < .01 | ||||
| Head circum | cm | Low | European | – | 17.63 ± 0.53 (27)c | 30.04 ± 0.92 (17)c | P < .001 |
| Andean | – | 18.78 ± 0.56 (25)c | 31.59 ± 1.04 (13) | P < .001 | |||
| P ancestry | – | NS | NS | ||||
| High | European | – | 19.11 ± 0.41 (25)c | 31.95 ± 0.42 (36)c | P < .001 | ||
| Andean | – | 20.46 ± 0.31 (42)c | 31.08 ± 0.39 (41) | P < .001 | |||
| P ancestry | – | P < .05 | NS | ||||
| Abdominal circum | cm | Low | European | – | 16.03 ± 0.49 (27)c | 31.92 ± 0.30 (17) | P < .001 |
| Andean | – | 16.87 ± 0.50 (26)c | 31.81 ± 0.34 (13) | P < .001 | |||
| P ancestry | – | NS | NS | ||||
| High | European | – | 18.00 ± 0.42 (24)c | 32.06 ± 0.28 (36) | P < .001 | ||
| Andean | – | 18.91 ± 0.32 (43)c | 31.42 ± 0.26 (41) | P < .001 | |||
| P ancestry | – | NSb | NS | ||||
| Femur length | cm | Low | European | – | 3.53 ± 0.12 (27) | 6.97 ± 0.05 (17) | P < .001 |
| Andean | – | 3.75 ± 0.12 (26) | 7.01 ± 0.06 (13) | P < .001 | |||
| P ancestry | – | NS | NS | ||||
| High | European | – | 3.71 ± 0.10 (25) | 7.07 ± 0.54 (36) | P < .001 | ||
| Andean | – | 4.12 ± 0.08 (43) | 7.66 ± 0.51 (41) | P < .001 | |||
| P ancestry | – | P < .01 | NS |
Abbreviations: Circum, circumference; UA, uterine artery.
Values are shown as mean ± SEM, with sample sizes in parentheses.
05 < P < .10. Corrected for actual gestational age. Values are displayed for correction within ancestry group.
P < .05.
P < .01.
P < .001 for altitude comparisons within the ancestry group and week of pregnancy.
Angiogenic and Anti-Angiogenic Factors
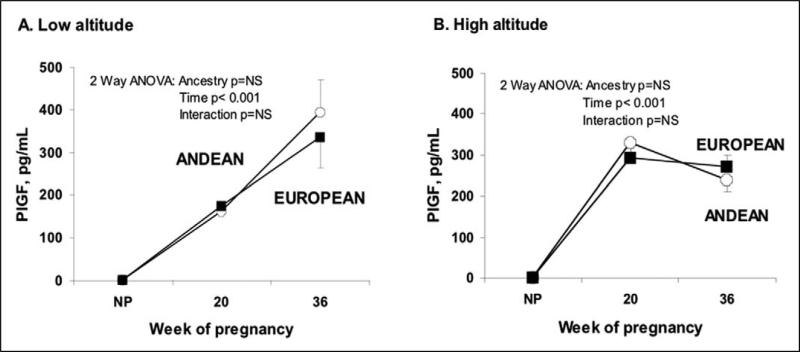
Pregnancy increased PlGF levels similarly in the Andeans and Europeans but the rise occurred earlier at high than low altitude (Figure 1, interaction between altitude and time, P < .001 for Andeans and P < .05 for Europeans). At low altitude, PlGF was positively associated with UA diameter or blood flow in all women at 20 weeks (partial R2 for Europeans = .48 P < .05 and for Andeans = .63 P < .01). No associations were observed at high altitude.
Figure 1.
At both altitudes and ancestry groups PlGF levels were greater during pregnancy than in the non-pregnant state. The pattern of increase across time differed such that PlGF increased early at high altitude (B) whereas levels rose throughout pregnancy at low altitude (A). This temporal difference resulted in greater PlGF levels at high than low altitude at 20 weeks in both ancestry groups (Andean: p<0.001, European: p<0.05).
