Araujia sericifera is a South American milkweed which is an invasive/exotic species in South Africa. This species relies on pollinators for sexual reproduction and we show it has co-opted native South African honeybees as primary pollinators in its adopted country. Moths also visit the flowers of this species, but contribute little to its relatively high pollination success.
Abstract
Background and aims
Successful invasive plants such as Araujia sericifera usually either are capable of automatic self-pollination or maintain pollinator services by having generalized pollination systems to make use of local pollinators in the invaded range. Alternatively, plants must co-opt new pollinators with similar morphology to native pollinators or reproduce asexually. We aimed to document the pollination biology of A. sericifera in South Africa. Given the success of this species as an invader, we predicted that sexual reproduction occurs either through self-pollination or because A. sericifera has successfully co-opted native insect pollinators.
Methodology
We examined the pollination biology of the South American A. sericifera in South Africa. We documented the effective pollinators including a comparison of the efficacy of nocturnal versus diurnal pollinators as well as the breeding system and long-term natural levels of the pollination success of this species.
Principal results
We found that native honeybees (Apis mellifera) were the main pollinators of A. sericifera in South Africa. Visiting moths are unimportant pollinators despite being attracted by the pale colour and nocturnal scent of the flowers. Plants from the Grahamstown population were incapable of autonomous self-pollination but pollinator-mediated self-pollination does occur. However, the highest fruit initiation resulted from out-crossed pollination treatments. The high pollen transfer efficiency of this species was comparable to other hymenopteran-pollinated exotic and native milkweeds, suggesting that A. sericifera maintains pollinator services at levels experienced by indigenous asclepiad species.
Conclusions
Araujia sericifera reproduces successfully in South Africa due to a combined ability of this species to attract and exploit native honeybees as its pollinators and of individual plants to set fruit from pollinator-mediated self-pollination.
Introduction
Invasive species introduced into new environments in small numbers could experience pollen limitation or pollination failure if they cannot shift to new pollinators (Parker 1997; Larson et al. 2002; Parker and Haubensak 2002). However, pollination failure (lack of seed set due to pollinator absence) rarely occurs in invasive species and is more likely to prevent species with highly specialized pollination systems and intricate flower morphologies (e.g. figs and orchids) from becoming invasive (Richardson et al. 2000), although exceptions occur (e.g. Ficus spp.: Nadel et al. 1992; Gardner and Early 1996; orchids: Liu and Pemberton 2010). Many invasive species typically either have generalized pollination systems and flowers with open accessible rewards (Richardson et al. 2000; Bjerknes et al. 2007) or overcome pollinator limitation through autonomous or pollinator-mediated self-pollination (Baker 1974; van Kleunen et al. 2008).
The mechanism of pollination in milkweeds (Asclepiadoideae-Apocynaceae) is mechanically complex and requires the accurate re-insertion of pollinia (aggregated compact pollen masses) that are removed as pairs and deposited individually into a snugly fitting stigmatic groove (Wyatt and Broyles 1994; Ollerton et al. 2003; Fig. 1C). Two pollinia are suspended off a clam-like mechanical clip (the corpusculum) that attaches to the pollinator, and constitute a single structure, the pollinarium, that is removed by pollinators. Pollinia are deposited when the insect that is already bearing a pollinarium drags a pollinium through one of the five specialized stigmatic grooves where it may become lodged, breaking off to effect pollination (Wyatt 1976; Wyatt and Broyles 1994). This relatively specialized floral morphology translates into specialized interactions with pollinators in 70 % of examined asclepiads that have less than five species of pollinators, while 38 % have only a single pollinator (Ollerton and Liede 1997). Nevertheless, several milkweeds, including the well-known North American species in the genus Asclepias, have highly generalized pollination systems (Ollerton and Liede 1997). Despite such generalizations, many of these are functionally specialized (sensu Fenster et al. 2004) to a group or family of pollinators with the right morphology and behaviour (Wolff et al. 2008).
Fig. 1.
The invasive A. sericifera is commonly found growing on urban fence-lines (A). Honeybees (A. mellifera) visit A. sericifera by initially hovering in front of the flower (B) and then landing on the petals (D). A flower of A. sericifera with the petals removed showing the gynostegium (C; aw = anther wing, ca = caudicle, cp = corpusculum, p = pollinium, sc = stigmatic chamber, the dashed oval indicates the position of a deposited pollinium). The photograph shows four whole pollinaria that have been deposited in two stigmatic chambers (two per chamber). This way of pollinarium deposition is considered unusual as pollinaria are typically deposited individually with only one pollinium lodged inside the stigmatic chamber (dashed oval, C), and not with one pollinium inside the stigmatic chamber while the other pollinarium is on the outside (see the text for further discussion). A sphingid moth, Temnora plagiata (E), and a noctuid, T. inferior (F, insert), found stuck inside the flower of A. sericifera (scale bars: A = 10 mm, D = 3 mm; all others = 5 mm).
Ten of the 94 species of milkweed occurring in Australia are naturalized invasive species (Forster 1994). In North America at least two species of Vincetoxicum are invasive (Daehler 1998; Cappuccino 2004), while there are two naturalized Asclepiadoideae in South Africa (Victor et al. 2000). Invasive milkweeds are likely to depend largely on co-opting new pollinators as few species can set seed through autonomous self-pollination (Wyatt and Broyles 1994).
Araujia sericifera (Brot.) is an invasive tropical vine that is famous for catching both diurnal and nocturnal Lepidopteran flower visitors. This results from the long proboscides of these insects becoming wedged between the rigid anther wings of its flowers, giving rise to common names of ‘mothcatcher’ or ‘cruelplant’ (Hicken 1928; Forster and Bruyns 1992). Smaller insects may also be trapped in the corpusculum and are incapable of escaping as these insects are too small to remove pollinaria. Araujia sericifera is pollinated by honeybees in Australia (Coleman 1935) and bumble bees (Bombus spp.) and Scoliid wasps (Scolia spp.—Scoliidae) in Europe (Romeo 1933). Several notes and papers have enumerated insects that visit the flowers of A. sericifera in other countries (see Romeo 1933 and references therein; Hicken 1928; Coleman 1935), although records from the native range are limited to a single observation (Morong 1889).
Given the success of this species as an invader in South Africa and the rarity of autonomous self-pollination in the Asclepiadoideae, we hypothesized that A. sericifera successfully utilizes native pollinators to maintain pollination success. We therefore set out to (i) determine the reliance of A. sericifera on pollinators by documenting its breeding system, (ii) determine the functional pollinators of A. sericifera in South Africa, (iii) quantify the consistency of pollination success in this species for several consecutive flowering seasons, (iv) determine the relative contribution of diurnal and nocturnal pollinators to pollination success and (v) compare whether the levels of pollination success in A. sericifera are similar to those of a native milkweed with similar growth form and pollination biology.
