Imprints of stress response by rice seedlings in terms of expression levels of stress response gene HSP70 are characterised . The response to arsenic and/or heat shock are shown to be additive for the same stress or the combined stresses, indicating a commonality of signalling pathways.
Abstract
Background and aims
Plants can withstand many abiotic stresses. Stress adaptation through retention of imprints of previous stress exposure has also been described in plants. We have characterized the imprint or memory of adaptive stress responses of rice seedlings to arsenic (As) and heat stress.
Methodology
Two-week-old rice seedlings (both with and without As) were given a 45 °C heat shock for 3 h. While under heat shock, the leafy portion of the seedlings was harvested at regular intervals. Subsequently, the seedlings were kept at room temperature for recovery and sampling continued over 3 h. Total RNA and protein were extracted from the leafy portion of the seedlings and complementary DNA (cDNA) was prepared from total RNA. The cDNA was used as a template for the polymerase chain reaction to identify the transcription level of HSP70. Protein extracted from the seedlings was western-blotted. HSP70 and actin (loading control) antibodies were used to recognize the proteins on the same blot.
Principal results
Our studies reveal that HSP70, a cellular chaperone gene, is over-expressed at the mRNA and protein levels when rice seedlings are exposed to As and heat. The effect is cumulative and increases with the duration of stress for 3 h. During 3 h recovery from heat stress at ambient temperatures for 3 h, the chaperone remains expressed at higher levels in plants pre-exposed to As.
Conclusions
Our findings demonstrate a retention of the imprint of previous stress exposure, perhaps through sustained activation of the signalling pathways upstream of over-expression of HSP70. Furthermore, stress-induced HSP70 expression was additive/cumulative for continued exposure to similar or different kinds of stress, indicating that a commonality of signal transduction networks is adopted when plants experience more than one stress.
Introduction
Plants experience continuous exposure to various biotic and abiotic stresses in their natural environment (Fujita et al. 2006). Abiotic stresses are primary causes of crop loss worldwide (Vinocur and Altman 2005). Plants must inevitably cope with frequent changes in their environment for their survival and consequently have evolved a wide range of mechanisms to deal with a variety of stresses (Fujita et al. 2006). Abiotic stress alters the levels of numerous soluble or structural proteins (Qureshi et al. 2007) that are important for adaptation. Following the recognition of environmental signals by the plant, the information contained therein may be retained for some time in the form of changes in concentration of small molecules, proteins, DNA methylation or histone modification (Trewavas 2005; Galis et al. 2009; Stork et al. 2009). Retention of the imprint of the stress exposure involves cellular- and molecular-level activation of signal transduction cascades and expression of specific stress genes (Trewavas 2005; Vinocur and Altman 2005). Retaining this information or the imprint/memory of the stress can be local and of either short- or long-term duration (Galis et al. 2009).
Transitory or sustained high temperatures cause an array of changes that affect plant growth and development (Wahid et al. 2007). In general, a transient elevation in temperature of 10–15 °C above ambient is considered to be a heat shock or heat stress. However, heat stress is a complex function of intensity (temperature in degrees), duration and rate of increase in temperature. Plants pre-exposed to heat stress will survive when, later, they are exposed to a higher temperature that would be lethal to a non-acclimated plant (Hong et al. 2003). The major aspect of this acquired thermo-tolerance involves expression of stress-responsive genes that help to maintain cellular homoeostasis (Hahn and Li 1990). In this regard, expression of heat shock protein (HSP) genes is known to be an important adaptive strategy (Feder and Hofmann 1999). The HSP family of proteins, with a molecular mass ranging from ∼10 to 200 kDa, have chaperone-like functions. They are also involved in signal transduction during heat stress (Schöffl et al. 1999). Heat shock proteins are encoded by a family of highly conserved genes and function as molecular chaperones in protein biosynthesis to facilitate protein folding, assembly, secretion, regulation, degradation and translocation. The 70 kDa HSP (HSP70) is the most abundant and best characterized protein family. Expression of HSP may result from a variety of stressors, including high temperatures, exposure to heavy metals or amino acid analogues, eukaryotic parasites or viral infection (Lindquist and Craig 1988; Feder and Hofmann 1999; Wang et al. 2004). Most HSPs have strong cytoprotective effects, maintaining proteins in their functional conformations, preventing aggregation of non-native proteins, refolding denatured proteins to regain their functional conformation and removal of non-functional but potentially harmful polypeptides (arising from misfolding, denaturation or aggregation). Thereby, HSPs ensure the maintenance of homoeostasis, protect cells and facilitate the return to equilibrium during recovery (Timperio et al. 2008). The over-expression of HSP70 genes correlates positively with the acquisition of thermotolerance and results in enhanced tolerance to salt, water and high-temperature stress in plants (Wang et al. 2004).