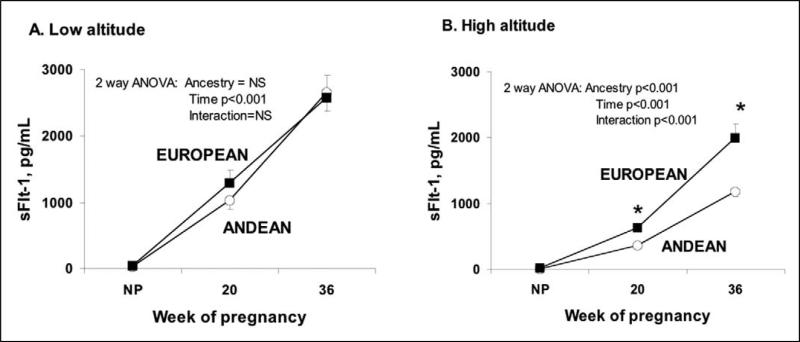
Pregnancy increased sFlt-1 levels at both altitudes (Figure 2). The pregnancy-associated rise was similar between ancestry groups at low but not high altitude, where Andeans showed a smaller rise and therefore lower sFlt-1 levels than Europeans at 20 and 36 weeks. sFlt-1 levels were lower at high than low altitude in both groups, with the reduction being greater in Andeans than Europeans (interaction between the effects of altitude, time, and ancestry, P < .05).
Figure 2.
At both altitudes and ancestry groups sFlt-1 levels increased across time. No differences were found between ancestry groups at low altitude (A). Pregnancy raised sFlt-1 at high altitude (B) to a greater extent in European than Andean women, with differences between groups being present at both weeks 20 and 36 (p<0.05).
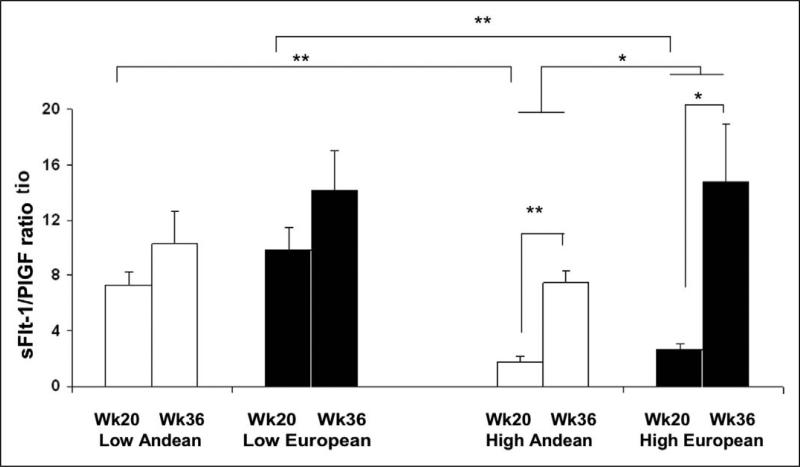
At low altitude, the sFlt-1/PlGF ratio was similar at 20 and 36 weeks of pregnancy and between ancestry groups (Figure 3). In contrast, pregnancy increased the sFlt-1/PlGF ratio at high altitude, with the ratio being lower in Andeans than Europeans at both 20 and 36 weeks (Figure 3), principally as a result of lower sFlt-1 (Figure 2).
Figure 3.
High altitude Andeans and Europeans had higher sFlt-1/PlGF levels at 36 than at 20 weeks of pregnancy (p<0.05; p<0.001 respectively). High altitude Europeans had higher sFlt-1/PlGF ratio than high altitude Andeans (p<0.05). Women living at high altitude had lower sFlt-1/PlGF ratio than their counterparts at low altitude (p<0.05).
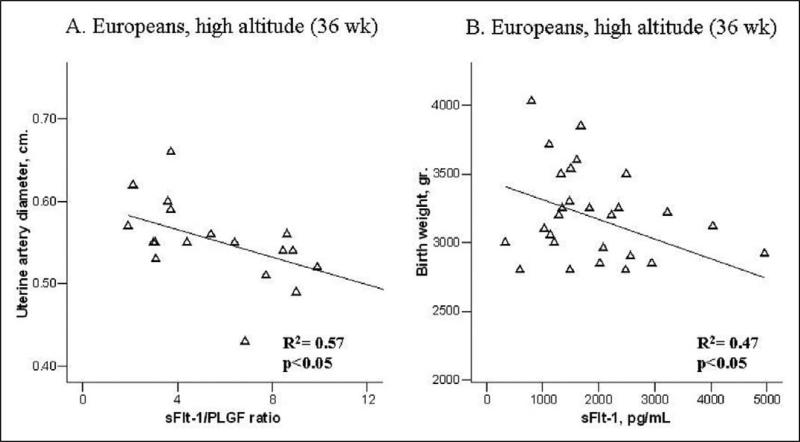
Among high-altitude Europeans, lower sFlt-1/PlGF ratios were associated with larger UA diameter at 36 weeks (Figure 4A). Heavier birth weights were associated with lower sFlt-1 levels at 36 weeks of pregnancy in high-altitude Europeans (Figure 4B), but no such association was present for Andeans or in either group at low altitude.