Materials and methods
Study species
Araujia sericifera (Apocynaceae-Asclepiadoideae) is indigenous to tropical (including Peru, Argentina, Paraguay and Brazil) and temperate (Uruguay) regions of South America, and has become invasive in several countries in Europe (France, Greece, Italy, Portugal and Spain), Australia, New Zealand, North America, Israel and South Africa (Forster and Bruyns 1992; EMPPO 2008). In South Africa, it commonly grows in abandoned fields and on fences in urban environments (Fig. 1A; Henderson and Anderson 1966). Flowers are white, streaked with light purple, and scented day and night. Flowers are borne on pedunculate axillary inflorescences (sensu Henderson and Anderson 1966). In South Africa, flowering begins in late November and ends in May, with the mid-season peak occurring in December (pers. obs.).
Cynanchum ellipticum (Apocynaceae-Asclepiadoideae) is a common milkweed, endemic to southern Africa (Liede 1993). Both species share broad similarities, including growth form and pollination biology. Cynanchum ellipticum also grows on fences in urban environments, forming large, dense floral displays. Cynanchum ellipticum flowers semi-continuously throughout the year whereas A. sericifera only flowers between November and March in Grahamstown.
Breeding systems
The breeding system of A. sericifera was determined using 20 wild plants growing around Grahamstown during the 2007–08 flowering season and again on 10 plants during the 2008–09 flowering season. During each year, the duration of the breeding system study was 6 weeks.
We performed three treatments per plant and replicated each treatment between one and four times per plant throughout the study period. Treatments were (i) out-crossed flowers pollinated with pollen from another plant, (ii) self-pollinated flowers pollinated with pollen from the same plant and (iii) unmanipulated control, where no pollination was carried out to test for autonomous self-pollination. Only one of each treatment was made per umbel. Following Wyatt (1976), we inserted only one pollinium per flower using small forceps. Due to the tubular shape of the flower, we made a longitudinal slit down one side of the corolla to access the gynostegium. We only bagged flowers with light nylon mesh bags until the buds opened. After treatments were performed, we prevented access to pollinators by wedging a cotton wool plug into the corolla. Because milkweeds often abort their fruit even after successful initial fertilization (Lipow and Wyatt 1998; Finer and Morgan 2003), we scored fruits as initial fertilizations and regularly inspected initiated fruits to record the proportion of initial fertilizations that matured into fruit.
We tested for differences in the number of fruits that were initiated between different pollination treatments by using two-sample t-tests based on different proportions of pollinations that initiated fruit (e.g. Lipow et al. 1999). In both years we only tested within-year differences between the number of fruits that were initiated from cross-pollinated flowers, self-pollinated flowers and unmanipulated controls. The results from the 2007–08 season indicated that A. sericifera does not undergo autonomous self-pollination, so we did not repeat unmanipulated controls during the 2008–09 season. All tests were at the 5 % level of significance.
Pollinators and pollinator behaviour
Diurnal visitors were caught while visiting individuals of A. sericifera in Grahamstown. Bees were the most abundant diurnal visitors and we limited our sampling to a total of 5 days in 2007 and 1 day in 2008. Bees were normally caught between 0800 and 1030 h, with most sampling periods not exceeding 1 h, for a total of ∼8h observation time. For all insects we counted the number of full pollinaria (pollinaria with no pollinia removed), half pollinaria (pollinaria with one pollinium removed) and corpuscula (pollinaria with both pollinia removed) present on the mouthparts.
Nocturnal visitors were collected during sampling periods ranging from 20 min to 2 h. All observations were made between 1930 (sunset) and 2200 h. During each observation period, we attempted to catch all observed moths and counted any additional visits where moths could not be caught. Moth visits were observed over 15 evenings (ca. 15 h observation time). Moths were only caught when visiting flowers, or collected after recently becoming stuck within a flower and were still alive.
Comparison between diurnal versus nocturnal pollination
We used seven large flowering individuals, of which three were exposed to nocturnal pollinators, three to diurnal pollinators and one to both (i.e. exposing part of the plant to nocturnal pollinators and another part to diurnal insects). Bagging consisted of either covering a large part of the plant with fine nylon mesh or bagging entire flowering stalks with large mesh bags. All open flowers were removed from the plants prior to bagging, and exclusion experiments were started once a sufficient number of flowers had opened per plant. Bags on plants exposed to nocturnal pollinators were removed at dusk (1900–1930 h) and replaced the next morning between 0440 and 0530 h before bees started visiting. Bags on plants that were only exposed to diurnal pollinators were removed at 0440–0530 h and then replaced again at dusk before moths started visiting. All plants were open to either nocturnal or diurnal pollinators for 3–5 days or nights. At the end of the bagging period, we randomly picked up to 50 open flowers from each of the four plants and picked another 50 flowers from an unbagged section on the same plant to serve as control flowers being open day and night to all pollinators. This resulted in a sample size of between 190 and 200 flowers for each of the four treatments. For statistical analysis we grouped flowers into those exposed to pollinators during the day or night only and the control flowers to either group.
We tested for differences between treatments by testing for differences in the percentage of flowers with pollinaria removed or deposited. Non-parametric analysis of variance was carried out using the program PERMANOVA (Anderson 2001; McArdle and Anderson 2001) because the small sample size for each category (N = 4) violated the assumptions of normality. Pairwise post hoc differences were tested using this program. For both the overall model and post hoc tests, we used 999 permutations to obtain accurate P values at the 5 % level of significance (Anderson 2001; McArdle and Anderson 2001).
Pollinarium removal, deposition and pollen transfer efficiency
Flowers of A. sericifera were sampled once at the beginning of 2007 and at three different dates within each of the 2007–08 and 2008–09 flowering seasons. During these later two seasons, the sampling intervals were spaced ∼1 month apart. At each sampling date we randomly picked three different flowers per plant from a subsample of plants (range: 9–28 for different sampling dates) growing on fences around Grahamstown. Due to the low number of flowering individuals during February 2007, we sampled up to 20 flowers per plant. For all flowers we scored pollination success by counting the number of pollinaria removed and the number of pollinia deposited per flower, and used this to calculate the average percentage of flowers with at least one pollinarium removed, one pollinium deposited and the pollen transfer efficiency (PTE). The PTE is the proportion of removed pollinia that are deposited on conspecific stigmas, calculated by dividing the number of deposited pollinia by the number of removed pollinia (removed pollinaria multiplied by two; Johnson et al. 2005). The PTE can be considered a population-level estimate of the efficiency with which pollinators move pollen between anthers and stigmas. It is a commonly used measure of pollination success in milkweeds (Shuttleworth and Johnson 2008,2009a, b; Coombs et al. 2009) and orchids (Peter and Johnson 2008a, b; Johnson et al. 2009; see Harder and Johnson 2008 for review).
