Abstract
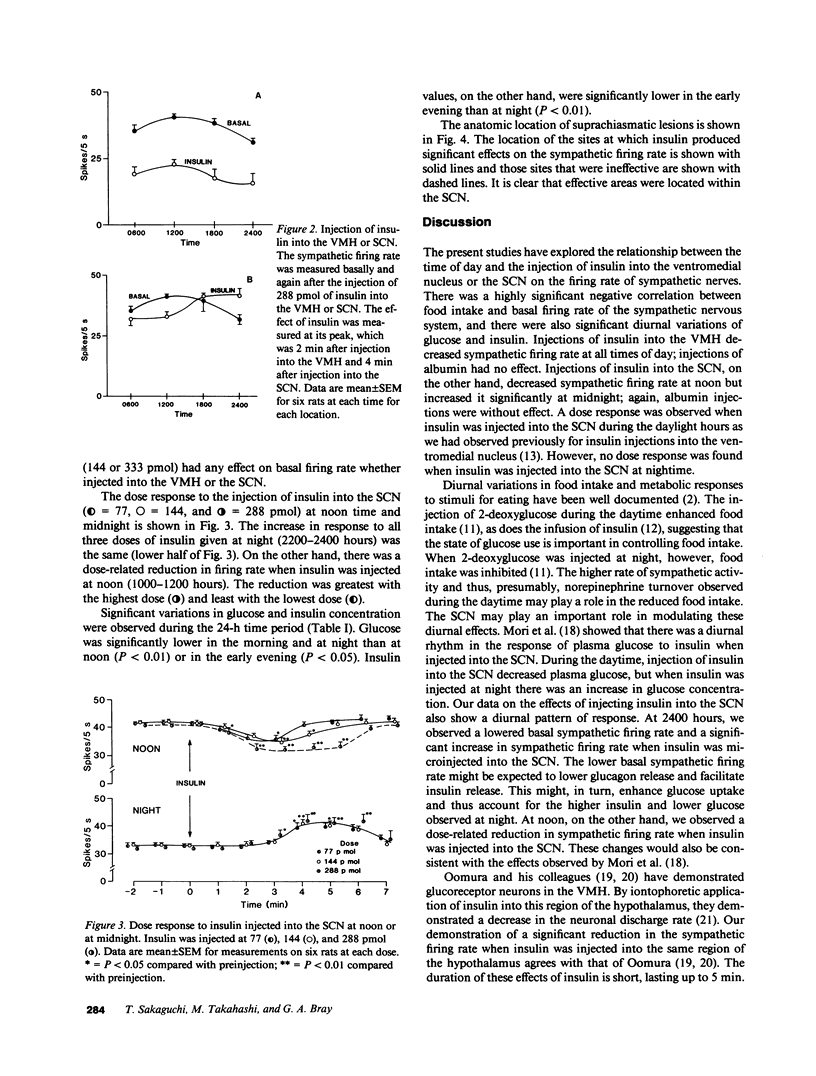
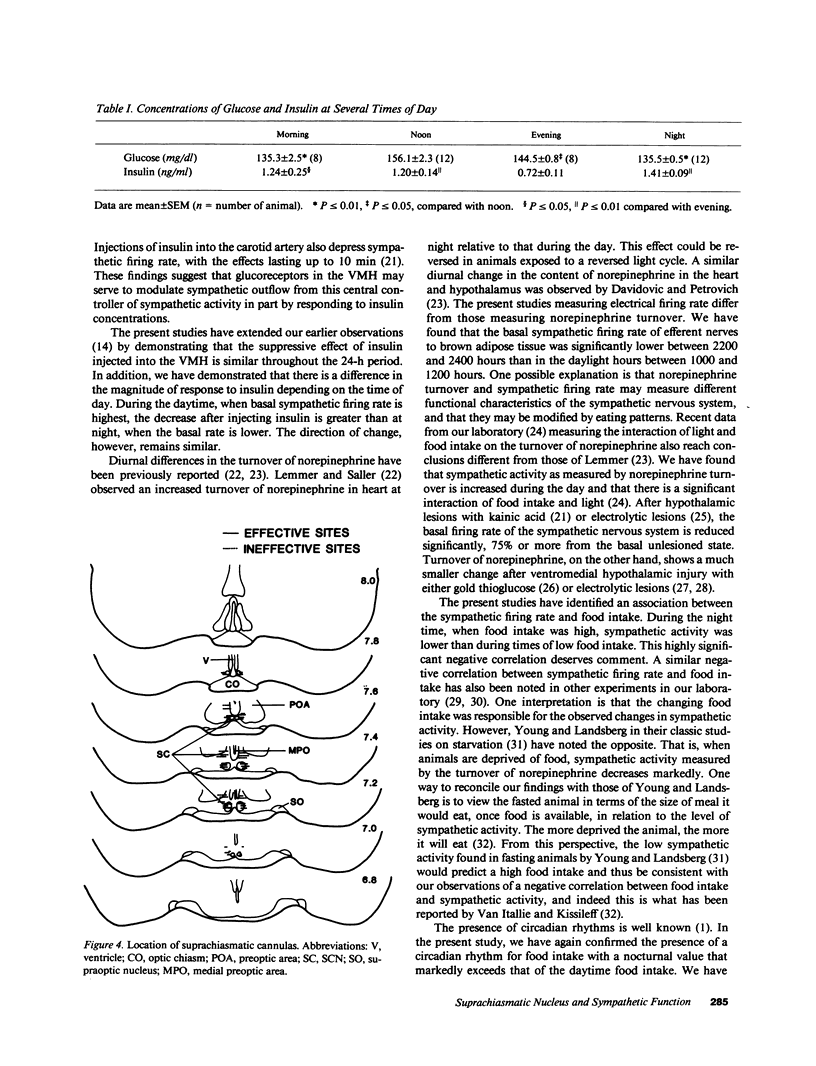
The present study was designed to test whether there are diurnal changes in the firing rate of sympathetic nerves to brown adipose tissue and whether these diurnal rhythms influenced the response to insulin injected into the suprachiasmatic nucleus or ventromedial hypothalamic nucleus (VMH). Food intake was highest at the beginning of the dark period (1800-2200 hours) and lowest during the daylight hours (0600-1000 and 1200-1600 hours). The basal sympathetic firing rate was highest at noon (1000-1200 hours) when food intake was lowest. At midnight, when food intake was highest, sympathetic firing rate was lowest. Injection of insulin (77, 144, and 288 pmol) into the VMH produced a dose-dependent depression of sympathetic firing rate at each of the four measurement periods (0400-0600 hours, 1000-1200 hours, 1600-1800 hours, and 2200-2400 hours), but the magnitude of the effect was greater at noon than at night. In contrast, insulin injections into the suprachiasmatic nucleus decreased the sympathetic firing rate at noon but produced a significant increase in the sympathetic firing rate at night. These data show that a diurnal rhythm exists for the sympathetic firing rate. The decrease in firing rate in response to insulin when injected into the VMH is in the same direction but varies in magnitude throughout the day, whereas the responsiveness of the suprachiasmatic nucleus to injections of insulin shows a reversal of response in relation to day/night cycles. The highly significant inverse relationship between basal sympathetic firing rate and food intake suggests that sympathetic activity may be part of an important control system for energy balance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balagura S., Harrell L. E. Neuroendocrine conditioning: conditioned feeding after alterations in glucose utilization. Am J Physiol. 1975 Feb;228(2):392–396. doi: 10.1152/ajplegacy.1975.228.2.392. [DOI] [PubMed] [Google Scholar]
- Bernardis L. L. Disruption of diurnal feeding and weight gain cycles in weanling rats by ventromedial and dorsomedial hypothalamic lesions. Physiol Behav. 1973 May;10(5):855–861. doi: 10.1016/0031-9384(73)90054-1. [DOI] [PubMed] [Google Scholar]
- Davidović V., Petrović V. M. Diurnal variations in the catecholamine content in rat tissues. Effects of exogenous noradrenaline. Arch Int Physiol Biochim. 1981 Dec;89(5):457–460. doi: 10.3109/13813458109082642. [DOI] [PubMed] [Google Scholar]