Figure 4.
High altitude Europeans with higher levels of sFlt1-PlGF ratio at 36 weeks of pregnancy had smaller UA diameters (A) and gave birth to smaller babies (B).
Fetal Growth Characteristics
There were no ancestry-related differences in fetal biometry at low altitude (Table 2). Among low-altitude Andeans, higher PlGF and lower sFlt-1/PlGF ratios at 20 weeks were associated with greater fetal abdominal circumference (R2: .59; R2: .37, respectively, both P < .05) and femur length (R2: .62; R2 .36, respectively, both P < .05). Among low-altitude Europeans, lower sFlt-1/PlGF ratios were also associated with greater head circumference (R2: .28 P < .05) and femur length (R2: .29 P < .05) at week 20. High-altitude Andeans had larger fetal head circumference and femur length relative to Europeans, and a trend toward greater abdominal circumference (Table 2). At high altitude, the PlGF levels at 20 weeks were positively associated with head circumference in Europeans (R2:.91 P < .001) and abdominal circumference in Andeans (R2: .53 P < .001).
Discussion
The current study presents novel evidence that high-altitude residence alters circulating levels of anti-angiogenic factors during pregnancy,2 the extent of these alterations is ancestry dependent, and likely contributes to greater UA blood flow and protection of fetal growth apparent in Andeans at high altitude. Specifically, in this study Andeans compared to Europeans at high altitude had lower sFlt-1 and sFlt-1/PlGF ratio levels at 20 and 36 weeks of pregnancy. Moreover, among high-altitude Europeans, lower anti-angiogenic factor sFlt-1 and sFlt-1/PlGF ratio levels were associated with heavier birth weights and greater UA diameters, respectively. Therefore, our data suggest that lower sFlt-1 and lower sFlt-1/PlGF ratio levels near term may contribute to greater UA blood flow and diameters seen in Andeans and thus the protection from altitude-associated reductions in fetal growth. To the best of our knowledge, this is the first study to demonstrate an association between circulating levels of angiogenic factors, fetal biometry, and UA blood flow during pregnancy.
Limitations of our study included smaller sample sizes at low compared to high altitude. Although we recruited an equal number of participants at both altitudes, greater admixture among the low-altitude Europeans (had >50% Indigenous American genetic ancestry) reduced our sample size slightly for that group. Another difficulty was that we had to rely on circulating levels of PlGF and sFlt-1 because only maternal blood samples could be obtained, and therefore could not distinguish between the contributions of a maternal versus fetal source of production for these substances. Second, contrary to our expectations, UA diameter and calculated UA blood flow values were greater at high than low altitude in the nonpregnant state in Europeans and Andeans. We were careful to have the same operators at a given altitude. However, we cannot disregard the possibility that operator- or equipment-related effects account, in part, for the variation between altitudes, given that even small differences in cursor placement can greatly influence the diameter and flow measurements, given that vessel radius is squared in flow calculations. Nonpregnant measurements were made postpartum, therefore we do not believe that menstrual cycle phase influenced our results. Furthermore, unlike our9,13,15 and other previous findings,14 adjusted birth weights were similar in Andean and European infants at high altitude, and we consider this result likely to be due to the smaller sample sizes in this versus previous report.