Given the broad similarities in growth form and pollination biology, we compared the pollination success of A. sericifera and the native C. ellipticum. Flowers of C. ellipticum were sampled during the closest peak flowering period of this species to A. sericifera, which, in Grahamstown, was from late February 2008 to May 2008. Cynanchum ellipticum flowers were sampled on three dates by picking three flowers per plant from 22–31 plants. We then compared the percentage of flowers with pollinaria removed, flowers with pollinaria deposited and PTE between these two species using the program PERMANOVA due to the non-parametric nature of the data. We used 720 permutations and calculated P-values using the Monte-Carlo method, which is advised for small samples (Anderson 2005).
Most pollinia of A. sericifera were deposited as whole pollinaria, with one pollinium inserted into the stigmatic chamber while the other pollinium and connected corpusculum remained on the outside of the anther wings (Fig. 1C). We believe this to be an unusual pattern of pollinium deposition for an asclepiad as the deposited pollinium typically breaks away from the caudicle and is left behind. Therefore, this pattern of pollinium deposition is likely the result of a morphological mismatch between A. sericifera and its co-opted pollinators. To document whether this pattern of pollinium deposition differs from that of C. ellipticum, we used the same flowers that were used to calculate PTE and for three of the sampling dates of both species, we counted the relative proportions of pollinaria that were deposited in this way and compared this with the proportion of pollinaria that were deposited ‘normally’, where a single pollinium is broken off from the corpusculum and seated within the stigmatic chamber.
Colours and reward
Flower colours of A. sericifera were measured on one flower selected randomly from each of 10 different plants (N= 10 flowers). Colour spectra of A. sericifera were measured using a USB 2000 photo spectrometer (Ocean Optics, Dunedin, FL; see Peter and Johnson 2008a for details). Two measurements were made: the first on the white part of the petal and the second on the inner corolla where flowers are frequently dappled with purple spots and streaks.
To measure the standing nectar volume and concentration of A. sericifera, we bagged 2 inflorescences per plant on 10 plants at 1800 h using nylon mesh bags. Inflorescences were harvested the following morning between 0800 and 0900 h. Nectar was extracted from one randomly selected flower per inflorescence using 10-µL micropipettes and the concentration measured as percentage sucrose equivalents using an Atago 0–50 % sucrose refractometer.
Results
Breeding system
Only cross-pollinated and self-pollinated treatments initiated fruit, suggesting that autonomous self-pollination or agamospermy does not occur (Table 1). During 2007–08, the percentage of successful fertilizations from cross-pollinated treatments was not significantly greater than that in self-pollinated treatments (P = 0.096, t76= 1.66). The percentage of flowers that received out-cross pollen and initiated fruit was 39.0 % (2007–08) compared with 20.5 % for self-pollinated flowers. The percentages of cross-pollinated and self-pollinated flowers that initiated fruit were both significantly greater than the unmanipulated control where none of the flowers initiated fruit (cross-pollination versus unmanipulated control: t88= 4.82, P < 0.001; self-pollination versus unmanipulated control: t86= 3.32, P < 0.001). During 2008–09, the percentage of flowers that initiated fruit from cross-pollination treatments significantly exceeded that initiated by self-pollination treatments (52 versus 21.4 %, t51= 2.32, P = 0.02). Fruit abortion was generally high in both out-cross and self-pollination treatments and only a fraction (maximum = 30.8 %) of successful pollinations matured into fruit. Only two cross-pollinated fruit matured in the 2007–08 flowering season and four cross-pollinated and one self-pollinated fruit matured in the 2008–09 flowering season. The small sample size of matured fruit precluded any statistical analyses on these data.
Table 1.
Results of a breeding system for 2 years on A. sericifera
| Flowering season (year) | Treatment | Number of flowers per treatment | Initiated fruit | % | Initiated fruit that matured | % |
|---|---|---|---|---|---|---|
| 2007–08 | Cross-pollination | 41 | 16 | 39.0a | 2 | 13.0 |
| Self-pollination | 39 | 8 | 20.5a | 0 | 0 | |
| Unmanipulated control | 49 | 0 | 0b | 0 | 0 | |
| 2008–09 | Cross-pollination | 25 | 13 | 52.0* | 4 | 30.8 |
| Self-pollination | 28 | 6 | 21.4** | 1 | 16.7 |
Araujia sericifera was not capable of autonomous self-pollination but was capable of geitonogamy, although out-cross pollination treatments had the highest percentage of successful fertilizations.
Superscript letters and symbols indicate significant differences using two-sample t-tests based on proportions. Different symbols were used for different years to indicate that tests were not done between different years.
Pollinators and pollinator behaviour
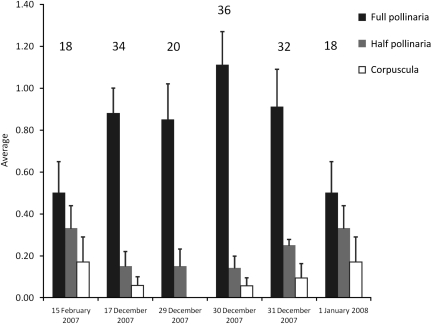
Honeybees (Apis mellifera) were the main diurnal visitors to A. sericifera, with 158 bees being caught in ∼8 h of sampling effort spread over six sampling dates (Fig. 1B and D; Table 2). The majority (69.6 %) of bees bore pollinaria that were carried exclusively on the proboscides. The average total number of pollinaria per bee ranged between 1.0 (SE = 0.2) and 1.30 (SE = 0.2) on different sampling dates. The mean number of full pollinaria always exceeded that of half pollinaria, which was in turn generally higher than the number of corpuscula carried (Fig. 2). Visits were initiated by first hovering in front of the flower before alighting on the dissected part of the petals and then crawling into the corolla tube because the proboscides of the bees were too short to access nectar merely by inserting the proboscis into the flower.
Table 2.