Arsenic (As) is one of the most injurious of global environmental toxicants. High concentrations in groundwater have been reported from many countries (Huang et al. 1992; Nickson et al. 1998; Chowdhury et al. 1999; Chowdhury et al. 2000; Smith et al. 2000; Anawar et al. 2002). Even though As is a human carcinogen, groundwater used for irrigating crops, especially rice, can be contaminated by As. The predominant form of As in soil solution is arsenite and rice takes up arsenite more readily than other forms of As. Thus, in areas where As concentrations are high, rice plants experience considerable exposure to the toxicant.
In the present study, we characterize the short-term adaptation to a stress or ‘memory’ of a stress in molecular terms, of plants exposed to repeated stress of a similar kind (heat or As) or to two different kinds of stress (heat and As).
Materials and methods
Plant material
Rice seeds [Oryza sativa ‘Kshitish’ (IET4094)] obtained from the Chinsura Rice Research Centre, Hoogly, India, were surface sterilized in 0.05 % HgCl2 solution for 15 min, thoroughly washed and then soaked for ∼3 h in distilled water. Imbibed seeds were germinated at 28 °C for 3 days in the dark on moist blotting paper in Petri dishes. Subsequently, the sprouted seeds were grown at 30 °C for ∼2 weeks with 13 h of light and 11 h of darkness in each 24 h period. Control or untreated seedlings were grown with water in the same Petri dishes. Arsenic was applied to the sprouted seedlings in the Petri dishes as 0.09 mM sodium arsenate (Na2HAsO4·7H2O) for up to 2 weeks.
Two-week-old seedlings (both with and without As) were given a 45 °C heat shock for 3 h. While under heat shock, leafy portions of the seedlings were harvested every 15 min (four samples per hour), except for the second hour when three instead of four samples per hour were taken, to monitor the development of the expression of HSPs. Subsequently, the seedlings were kept at room temperature and the sampling (four samples per hour) continued at 15-min intervals (except for the second hour when three samples were taken) over 3 h to determine the rate at which the expression levels reverted to their normal basal values. The first sample collected during recovery (0 h recovery) was taken 5 min after cessation of the heat shock treatment. On completion of a treatment cycle, seedlings were kept overnight at 30 °C and the same series of treatments was then repeated to establish any changes in the expression pattern compared with the previous day's exposure to the same stress.
RNA and protein extraction
Total RNA and protein were extracted from the leafy portion of the seedlings using TRI reagent (AMBION, CA, USA) according to the manufacturer's protocol. Samples for RNA were quantified by absorbance at 260 nm (1 OD corresponds to 40 μg of RNA) and purity was assessed by the ratio of absorbance at 260 to that at 280 nm. Total protein estimations were made according to Lowry et al. (1951).
Preparation of complementary DNA from total RNA
Complementary DNA (cDNA) was prepared from total RNA following the standard protocol. Five micrograms of RNA and 100 ng of oligo (dT)18 primer were taken in a total volume of 11 μL, heated at 70 °C for 10 min, followed by 1 min chilling on ice. Nine microlitres of reaction mixture containing first-strand buffer, reverse transcriptase (100 U of Moloney murine leukemia virus reverse transcriptase), dithiothreitol and deoxynucleotide triphosphate (dNTP) were added and kept at room temperature for 15 min. This mixture was then incubated at 37 °C for 1 h, heated at 90 °C for 5 min and cooled at 4 °C for 10 min before storing at −20 °C prior to use as a template for polymerase chain reactions (PCRs) (Brown 2000).