Our low-altitude data agree with previous studies of normal pregnancy in terms of the gradual nature of the rise in PlGF and the absolute values achieved.21,28 At high altitude, however, PlGF increased earlier than at low altitude in both ancestry groups. We consider that the more abrupt increase in PlGF at 20 weeks may reflect a longer vasculogenic phase of placental growth; this idea is in accordance with the hypothesized 2-stage model which postulates that high altitude exerts a greater stimulatory effect on angiogenic factors early in pregnancy as a compensatory response.29 Existing data suggest that PlGF levels are lower and sFlt-1 greater, in preeclampsia and/or fetal growth restriction, both of which are often marked by reduced uteroplacental blood flow resulting in reduced oxygen and available nutrients.30-33 We believe that the greater PlGF levels seen at 20 weeks at high altitude may be due to lower levels of sFlt-1, suggesting that the normal binding that occurs between such factors34,35 may not be happening by the middle of pregnancy at high altitude (ie, 20 weeks). The fact that greater PlGF levels at high compared to low altitude were evident by 20 weeks is important given that this is prior to the time when fetal growth is thought to decline in altitude-associated fetal growth restriction.7
Studies conducted in human placental villous explants,36 primary cytotrophoblast cell cultures,20 and intact animals27 demonstrate that hypoxia upregulates sFlt-1 expression. Such upregulation would be anticipated to antagonize the normal pregnancy-associated rise in vascular endothelial growth factor (VEGF) and/or PlGF required for vascular remodeling and angiogenesis.20,24,27,36-38 We, therefore, expected to find higher sFlt-1 in the maternal circulation at high than low altitude but, in contrast, we found lower sFlt-1 levels in both ancestry groups. One explanation for this may be that the production of sFlt-1 by peripheral blood mononuclear cells (PBMCs) is altered under conditions of hypoxia and preeclampsia and may be cell-type specific.38 Rajakumur and colleagues38 report that hypoxia increases sFlt-1 production by PBMCs, however because they did not report cell differentials it is unknown what kind of PBMCs was producing sFlt-1. This is an important consideration for interpreting our results, given that acute and chronic hypoxia decrease the number as well as the proportion of T-lymphocytes (CD3+ and CD4+) among total PBMCs, whereas the opposite is true for natural killer (NK) lymphocytes (CD16 and CD56).39 Thus, we consider that the lower sFlt-1 values at high versus low altitude reported in this study, may reflect differences in the composition of PBMCs at high relative to low altitude. Our data also demonstrate that sFlt-1 levels in European women were elevated relative to Andean values. Such higher sFlt-1 levels were seen in combination with reduced UA blood flow, similar to what has been observed in preeclampsia and Small for Gestational Age (SGA) compared with normal pregnancies31,40; this literature, in combination with our data, support the likelihood that higher sFlt-1 levels and sFlt-1/PlGF ratios contributed to the lower UA blood flow and birth weights observed in previous studies of European versus Andean women at high altitude.9,13,14
Alterations in PlGF as well as sFlt-1 characterize pregnancies complicated by preeclampsia and fetal growth restriction, both conditions with marked reductions in uteroplacental blood flow. Consistent with this, we found that European women with greater sFlt-1 levels at 36 weeks delivered infants of lower birth weight at high altitude. Because previous studies show that the ratio of sFlt-1-to-PlGF levels are altered in pregnancies where there is reduced uteroplacental blood flow and fetal growth,41-43 we also asked whether their ratio was influenced by effects of altitude or ancestry. Consistent with our hypothesis, Andean sFlt-1/PlGF ratios did not rise to as great an extent from 20 to 36 weeks as they did in the European participants. As a result, Andean sFlt-1 relative to PlGF levels were only half of those seen in Europeans near term (Figure 3). Given the association between higher sFlt-1/PlGF values and smaller UA diameter in the European women, we considered that these group differences in the sFlt-1/PlGF ratio were likely important contributors to the one-third smaller UA diameters and markedly lower UA blood flow values reported previously13,15 and seen here in the Europeans compared to Andeans near term at high altitude.
In summary, Andean relative to European women have lower sFlt-1 and sFlt-1/PlGF ratios at high altitude, supporting the idea that these factors are related to Andean protection from altitude-associated reductions in fetal growth. Further study is needed to determine the mechanisms by which ancestry and altitude influence the pregnancy-related changes in these factors. The contribution of other factors also influencing maternal vascular response to pregnancy is also of interest. In particular, given the important role of soluble-endoglin, an antiangiogenic molecule of importance to preeclampsia,43 and cytokines as local mediators of placental development and maternal immune response during normal and complicated pregnancies,18,19,44-46 future studies are needed for determining their role in accounting for the effects of altitude and ancestry on UA blood flow and fetal growth.
Acknowledgments
We thank all the women who kindly agreed to participate in this study, as well as to the many technicians for their invaluable technical support. In particular, we thank Martha Aguilar, Ana-María Alarcón, Jose Luis Casanova, Dolly Condori, Cristina Gonzáles, Jennifer Hageman, Freddy Limachi, Lourdes Mabrich, Zaida Martínez, Gene and Rosann McCullough, Julio Roca, Armando Rodriguez, Jessica Schaymann and Wilmar Velásquez. We also express our appreciation to the several physicians who helped with participant recruitment and study conduct, including Drs J. Fernando Armaza, María del Pilar García, Jessica Pardo, Marco Vargas, and Dra. Elizabeth Zelada.