- Hara E., Saito M. Diurnal changes in plasma glucose and insulin responses to oral glucose load in rats. Am J Physiol. 1980 May;238(5):E463–E466. doi: 10.1152/ajpendo.1980.238.5.E463. [DOI] [PubMed] [Google Scholar]
- Kakolewski J. W., Deaux E., Christensen J., Case B. Diurnal patterns in water and food intake and body weight changes in rats with hypothalamic lesions. Am J Physiol. 1971 Sep;221(3):711–718. doi: 10.1152/ajplegacy.1971.221.3.711. [DOI] [PubMed] [Google Scholar]
- Kimura T., Maji T., Ashida K. Periodicity of food intake and lipogenesis in rats subjected to two different feeding plans. J Nutr. 1970 Jun;100(6):691–697. doi: 10.1093/jn/100.6.691. [DOI] [PubMed] [Google Scholar]
- Larue-Achagiotis C., Le Magnen J. Dual effects of 2-deoxy-D-glucose on food intake in the rat: inhibition at night and stimulation in the day-time. Physiol Behav. 1979 Nov;23(5):865–869. doi: 10.1016/0031-9384(79)90192-6. [DOI] [PubMed] [Google Scholar]
- Larue-Achagiotis C., le Magnen J. The different effects of continuous night and day-time insulin infusion on the meal pattern of normal rats: comparison with the meal pattern of hyperphagic hypothalamic rats. Physiol Behav. 1979 Mar;22(3):435–439. doi: 10.1016/0031-9384(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Body energy balance and food intake: a neuroendocrine regulatory mechanism. Physiol Rev. 1983 Jan;63(1):314–386. doi: 10.1152/physrev.1983.63.1.314. [DOI] [PubMed] [Google Scholar]
- Le Magnen J., Devos M., Gaudillière J. P., Louis-Sylvestre J., Tallon S. Role of a lipostatic mechanism in regulation by feeding of energy balance in rats. J Comp Physiol Psychol. 1973 Jul;84(1):1–23. doi: 10.1037/h0035019. [DOI] [PubMed] [Google Scholar]
- Lemmer B., Saller R. Influence of light and darkness on the turnover of noradrenaline in the rat heart. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):75–84. doi: 10.1007/BF00647404. [DOI] [PubMed] [Google Scholar]
- Mori T., Nagai K., Hara M., Nakagawa H. Time-dependent effect of insulin in suprachiasmatic nucleus on blood glucose. Am J Physiol. 1985 Jul;249(1 Pt 2):R23–R30. doi: 10.1152/ajpregu.1985.249.1.R23. [DOI] [PubMed] [Google Scholar]
- Nagai K., Nishio T., Nakagawa H., Nakamura S., Fukuda Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978 Feb 24;142(2):384–389. doi: 10.1016/0006-8993(78)90648-0. [DOI] [PubMed] [Google Scholar]
- Niijima A., Rohner-Jeanrenaud F., Jeanrenaud B. Role of ventromedial hypothalamus on sympathetic efferents of brown adipose tissue. Am J Physiol. 1984 Oct;247(4 Pt 2):R650–R654. doi: 10.1152/ajpregu.1984.247.4.R650. [DOI] [PubMed] [Google Scholar]