Summary of the total number of different species of insects caught visiting the flowers of A. sericifera, sampling effort (sampling days and hours) and the average number of whole, and half pollinaria and corpuscula borne by each taxon
| Species | Order | Family | Number of days sampled | Total number of hours sampled | Number of individuals caught | Number of individuals bearing pollinaria | Whole pollinaria (mean ± 1 SE) | Half pollinaria (mean ± 1 SE) | Corpuscula (mean ± 1 SE) | Total pollinium load (mean ± 1 SE) |
|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal visitors | ||||||||||
| A. mellifera | Hymenoptera | Apidae | 6 | 8 | 158 | 110 | 0.85 ± 0.84 | 0.21 ± 0.42 | 0.082 ± 0.34 | 1.14 ± 0.1 |
| X. caffra | Hymenoptera | Apidae | 6 | 8 | 1 | 0 | 0 | 0 | 0 | 0 |
| X. flavicollis | Hymenoptera | Apidae | 6 | 8 | 1 | 0 | 0 | 0 | 0 | 0 |
| Nocturnal visitors | ||||||||||
| A. sabulifera | Lepidoptera | Noctuidae | 15 | 15 | 1 | 0 | 0 | 0 | 0 | 0 |
| Athetis pigra | Lepidoptera | Noctuidae | 15 | 15 | 1 | 0 | 0 | 0 | 0 | 0 |
| Borolia spp. | Lepidoptera | Noctuinae | 15 | 15 | 1 | 1 | 0 | 0 | 1 | 1 |
| E. congressa | Lepidoptera | Noctuidae | 15 | 15 | 1 | 0 | 0 | 0 | 0 | 0 |
| E. congressa or E. sobria | Lepidoptera | Noctuidae | 15 | 15 | 2 | 0 | 0 | 0 | 0 | 0 |
| H. armigera | Lepidoptera | Noctuidae | 15 | 15 | 2 | 1 | 0.50 ± 0.50 | 0 | 0 | 0.50 ± 0.50 |
| S. cilium | Lepidoptera | Noctuidae | 15 | 15 | 3 | 2 | 0.67 ± 0.33 | 0 | 0 | 0.67 ± 0.33 |
| T. capensis | Lepidoptera | Sphingidae | 15 | 15 | 1 | 0 | 0 | 0 | 0 | 0 |
| T. inferior | Lepidoptera | Noctuinae | 15 | 15 | 5 | 4 | 0.60 ± 0.24 | 0.2 ± 0.20 | 0 | 0.8 ± 0.20 |
| Additional collectionsa | ||||||||||
| A. horta | Lepidoptera | Nymphalidae | 2 | 2 | 1 | 0.50 ± 0.50 | 0 | 0 | 0.50 ± 0.50 | |
| Borolia spp. | Lepidoptera | Noctuinae | 2 | – | 2 | 1 | 0.50 ± 0.50 | 0 | 0 | 0.50 ± 0.50 |
| C. florella | Lepidoptera | Pieridae | 1 | – | 1 | 0 | 0 | 0 | 0 | 0 |
| C. hylasb | Lepidoptera | Sphingidae | 1 | – | 1 | 1 | 0 | 0 | 1 | 1 |
| T. plagiata | Lepidoptera | Sphingidae | 1 | – | 1 | 1 | 1 | 1 | 1 | 3 |
| T. pylas | Lepidoptera | Sphingidae | 1 | – | 1 | 1 | 0 | 0 | 0 | 0 |
| T. capensis | Lepidoptera | Sphingidae | 1 | – | 1 | 1 | 1 | 0 | 0 | 1 |
Honeybees were the most abundant flower visitors and the majority of these insects bore pollinaria. A wide diversity of moths visited A. sericifera, but moths were less abundant than honeybees and generally carried lower numbers of pollinaria.
aAdditional collections refer to insects not collected during sampling times.
bDay-flying hawkmoth.
Fig. 2.
Changes in the mean number of full pollinaria, half pollinaria and corpuscula carried by honeybees visiting A. sericifera. Pollinarium loads on honeybees caught on different days indicated that honeybees mostly carried full pollinaria. The lower number of half pollinaria and corpuscula present on the mouthparts results from most pollinaria being deposited as full pollinaria due to the morphological mismatch between pollinaria and native honeybees (see the text for details). Numbers appearing above the bars indicate the number of bees caught at each sampling date (bars = mean ± 1 SE).
Other diurnal visitors collected include single individuals of the day-flying Cephanodes hylas (Sphingidae) and two butterfly species, Acraea horta (Nymphalidae) and Catopsilla florella (Pieridae). Two other individuals of C. hylas were observed visiting A. sericifera during the day but were not captured (Table 2).
Nocturnal visitors included at least 11 different species of moths visiting A. sericifera, most of which were small settling Noctuids (Table 2). The most abundant noctuid species were Tycomarptes inferior (Fig. 1F), Spodoptera cilium and Helicoverpa armigera. Pollinarium loads borne by these moths ranged from a maximum of 0.8 (SE = 0.2) in T. inferior to 0.5 (SE = 0.50) in H. armigera. Larger noctuids (Ericeia congressa or E. sobria and Anomis subulifera) and one hawkmoth (Theretra capensis—Sphingidae) were also caught visiting A. sericifera. Moths were less abundant than bees, and in 15 h we caught 17 moths and saw ∼50 visits. Another five moth species were collected while stuck in flowers during the day and are listed as ‘additional collections’ in Table 1. Moths carried pollinaria on the tip of the tongue with the corpusculum either surrounding the tip or clipping onto the side of the proboscis tip in larger Sphingids. The abundance of moths was typically low and very variable (range: 0–24 observations per evening). During 5 of the 15 evenings, no moth visits were seen. The highest visitation rate was recorded on one large plant on the evenings of 29 January 2008, 30 January 2008 and on 1 February 2008, where we saw 6, 24 and 8 visits, respectively. Moths visited the flowers of A. sericifera by making hovering approaches before alighting on the petals and extending their proboscides into the basal nectar cavities of the flower. Smaller-sized moths also crawled into the short tubular corona in order to reach nectar. We inspected several moths that were caught within the flowers and found that either the tongue itself was wedged between the anther wings or moths carrying a pollinarium are caught when the entire pollinarium is dragged into the stigmatic chamber and wedged behind the anther wings (Fig. 1E). Smaller moths may be too weak to break the caudicle when a pollinium is deposited correctly (Fig. 1F).
Comparison between diurnal and nocturnal pollination
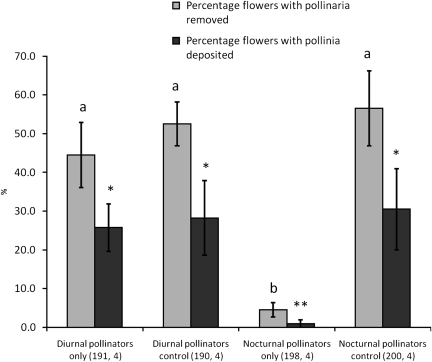
The average percentage of flowers with pollinaria removed from plants that were only exposed to nocturnal pollination was 4.5 % (SE = 1.9; Fig. 3). This was significantly lower than the percentage of flowers with removals from plants exposed only to diurnal pollinators (average ‘Diurnal pollinators only’ = 44.5 %, SE = 8.4, t4 =4.65, P = 0.034) and the control flowers exposed to nocturnal pollinators only (average control = 56.5 %, SE = 9.7, t4 = 5.27, P = 0.030). The average percentage of flowers with pollinaria removed from plants exposed only to diurnal pollinators was 44.5 % (SE = 8.4) and was not significantly different from its control (average control = 52.5 %, SE = 5.7, t4 = 0.79, P = 0.47).
Fig. 3.
Differences in the percentage of flowers with pollinaria removed and the percentage of flowers with pollinia deposited in plants that have been exposed to diurnal pollinators only, nocturnal pollinators only, or both (control). Plants exposed to diurnal pollinators only had a significantly higher percentage of flowers with pollinaria removed and deposited than plants exposed only to nocturnal pollinators. Asterisks above the bars correspond to significant differences between treatments. Sample sizes (n flowers, n plants) for each treatment are included in parentheses below the bars. All bars = mean ± 1 SE.