Gene expression study by reverse transcriptase–polymerase chain reaction
The reverse transcriptase (RT) products (cDNA) were used as a template for PCR to identify the transcription level of HSP70 genes (GenBank Accession No. CAA47948). Rice actin genes (Rac1) (Gu et al. 2005) were used as a positive internal control. Two microlitres of cDNA from the RT reaction mixture were amplified in a 25 μL reaction volume containing 10 pmol of each primer, 200 μM dNTP, in buffer containing 1.5 mM MgCl2 and 5 U of Taq polymerase (Fermentas). The PCR products were visualized by running a 1 % agarose gel stained with ethidium bromide (EtBr). The stained gels were visualized under an ultraviolet trans-illuminator and photographed in GelDoc (Bio-Rad). The primer sequences are given in Table 1. For both the PCR programmes, the reaction mixtures were initially denatured at 95 °C for 5 min followed by 30 cycles of 95 °C (30 s), 55 °C (30 s) and 72 °C (1 min) and culminating in 10 min of extension at 72 °C.
Table 1.
Primer sequences
| Gene | Primer sequence |
|---|---|
| Rice actin (Rac1) | FWD 5′-GGAACTGGTATGGTCAAGGC-3′ |
| REV 5′-AGTCTCATGGATAACCGCAG-3′ | |
| Rice HSP70 | FWD 5′-TGTTGTTCTTGTTGGTGGCT-3′ |
| REV 5′-GACTCCACCTTCTTCTTGT-3′ |
FWD, forward primer; REV, reverse primer.
Protein immunoblotting
Protein estimations used 10 µg of protein separated by 12 % sodium dodecyl sulphate polyacrylamide gel electrophoresis. The protein marker used was unstained SM0438 from Fermentas. After electrophoresis, the gel was transferred to a polyvinylidene difluoride (PVDF) membrane at 300 mA current in a semi-wet transfer system for 90 min. The PVDF membrane was treated earlier with absolute methanol for 10 s and immediately washed with deionized water and soaked in transfer buffer. The membrane treated in this way was used to transfer proteins from the gel. To check proper transfer of the proteins, the membrane was drenched with methanol and stained with 1×PonceauS. After washing out the 1×PonceauS, the membrane was blocked with 3 % bovine serum albumin in TBST (25 mM Tris-Cl pH 7.5, 0.15 M NaCl pH 7.40; 0.05 % Tween-20) for 2 h at room temperature. Subsequently, the blot was cut between the markers 66 and 45 for the determination of HSP70 and actin values from the same blot. The two parts of the blot were incubated overnight with primary antibody at a specific dilution (as directed by the manufacturer) in TBST accompanied by constant shaking. The two primary antibodies used were mouse anti-HSP70 antibody (BD-Pharmingen) at 1:1000 dilution for observing HSP70 expression and mouse anti-actin antibody (Abcam) at 1:500 dilution for internal actin control. The upper part of the blot was treated with mouse anti-HSP70 antibody and the lower part was treated with mouse anti-actin antibody. The immune complex was detected by anti-mouse horseradish peroxidase (HRP)-conjugated (Cell Signalling Tech.) secondary antibody at 1:5000 dilution at room temperature with constant shaking of the membrane for 2 h. The membrane was then washed four times with TBST and developed using ECL western blotting detection reagents for HRP-conjugated secondary antibody.
Quantification of the western blot
Western blots were scanned by a UMAX Astra Scanner and the bands were quantified by using Image J software. The western images were transferred to the graph images by using Image J software. Band intensities were measured by using the software (both HSP70 and actin:protein and transcript data). The HSP70/actin value data were represented graphically using Microsoft Excel. Therefore, the graphs represent HSP70 values normalized to respective actin values.