- Nishio T., Shiosaka S., Nakagawa H., Sakumoto T., Satoh K. Circadian feeding rhythm after hypothalamic knife-cut isolating suprachiasmatic nucleus. Physiol Behav. 1979 Oct;23(4):763–769. doi: 10.1016/0031-9384(79)90172-0. [DOI] [PubMed] [Google Scholar]
- Oomura Y. Glucose as a regulator of neuronal activity. Adv Metab Disord. 1983;10:31–65. doi: 10.1016/b978-0-12-027310-2.50008-6. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ono T., Ooyama H., Wayner M. J. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969 Apr 19;222(5190):282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Bray G. A. Intrahypothalamic injection of insulin decreases firing rate of sympathetic nerves. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2012–2014. doi: 10.1073/pnas.84.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Bray G. A. The effect of intrahypothalamic injections of glucose on sympathetic efferent firing rate. Brain Res Bull. 1987 May;18(5):591–595. doi: 10.1016/0361-9230(87)90128-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Shimojo E. Inhibition of gastric motility induced by hepatic portal injections of D-glucose and its anomers. J Physiol. 1984 Jun;351:573–581. doi: 10.1113/jphysiol.1984.sp015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie T. B., Kissileff H. R. Physiology of energy intake: an inventory control model. Am J Clin Nutr. 1985 Nov;42(5 Suppl):914–923. doi: 10.1093/ajcn/42.5.914. [DOI] [PubMed] [Google Scholar]
- Vander Tuig J. G., Kerner J., Romsos D. R. Hypothalamic obesity, brown adipose tissue, and sympathoadrenal activity in rats. Am J Physiol. 1985 May;248(5 Pt 1):E607–E617. doi: 10.1152/ajpendo.1985.248.5.E607. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Bray G. A. Catecholamine turnover in rats with ventromedial hypothalamic lesions. Am J Physiol. 1984 Apr;246(4 Pt 2):R558–R565. doi: 10.1152/ajpregu.1984.246.4.R558. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Fisler J. S., Fukushima M., Bray G. A., Schemmel R. A. Diet, lighting, and food intake affect norepinephrine turnover in dietary obesity. Am J Physiol. 1987 Feb;252(2 Pt 2):R402–R408. doi: 10.1152/ajpregu.1987.252.2.R402. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Impaired suppression of sympathetic activity during fasting in the gold thioglucose-treated mouse. J Clin Invest. 1980 May;65(5):1086–1094. doi: 10.1172/JCI109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977 Jun 24;196(4297):1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]