The average percentage of flowers with pollinia deposited in plants exposed only to nocturnal pollination was 1 % (SE = 1.0) and was significantly lower than the control flowers of this group (average control = 30.5 %, SE = 10.5, t4 = 2.79, P = 0.03) and the percentage of pollinated flowers exposed to diurnal pollinators (t4 = 3.97, P = 0.034). The average percentage of flowers that received at least one pollinium was 25.7 % (SE = 6.1) for plants exposed only to diurnal pollinators and did not differ significantly from the control flowers of this group (average control = 28.1 %, SE = 9.6, t4= 0.21, P = 0.94).
Pollinarium removal, deposition and PTE
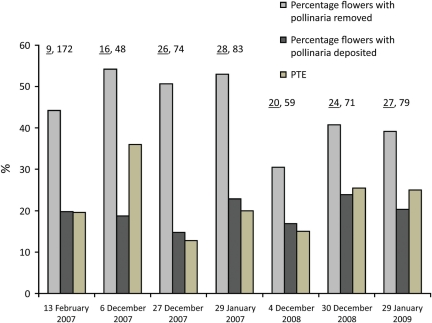
In A. sericifera, the percentage of flowers with at least one pollinarium removed ranged between 30.5 and 54.2 % on different sampling dates. The percentage of pollinated flowers ranged from a minimum of 14.7 % to a maximum of 23.9 % (Fig. 4). The PTE was generally high, and ranged from a minimum of 12.8 % to a maximum of 36 % across both years. The trend of PTE, however, did not vary predictably across sampling dates.
Fig. 4.
Changes in the percentage of flowers with at least one pollinarium removed, with at least one pollinium deposited and PTE (percentage of removed pollinia that are deposited on conspecific stigmas) at different sampling dates between February 2007 and January 2009. The pollination success of A. sericifera was generally high, suggesting that this species effectively maintains pollination service to its flowers outside its native range by attracting native diurnal numbers (numbers above each sampling date contain the number of plants (underlined) followed by the number of flowers for each sampling date).
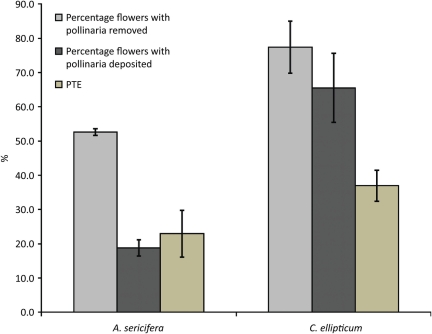
The average percentage of flowers with at least one pollinarium removed was 77.4 % (SE = 7.6) for C. ellipticum, which was significantly higher than the 52.6 % (SE = 1.0) obtained for A. sericifera (t5= 3.25, P = 0.035; Fig. 5). The percentage of flowers that received pollinia was 65.5 % (SE = 10) in C. ellipticum and was significantly higher than the 18.8 % (SE = 2.4) received by A. sericifera (t5= 4.53, P = 0.007). The average PTE of C. ellipticum was 36.9 % (SE = 4.5), which was not significantly higher than that of A. sericifera (mean = 22.9.0 %, SE = 6.9; t5= 1.71, P = 0.17).
Fig. 5.
Comparison of the average percentage of flowers with pollinaria removed, flowers with pollinia deposited and PTE between the exotic A. sericifera and native C. ellipticum. Pollinarium removal and deposition were significantly higher in C. ellipticum but PTE was similar and not significantly different between these two species (bars = mean ± 1 SE). Flowers of A. sericifera were sampled on 6 December 2007 (N = 16 plants, 48 flowers), 27 December 2007 (N = 26 plants, 74 flowers) and 29 January 2008 (N = 28 plants, 83 flowers). Flowers of C. ellipticum were sampled on 17 March 2008 (N = 22 plants, 64 flowers), 29 March 2008 (N = 31 plants, 87 flowers) and 14 April 2008 (N = 31 plants, 92 flowers).
In A. sericifera, the majority (61.2 %; 30 of 49) of pollinaria were deposited as whole pollinaria with one pollinium inserted in the stigmatic chamber while the other remaining pollinium and corpusculum were left outside (Fig. 1C). This was significantly lower than the 1.9 % (8 of 427; t-test based on proportions, P < 0.0001) of C. ellipticum pollinia deposited in this manner, the majority being deposited as single pollinia. The remaining percentage of depositions in A. sericifera were either deposited normally (22 %, 11 of 49) or were deposited as either the entire pollinarium (i.e. both pollinia and corpusculum) inside the stigmatic chamber or as a half pollinarium. The vast majority of C. ellipticum depositions (97.9 %, 418 of a total of 427) were deposited ‘normally’ as explained in the methods.
Colours and reward
Colours for the tips of the corolla and the centre varied between purple and white. Both areas only reflected above 400 nm, indicating no UV reflectance from the petal. Nectar volumes were large (average = 17.27 µL; SE = 2.54, N = 19), but highly variable (range: 0.97–48.81 µL). The concentration per flower ranged from 5.90 to 50.75 sucrose equivalents with an average concentration of 22.0 % sucrose equivalents (SE = 2.66, N = 19).
Discussion
In South Africa, A. sericifera is pollinated primarily by native honeybees (A. mellifera) while nocturnal moths are relatively ineffectual pollinators. Other diurnal flower visitors such as carpenter bees, day-flying hawkmoths and butterflies were sometimes seen visiting this species, but only did so infrequently and rarely carried pollinaria. Honeybees have learnt to access the nectar of the oversized flowers, but like moths, bees were sometimes ‘caught’ by the anther wings of the flower; however, most freed themselves after a brief struggle. Observations made by Coleman (1935) in Australia and Romeo (1933) in Europe indicated that larger Hymenoptera such as carpenter bees (Xylocopa violacea), bumblebees (Bombus pascuorum and B. terrestris), Scoliidae (Scolia flavifrons and S. sexmaculata) and honeybees manage to escape from the anther wings more often than not.
Moths visiting A. sericifera in Grahamstown removed and deposited only a fraction of pollinaria when compared with honeybees. Moths and butterflies have also been observed visiting this species in Europe (Hicken 1928; Romeo 1933), although these authors report more butterflies than were observed in the current study. Moths bearing pollinaria had the corpuscula attached around the tip of the tongue, similar to pollinaria of the moth-pollinated vine Metaplexis japonica (Asclepiadoideae; Sugiura and Yamazaki 2005). The efficacy of moths in pollinating A. sericifera is limited due to the tendency of these insects to get stuck and die within the flowers. This ineffectiveness of moths in depositing pollinia is further confirmed by the relatively few half pollinaria carried by these insects. Similarly, Romeo (1933) found that several genera of Noctuidae (e.g. Plusia spp., Heliothis spp. and Caradrina spp.) and Sphingidae (Deilephila spp. and Macroglossa spp.) visited the flowers of A. sericifera in Europe and supposedly also play a minor role in the pollination of this species. It is worth noting that the appendages of both pollinating and non-pollinating insects regularly become stuck between the anther wings or within the corpuscular groove of milkweed flowers, and this does not only occur in invasive species (see Robertson 1887; Hicken 1928; Frost 1965; Morse 1981; Shuttleworth and Johnson 2009b).