Results
Changes in HSP70 protein levels during heat shock and recovery in plants receiving no As pre-treatments and in plants pre-treated with As
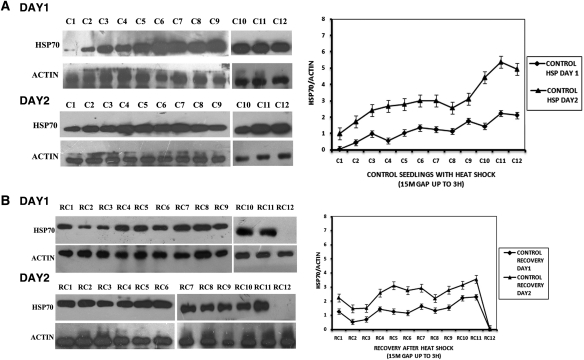
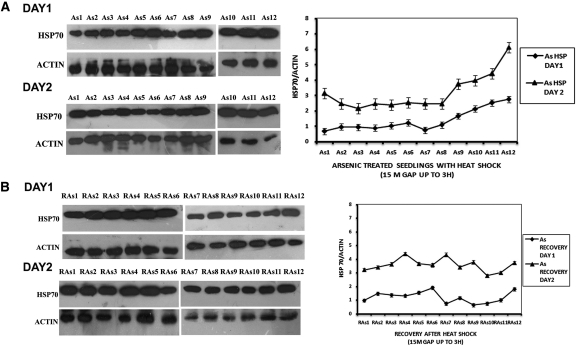
Figure 1 shows HSP70 protein levels in rice seedlings subjected only to heat shock (A) and recovery after withdrawal of heat shock (B). Levels of HSP70 protein were quantified every 15 min (four samples per hour) during the 3-h heat shock period, except for the second hour when three samples were taken. On the first day of heat exposure, HSP70 protein levels increased moderately for the first 2 h, but on prolongation of the heat shock to 3 h, HSP70 levels became noticeably higher. On Day 2, when the heat treatment was repeated, initial HSP70 levels were already markedly higher than the initial levels of Day 1 plants and the difference was maintained throughout the 3 h of heat shock (Fig. 1A). When heat shock was combined with As treatment (Fig. 2A), heat shock on Day 1 resulted in considerably higher HSP70 levels than in plants given heat shock alone. For the first 2 h of combined treatment, there was little additional change in the HSP70 levels. However, on prolongation of the heat shock period to 3 h, moderate increases in HSP70 levels were observed. Within the first 2.75 h of recovery at 30 °C (Fig. 1B), fluctuation in the HSP70 levels was observed but within limits similar to those of seedlings treated with 3 h of heat stress only. But, after ∼3 h of recovery time, HSP70 levels fell to pre-heat shock values. However, HSP70 reappeared at 3 h 15 min (data not shown), indicating fluctuations in HSP70 levels. Arsenic-treated plants presented a different picture (Fig. 2B). During recovery, plants with combined heat and As treatment maintained a higher level of HSP70 than plants given only heat shock. Moreover, during the last hour of recovery the decline in HSP70 was marked only in plants subjected to heat shock alone. In plants given both stresses, a very modest decline in HSP70 was seen.
Fig. 1.
Protein expression in control seedlings on two consecutive days. (A) HSP70 protein expression in control seedlings with heat shock. C1—at 0 h of heat shock; C2—after 15 min of heat shock; C3—after 30 min of heat shock; C4—after 45 min of heat shock; C5—after 1 h of heat shock; C6—after 1 h 15 min of heat shock; C7—after 1 h 45 min of heat shock; C8—after 2 h of heat shock; C9—after 2 h 15 min of heat shock; C10—after 2 h 30 min of heat shock; C11—after 2 h 45 min of heat shock; C12—after 3 h of heat shock. (B) HSP70 protein expression in control seedlings in recovery at ambient temperature for 3 h after withdrawal of heat shock. RC1—at 0 h after withdrawal of heat shock; RC2—at 15 min after withdrawal of heat shock; RC3—at 30 min after withdrawal of heat shock; RC4—at 45 min after withdrawal of heat shock; RC5—at 1 h after withdrawal of heat shock; RC6—at 1 h 15 min after withdrawal of heat shock; RC7—at 1 h 45 min after withdrawal of heat shock; RC8—at 2 h after withdrawal of heat shock; RC9—at 2 h 15 min after withdrawal of heat shock; RC10—at 2 h 30 min after withdrawal of heat shock; RC11—at 2 h 45 min after withdrawal of heat shock; RC12—at 3 h after withdrawal of heat shock. The graphs on the right represent data expressed as band intensities of HSP70 normalized to actin. The error bars are standard errors of the means of two measurements (n= 2) from band density measurements of the same blot. The data are representative of 2–3 experiments.
Fig. 2.