Funding
Financial support was received from grants from the National Institutes of Health (HL60131, HL07171, and HL079647), a National Sciences Foundation predoctoral fellowship (MJW) and Graduate Research support (CGJ), and an American Heart Association predoctoral fellowship (CGJ) (0610129Z).
References
- 1.Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(suppl 1):S10–S18. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 2.Cross JC, Hemberger M, Lu Y, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187(1-2):207–212. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 3.Osol G, Celia G, Gokina N, et al. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol. 2008;294(3):H1381–H1387. doi: 10.1152/ajpheart.00922.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25(2-3):103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 1995;79(1):7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Julian CG, Galan HL, Wilson MJ, et al. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R906–R915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol. 2000;16(1):9–18. doi: 10.1046/j.1469-0705.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54(1):20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 9.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F372–F377. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 m) in Colorado. Am J Obstet Gynecol. 1999;180(5):1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol. 2003;4(2):141–156. doi: 10.1089/152702903322022767. [DOI] [PubMed] [Google Scholar]
- 12.Moore LG, Shriver M, Bemis L, et al. Maternal adaptation to high-altitude pregnancy: an experiment of nature—a review. Placenta. 2004;25(suppl A):S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MJ, Lopez M, Vargas M, et al. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1313–R1324. doi: 10.1152/ajpregu.00806.2006. [DOI] [PubMed] [Google Scholar]
- 14.Zamudio S, Postigo L, Illsley NP, et al. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582(pt 2):883–895. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julian CG, Wilson MJ, Lopez M, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1564–1575. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szukiewicz D, Szewczyk G, Watroba M, Kurowska E, Maslinski S. Isolated placental vessel response to vascular endothelial growth factor and placenta growth factor in normal and growth-restricted pregnancy. Gynecol Obstet Invest. 2005;59(2):102–107. doi: 10.1159/000082622. [DOI] [PubMed] [Google Scholar]
- 17.Cooper B. Mechanism of vasodilation by placental growth factor (PIGF) in the uterine circulation. J Soc Gynecol Invest. 2005;12(2) [Google Scholar]
- 18.Bulla R, Bossi F, Radillo O, de Seta F, Tedesco F. Placental trophoblast and endothelial cells as target of maternal immune response. Autoimmunity. 2003;36(1):11–18. doi: 10.1080/0891693031000067331. [DOI] [PubMed] [Google Scholar]
- 19.Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75(3):243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 20.Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145(11):4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109(6):1316–1324. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta. 2000;21(suppl A):S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 25.Lash GE, Taylor CM, Trew AJ, et al. Vascular endothelial growth factor and placental growth factor release in cultured trophoblast cells under different oxygen tensions. Growth Factors. 2002;20(4):189–196. doi: 10.1080/0897719021000069560. [DOI] [PubMed] [Google Scholar]
- 26.Shriver MD, Kennedy GC, Parra EJ, et al. The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum Genomics. 2004;1(4):274–286. doi: 10.1186/1479-7364-1-4-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahtiyar MO, Buhimschi C, Ravishankar V, et al. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol. 2007;196(1):72.e1–72.e6. doi: 10.1016/j.ajog.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23(1):101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 29.Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4(2):171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 30.Schlembach D, Wallner W, Sengenberger R, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007;29(4):407–413. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 31.Savvidou MD, Yu CK, Harland LC, Hingorani AD, Nicolaides KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol. 2006;195(6):1668–1673. doi: 10.1016/j.ajog.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 32.Chaiworapongsa T, Espinoza J, Gotsch F, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21(1):25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49(4):818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 34.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9(4):225–230. doi: 10.1007/s10456-006-9055-8. discussion 231. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 36.Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26(2-3):210–217. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Rajakumar A, Michael HM, Rajakumar PA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26(7):563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Med Sci Sports Exerc. 2005;37(5):768–774. doi: 10.1249/01.mss.0000162688.54089.ce. [DOI] [PubMed] [Google Scholar]
- 40.Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50(1):137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 42.Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112(1):51–57. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 43.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 44.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84(6):937–940. [PubMed] [Google Scholar]
- 45.Laskowska M, Leszczynska-Gorzelak B, Laskowska K, Oleszczuk J. Evaluation of maternal and umbilical serum TNFal-pha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J Matern Fetal Neonatal Med. 2006;19(6):347–351. doi: 10.1080/14767050600637937. [DOI] [PubMed] [Google Scholar]
- 46.Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82(12):1099–1102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]