Understanding the pollination biology of A. sericifera requires examining pollinator records from its native range. Most of the records of insects pollinating A. sericifera are old (1825–1935) and are confined to areas where it is exotic (e.g. Hicken 1928; Romeo 1933; Coleman 1935). Honeybees that frequently pollinate A. sericifera in its invasive range are not native to its region of origin in South America (Ruttner 1988). Bumblebees are native to South America (Michener 2000), and were proposed by Coleman (1935) to be the pollinator in the native range. The only record of a potential pollinator in its natural range was a visit by a day-flying hawkmoth in Paraguay (Morong 1889). The large nectar volume, white flowers and nocturnal scent are typical of moth-pollinated flowers (Faegri and van der Pijl 1979), and may explain the attractiveness of these flowers to moths around the world. The nectar concentration is relatively low and typical of hawkmoth-pollinated species (Cruden et al. 1983). White-coloured flowers, bulbous nectar cavities and filaments emerging from the top of the pistil are also present in the moth-pollinated M. japonica (see Sugiura and Yamazaki 2005), suggesting that moths could be the natural pollinators. Pollination by Hymenoptera is equally likely—the large, sharply pointed and rigid anther wings are also present in some Pachycarpus spp. that are pollinated by large Pompilid wasps (Shuttleworth and Johnson 2006).
The large flower size of A. sericifera suggests that it is not optimized for pollination by relatively small honeybees. Despite this, honeybees are efficient at removing and depositing pollen. The nectar volumes of this species were generally large but highly variable, making it difficult to say whether these nectar volumes point to larger insects being the natural pollinators. Inferring the natural pollinator from the size of the nectar reward is also difficult as the standing crop of nectar is known to be variable (Keasar et al. 2008). The range of nectar concentrations recorded for flowers of this species is, however, well within the range of most bee-pollinated plants (Cruden et al. 1983).
One possibility is that A. sericifera is highly generalized in its native range, which enables it to exploit diverse assemblages of pollinators in various parts of the world where it has become invasive. It seems likely from the morphological evidence presented above (white, scented flowers, a long corolla tube for an asclepiad, abundant nectar, large pollinaria) that native pollinators are either relatively large moths with relatively short tongues such as large noctuids or relatively large, long-tongued bees (Bombus or Euglossine bees). As noted above, honeybees do not occur in South America, where eusocial bees include only smaller Meliponini stingless bees or larger Bombus bees (Michener 2000). Honeybees mismatch with morphological aspects of the flower such as the large corolla tube and large pollinaria, which attach poorly to the bee resulting in messy deposition of whole pollinaria—all of these features point to A. sericifera being adapted to pollinators larger than honeybees.
The interaction of A. sericifera with native honeybees in South Africa and with honeybees and bumblebees in other invaded areas confirms that the intricate flower morphology of milkweeds is not a barrier to co-opting new pollinators, particularly in species that attract honeybees and other generalist Hymenoptera. For instance, exotic honeybees are one of the most effective pollinators of Asclepias incarnata within its home range (Ivey et al. 2003). Similar groups of Hymenoptera (pompilids, vespids and ichneumonids) pollinate Gomphocarpus physocarpus in its invasive (Australia) and native (South Africa) ranges (Forster 1994; Coombs et al. 2009). Milkweeds that are pollinated by pollinators other than the Hymenoptera have also become invasive. One species, Vincetoxicum nigrum, is an invasive fly-pollinated vine occurring in the USA (Lumer and Yost 1995), while Herrera and Nassar (2009) have reported fly pollination (Muscidae, Calliphoridae and Sarcophagidae) in naturalized populations of Stapelia gigantea in Venezuela.
Despite having to co-opt native honeybees as pollinators, A. sericifera maintains relatively high levels of pollination success that are lower but still comparable to a native honeybee-pollinated milkweed. During some periods, over half of all flowers of A. sericifera had pollinaria removed and more than a third of all removed pollinia were subsequently deposited. Although the estimates of pollen removal and deposition were higher in C. ellipticum, this is to be expected as bees pollinating C. ellipticum carry some of the largest numbers of pollinaria recorded for any African milkweed (G. Coombs, A.P. Dold and C.I. Peter, unpubl. res.). The PTE of C. ellipticum was not significantly higher than that of A. sericifera. This is impressive considering that A. sericifera is exotic and has inherent pollination inefficiency introduced by honeybees which frequently deposit whole pollinaria with one of the paired pollinia positioned outside of the stigmatic groove, thereby wasting half of the pollinia. Although it is tempting to conclude that this pattern of pollinium deposition is entirely due to a mismatch between honeybees and the pollinaria of A. sericifera, regular deposition of entire pollinaria (i.e. both pollinaria and the corpusculum) has been reported in wasps (Polybia spp.) pollinating Oxypetalum appendiculatum (Viera and Shepherd 1999).
The seasonal variability in pollination success of A. sericifera is not uncommon in plants. Peter and Johnson (2008b) demonstrated that PTE in Acrolophia cochlearis (Orchidaceae) ranged from 0 to 60 % throughout the 5-month flowering period of this species. Similar results have been reported for milkweeds (Ivey et al. 2003). Estimates of pollen removal and deposition for other invasive milkweeds include those made by Coleman (1935), who indicated that on average 80 % of the pollinaria had been removed and 40 % deposited in flowers of A. sericifera that apparently showed signs of being fertilized. Forster (1994) reported that 38.9 % of flowers had been pollinated in an Australian population of G. physocarpus and the average PTE was 24.9 % per plant. Although data are clearly limited, our findings suggest that the measures of pollination success in A. sericifera are comparable to those experienced by other invasive milkweeds, both in magnitude and variability.
Unlike the breeding systems of many other invasive species, A. sericifera is not capable of autonomous self-pollination, making this species entirely reliant on bees for pollination and fruit set. This type of breeding system is, however, expected within the Asclepiadaceae, where automatic self-pollination is rare (Wyatt and Broyles 1994). To our knowledge, the only exotic milkweeds that have been reported to have this ability have been V. nigrum (Lumer and Yost 1995) and observations by Cappuccino (2004) that suggested automatic self-pollination is present in V. rossicum. Araujia sericifera is, however, genetically self-compatible and capable of pollinator-facilitated self-pollination (geitonogamy), a trait present in most invasive species (van Kleunen et al. 2008), but relatively rare in the Asclepiadoideae, although this mode of reproduction is known from some weedy North American milkweeds (e.g. A. exaltata, A. speciosa, A. currassavica and A. fruticosa; Wyatt and Broyles 1997; Lipow et al. 1999; Finer and Morgan 2003). The ability of A. sericifera to self-pollinate could facilitate reproduction in the early stages of invasion, although the tendency for geitonogamous pollinations to initiate and mature less fruit leads us to conclude that in larger, well-established populations with relatively high and consistent pollen transfer, most fruit set is likely to come from cross-pollinations carried out by honeybees.