Protein expression in As-treated seedlings on two consecutive days. (A) HSP70 protein expression in As-treated seedlings with heat shock. As1—at 0 h of heat shock; As2—after 15 min of heat shock; As3—after 30 min of heat shock; As4—after 45 min of heat shock; As5—after 1 h of heat shock; As6—after 1 h 15 min of heat shock; As7—after 1 h 45 min of heat shock; As8—after 2 h of heat shock; As9—after 2 h 15 min of heat shock; As10—after 2 h 30 min of heat shock; As11—after 2 h 45 min of heat shock; As12—after 3 h of heat shock. (B) HSP70 protein expression in As-treated seedlings measured for 3 h in recovery at ambient temperature after 3 h of heat shock. RAs1—at 0 h after withdrawal of heat shock; RAs2—at 15 min after withdrawal of heat shock; RAs3—at 30 min after withdrawal of heat shock; RAs4—at 45 min after withdrawal of heat shock; RAs5—at 1 h after withdrawal of heat shock; RAs6—at 1 h 15 min after withdrawal of heat shock; RAs7—at 1 h 45 min after withdrawal of heat shock; RAs8—at 2 h after withdrawal of heat shock; RAs9—at 2 h 15 min after withdrawal of heat shock; RAs10—at 2 h 30 min after withdrawal of heat shock; RAs11—at 2 h 45 min after withdrawal of heat shock; RAs12—at 3 h after withdrawal of heat shock. The graphs on the right represent data expressed as band intensities of HSP70 normalized to actin. The error bars are standard errors of the means from band density measurements of two measurements (n= 2) of the same blot. The data are representative of 2–3 experiments.
Changes in HSP70 mRNA levels during heat shock and recovery in plants receiving no As pre-treatment and in plants pre-treated with As
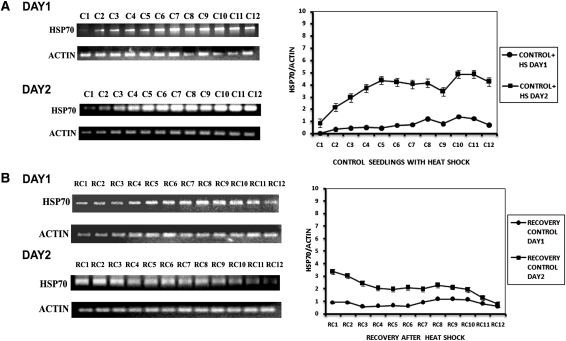
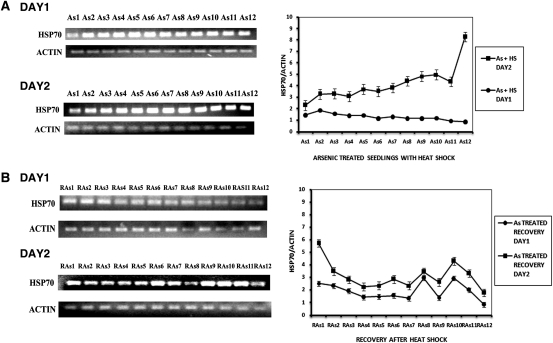
Figure 3A shows the mRNA expression levels of HSP70 in plants given heat shock but without As. Changes in the levels of mRNA expression were in keeping with the rise in protein levels. Day 1 heat shock produced a moderate increase in HSP70 mRNA, in contrast to Day 2 where the response was more robust. On recovery from heat shock, only heat-shocked plants maintained the modestly elevated HSP70 levels for ∼2 h and in the third hour showed a slow decline (Fig. 3B). On Day 2, the initial values for HSP70 mRNA were high but underwent a slow decline for the first 2 h and thereafter HSP70 mRNA levels became attenuated. The rise in HSP70 mRNA was moderate during Day 1 in As- and heat-shocked plants but climbed sharply on Day 2 of the dual stress treatment (Fig. 4A). The seedlings receiving dual treatment (As and heat shock) also showed a decline of HSP70 levels during recovery, but the HSP70 mRNA levels were distinctly higher than in seedlings receiving only heat shock (Fig. 4B).
Fig. 3.
HSP70 mRNA expression in control seedlings on two consecutive days. (A) HSP70 mRNA expression in control seedlings with heat shock. C1—at 0 h of heat shock; C2—after 15 min of heat shock; C3—after 30 min of heat shock; C4—after 45 min of heat shock; C5—after 1 h of heat shock; C6—after 1 h 15 min of heat shock; C7—after 1 h 45 min of heat shock; C8—after 2 h of heat shock; C9—after 2 h 15 min of heat shock; C10—after 2 h 30 min of heat shock; C11—after 2 h 45 min of heat shock; C12—after 3 h of heat shock. (B) HSP70 mRNA expression in control seedlings in recovery at ambient temperature for 3 h after withdrawal of heat shock. RC1—at 0 h after withdrawal of heat shock; RC2—at 15 min after withdrawal of heat shock; RC3—at 30 min after withdrawal of heat shock; RC4—at 45 min after withdrawal of heat shock; RC5—at 1 h after withdrawal of heat shock; RC6—at 1 h 15 min after withdrawal of heat shock; RC7—at 1 h 45 min after withdrawal of heat shock; RC8—at 2 h after withdrawal of heat shock; RC9—at 2 h 15 min after withdrawal of heat shock; RC10—at 2 h 30 min after withdrawal of heat shock; RC11—at 2 h 45 min after withdrawal of heat shock; RC12—at 3 h after withdrawal of heat shock. The graphs on the right represent data expressed as band intensities of HSP70 normalized to actin. The error bars are standard errors of the means of two measurements (n= 2) from band density measurements of the same gel. The data are representative of 2–3 experiments.