Conclusions and forward look
We have shown support for our hypothesis that A. sericifera has successfully co-opted a native generalist pollinator (honeybee) in its invaded range in South Africa. The high pollination success of A. sericifera suggests that it does not suffer pollination failure in South Africa and consistently maintains relatively high levels of PTE throughout several flowering seasons. The species is also able to reproduce in small populations owing to the ability of single individuals to set fruit through geitonogamous pollinations. The results of this study combined with others (e.g. Liu and Pemberton 2010) presents mounting evidence that invasive plants are not necessarily prevented from invading new regions to specialized flower morphologies. Future studies should focus on documenting the natural pollinators, pollination success and breeding system of A. sericifera in its natural range. These data would reveal whether this species maintains equally high levels of pollination success in its native versus exotic ranges and whether geitonogamy is present in natural populations or is an acquired trait present only in exotic populations (e.g. van Kleunen et al. 2008). A further point of interest will be to examine the degree to which invasive asclepiads have generalist pollination systems as a pre-adaptation to exploiting novel pollinators when invading new areas.
Sources of funding
This work was funded by the Henderson Foundation (Rhodes University, South Africa) and the Rhodes University Joint Research Council.
Contributions by the authors
Both authors were involved in planning the research and writing the manuscript. The first author conducted the majority of the fieldwork.
Conflict of interest statement
None declared.
Acknowledgements
We thank William Coombs Jr and William Sr for help with fieldwork and technical aspects of the project. We thank Martin Kruger from the Transvaal Museum for help with identifying moths and also Steve Johnson and Robert Pemberton for comments on the manuscript.
References
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. doi:10.1046/j.1442-9993.2001.01070.x. [Google Scholar]
- Anderson MJ. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. New Zealand: Department of Statistics, University of Auckland; 2005. [Google Scholar]
- Baker HG. The evolution of weeds. Annual Review of Ecology and Systematics. 1974;5:1–24. doi:10.1146/annurev.es.05.110174.000245. [Google Scholar]
- Bjerknes A-L, Totland O, Hegland SJ, Nielsen A. Do alien plant invasions really affect pollination success in native plant species? Biological Conservation. 2007;138:1–12. doi:10.1016/j.biocon.2007.04.015. [Google Scholar]
- Cappuccino N. Allee effect in an invasive alien plant pale swallow wort Vincetoxicum rossicum (Asclepiadaceae) Oikos. 2004;106:3–8. doi:10.1111/j.0030-1299.2004.12863.x. [Google Scholar]
- Coleman E. Pollination in Australia of Araujia sericifera Brothero. The Victorian Naturalist. 1935;52:3–7. [Google Scholar]
- Coombs G, Peter CI, Johnson SD. A test for Allee effects in the self-incompatible wasp-pollinated milkweed Gomphocarpus physocarpus (Asclepiadoideae) Austral Ecology. 2009;34:688–697. doi:10.1111/j.1442-9993.2009.01976.x. [Google Scholar]
- Cruden RW, Hermann SM, Peterson S. Patterns of nectar production and plant-pollinator coevolution. In: Bentley B, Elias T, editors. The biology of nectaries. New York: Columbia University Press; 1983. [Google Scholar]
- Daehler CC. The taxonomic distribution of invasive angiosperm plants: ecological insights and comparison to agricultural weeds. Biological Conservation. 1998;84:167–180. doi:10.1016/S0006-3207(97)00096-7. [Google Scholar]
- European and Medditeranean Plant Protection Organization (EMPPO) 2008. www.eppo.org/QUARANTINE/Alert_List/invasive_plants/Araujia_sericifera.htm. (5 May 2009)
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thompson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. doi:10.1073/pnas.0607306104. [Google Scholar]
- Finer MS, Morgan MT. Effects of natural rates of Geitonogamy on fruit set in Asclepias speciosa (Apocynaceae): evidence favoring the plant's dilemma. American Journal of Botany. 2003;90:1746–1750. doi: 10.3732/ajb.90.12.1746. doi:10.3732/ajb.90.12.1746. [DOI] [PubMed] [Google Scholar]
- Forster PI. Diurnal insects associated with the flowers of Gomphocarpus physocarpus E. Mey. (Asclepiadaceae), an introduced weed in Australia. Biotropica. 1994;26:214–217. doi:10.2307/2388811. [Google Scholar]
- Forster PI, Bruyns PV. Clarification of synonymy for the common moth-vine Araujia sericifera (Asclepiadaceae) Taxon. 1992;41:746–749. doi:10.2307/1222403. [Google Scholar]
- Frost SW. Insects and pollinia. Ecology. 1965;46:556–558. doi:10.2307/1934896. [Google Scholar]
- Gardner RO, Early JW. The naturalization of Banyan figs (Ficus spp. Moraceae) and their pollinating wasps (Hymenoptera: Agaonidae) in New Zealand. New Zealand Journal of Botany. 1996;34:103–110. [Google Scholar]
- Harder LD, Johnson SD. Function and evolution of aggregated pollen in angiosperms. International Journal of Plant Sciences. 2008;169:59–78. doi:10.1086/523364. [Google Scholar]
- Henderson M, Anderson JG. Common weeds in South Africa. South Africa: Department of Agricultural and Technical Sciences; 1966. Botanical Research Institute. [Google Scholar]
- Herrera I, Nassar JM. Reproductive and recruitment traits as indicators of the invasive potential of Kalanchoe daigremontiana (Crassulaceae) and Stapelia gigantea (Apocynaceae) in a Neotropical arid zone. Journal of Arid Environments. 2009;73:978–986. doi:10.1016/j.jaridenv.2009.05.004. [Google Scholar]
- Hicken CM. La planta cruel ‘El Tasi’ (Araujoa sericifera Brot.) Darwiniana, Carpeta del ‘Darwinion’. 1928;2:24–29. [Google Scholar]
- Ivey CT, Martinez P, Wyatt R. Variation in pollinator effectiveness in swamp milkweed, Asclepias incarnata (Apocynaceae) American Journal of Botany. 2003;90:214–225. doi: 10.3732/ajb.90.2.214. doi:10.3732/ajb.90.2.214. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Neal PR, Harder LD. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnaean Society. 2005;86:175–190. doi:10.1111/j.1095-8312.2005.00541.x. [Google Scholar]
- Johnson SD, Torninger E, Agren J. Relationship between population size and pollen fates in a moth pollinated orchid. Biology Letters. 2009;5:282–285. doi: 10.1098/rsbl.2008.0702. doi:10.1098/rsbl.2008.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasar T, Sadeh A, Shmida A. Variability in nectar production and standing crop, and their relation to pollinator visits in a Mediterranean shrub. Arthropod–Plant Interactions. 2008;2:117–123. doi:10.1007/s11829-008-9040-9. [Google Scholar]
- Larson KC, Fowler SP, Walker JC. Lack of pollinators limits fruit set in the exotic Lonicera japonica. American Midland Naturalist. 2002;148:54–60. doi:10.1674/0003-0031(2002)148[0054:LOPLFS]2.0.CO;2. [Google Scholar]
- Liede S. A taxonomic revision of the genus Cynanchum, L. (Asclepiadaceae) in southern Africa. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1993;114:503–550. [Google Scholar]
- Lipow SR, Wyatt R. Reproductive biology and breeding system in Gonolobus suberosus (Asclepiadaceae) Journal of the Torrey Botanical Society. 1998;125:183–193. doi:10.2307/2997216. [Google Scholar]
- Lipow SR, Broyles SB, Wyatt R. Population differences in self-fertility in the ‘self-incompatible’ milkweed Asclepias exaltata (Asclepiadaceae) American Journal of Botany. 1999;86:1114–1120. doi:10.2307/2656974. [PubMed] [Google Scholar]
- Liu H, Pemberton R. Pollination of an invasive orchid, Cyrtopodium polyphyllum (Orchidaceae), by an invasive oil-collecting bee, Centris nitida, in southern Florida. Botany. 2010;88:290–295. doi:10.1139/B10-017. [Google Scholar]
- Lumer C, Yost SE. The reproductive biology of Vincetoxicum nigrum (L.) Moench (Asclepiadaceae), a Mediterranean weed in New York State. Bulletin of the Torrey Botanical Club. 1995;122:15–23. doi:10.2307/2996399. [Google Scholar]
- McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. doi:10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [Google Scholar]
- Michener CD. The bees of the world. Baltimore: Johns Hopkins University Press; 2000. [Google Scholar]
- Morong I. Paraguay and its flora (II) Botanical Gazette. 1889;14:245. [Google Scholar]
- Morse DH. Modification of bumblebee foraging: the effect of milkweed pollinia. Ecology. 1981;62:89–97. doi:10.2307/1936672. [Google Scholar]
- Nadel H, Frank JH, Knight RJ. Escapees and accomplices: the naturalization of exotic Ficus and their associated faunas in Florida. Florida Entomolomologist. 1992;75:29–38. doi:10.2307/3495478. [Google Scholar]
- Ollerton J, Liede S. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. Biological Journal of the Linnean Society. 1997;62:593–610. doi:10.1093/aob/mcg206. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany. 2003;92:807–834. doi: 10.1093/aob/mcg206. doi:10.1093/aob/mcg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker IM. Pollinator limitation of Cytisus scoparius (Scotch broom), an invasive exotic shrub. Ecology. 1997;78:1457–1470. [Google Scholar]
- Parker IM, Haubensak KA. Comparative pollinator limitation of two non-native shrubs: do mutualisms influence invasions? Oecologia. 2002;130:250–258. doi: 10.1007/s004420100799. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology. 2008a;89:1583–1595. doi: 10.1890/07-1098.1. doi:10.1890/07-1098.1. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Reproductive biology of Acrolophia cochlearis (Orchidaceae): estimating rates of cross-pollination in epidendroid orchids. Annals of Botany. 2008b;104:573–581. doi: 10.1093/aob/mcn218. doi:10.1093/aob/mcn218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmanek M. Plant invasions—the role of mutualisms. Biological Reviews. 2000;75:65–93. doi: 10.1017/s0006323199005435. doi:10.1017/S0006323199005435. [DOI] [PubMed] [Google Scholar]
- Robertson C. Insect relations of certain Asclepiads II. Botanical Gazette. 1887;12:244–250. doi:10.1086/326179. [Google Scholar]
- Romeo A. Contributo alla biologia fiorale dell ‘Araujia sericifera’ Brot. La pianta catturatrice d'insect. Annali del Regio Instituto Superiore Agrario di Portici. 1933;6:79–97. [Google Scholar]
- Ruttner F. Biogeography and taxonomy of honeybees. Berlin: Springer; 1988. [Google Scholar]
- Shuttleworth A, Johnson SD. Specialized pollination by large spider-hunting wasps and self-incompatibility in the African milkweed Pachycarpus asperifolius. International Journal of Plant Sciences. 2006;167:1177–1186. doi:10.1086/507685. [Google Scholar]
- Shuttleworth A, Johnson SD. Bimodal pollination by wasps and beetles in the African milkweed, Xysmalobium undulatum. Biotropica. 2008;40:568–574. doi:10.1111/j.1744-7429.2008.00418.x. [Google Scholar]
- Shuttleworth A, Johnson SD. New records of insect pollinators for South African Asclepiads (Apocynaceae – Asclepiadoideae) South African Journal of Botany. 2009a;75:689–698. doi:10.1016/j.sajb.2009.07.017. [Google Scholar]
- Shuttleworth A, Johnson SD. Palp-faction: an African milkweed dismembers its wasp pollinators. Environmental Entomology. 2009b;38:741–747. doi: 10.1603/022.038.0326. doi:10.1603/022.038.0326. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Yamazaki K. Moth pollination of Metaplexis japonica (Apocynaceae): pollinaria transfer on the tip of the proboscis. Journal of Plant Research. 2005;118:257–262. doi: 10.1007/s10265-005-0216-4. doi:10.1007/s10265-005-0216-4. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Manning JC, Pasqualetto V, Johnson SD. Phylogenetically independent associations between autonomous self-fertilization and plant invasiveness. American Naturalist. 2008;171:195–201. doi: 10.1086/525057. doi:10.1086/525057. [DOI] [PubMed] [Google Scholar]
- Victor JE, Bredenkamp CL, Venter HJT, Bruyns PV, Nicholas A. Apocynaceae. In: Leistner OA, editor. Seed plants of southern Africa: families and genera, Vol. 10. Pretoria: National Botanical Institute; 2000. [Google Scholar]
- Viera MF, Shepherd GJ. Pollinators of Oxypetalum (Asclepiadaceae) in Southeastern Brazil. Revista Brasileira de Biologia. 1999;59:693–704. doi: 10.1590/s0034-71081999000400018. [DOI] [PubMed] [Google Scholar]
- Wolff D, Meve U, Liede-Schumann S. Pollination of Ecaudorian Asclepiadoideae (Apocynaceae): how generalized are morphologically specialized flowers? Basic and Applied Ecology. 2008;9:24–34. doi:10.1016/j.baae.2007.06.013. [Google Scholar]
- Wyatt R. Pollination and fruit set in Asclepias: a reappraisal. American Journal of Botany. 1976;63:845–851. doi:10.2307/2442044. [Google Scholar]
- Wyatt R, Broyles SB. Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics. 1994;25:423–441. doi:10.1146/annurev.es.25.110194.002231. [Google Scholar]
- Wyatt R, Broyles SB. The weedy tropical milkweeds Asclepias curassavica and A. fruticosa are self-compatible. Biotropica. 1997;29:232–234. doi:10.1111/j.1744-7429.1997.tb00029.x. [Google Scholar]