Fig. 4.
HSP70 mRNA expression in As-treated seedlings on two consecutive days. (A) HSP70 mRNA expression in As-treated seedlings with heat shock. As1—at 0 h of heat shock; As2—after 15 min of heat shock; As3—after 30 min of heat shock; As4—after 45 min of heat shock; As5—after 1 h of heat shock; As6—after 1 h 15 min of heat shock; As7—after 1 h 45 min of heat shock; As8—after 2 h of heat shock; As9—after 2 h 15 min of heat shock; As10—after 2 h 30 min of heat shock; As11—after 2 h 45 min of heat shock; As12—after 3 h of heat shock. (B) HSP70 mRNA expression in As-treated seedlings in recovery for 3 h at ambient temperature after 3 h of heat shock. RAs1—at 0 h after withdrawal of heat shock; RAs2—at 15 min after withdrawal of heat shock; RAs3—at 30 min after withdrawal of heat shock; RAs4—at 45 min after withdrawal of heat shock; RAs5—at 1 h after withdrawal of heat shock; RAs6—at 1 h 15 min after withdrawal of heat shock; RAs7—at 1 h 45 min after withdrawal of heat shock; RAs8—at 2 h after withdrawal of heat shock; RAs9—at 2 h 15 min after withdrawal of heat shock; RAs10—at 2 h 30 min after withdrawal of heat shock; RAs11—at 2 h 45 min after withdrawal of heat shock; RAs12—at 3 h after withdrawal of heat shock. The graphs on the right represent data expressed as band intensities of HSP70 normalized to actin. The error bars are standard errors of the means of two measurements (n= 2) from band density measurements of the same gel. The data are representative of 2–3 experiments.
Discussion
There are several organizational and temporal levels in the adaptation of the plant to an incoming environmental signal. Initially, the response could be in terms of reversible modification of ion fluxes and signal transduction networks, followed by changes in gene expression. In the very long term, stress may lead to the selection of genetic mutations that give rise to phenotypic changes of adaptive value. These changes may be calibrated by the sustained strength and continued presence of the environmental signal (Trewavas 2003, 2005; Galis et al. 2009).
Plants can withstand various forms of abiotic stresses, and this property is extremely important for crop health and yield. The key element in suppressing the damage plants sustain under stress is the ability to retain an imprint or memory of previous stressful conditions through the modulation of gene expression. The present work contributes to a better understanding of this mechanism underlying short-term adaptation by plants undergoing sub-lethal stresses. Our study involved the interaction of two different forms of stress since field crops may often encounter more than one form of stress. This revealed an element of synergistic cross-tolerance between the effects of As and heat stress on rice seedlings. This is relevant to practical farming because contamination of groundwater and soil is a common occurrence in West Bengal, India, where rice, the chief staple crop, is subject to summer temperatures of 43–45 °C and to As in the soil.
Our study clarifies the extent to which one sub-lethal stress may render the plant better able to cope with a subsequent stress that is either the same as, or different from, the first. Our measure was the expression of the survival gene HSP70. The findings demonstrated that on Day 2 of heat exposure, the initial values of the HSP protein (Figs 1A and 2A) and its mRNA levels (Figs 3A and 4A) were higher than the initial values on Day 1. This indicates that the imprint of pre-exposure would have rendered the plants better able to withstand subsequent stress by over-expressing HSP70. Secondly, and perhaps more importantly, we determined the stability of the response/imprints and whether or not these imprints are cumulative. We found that during heat shock, a time-dependent elevation in HSP70 took place (Figs 1A and 2A), indicating the cumulative nature of the stress imprint. Also, the stress stimulus became saturated after imposing heat stress for 3 h on the second day (Fig. 1A), showing an attenuation of the rise in HSP70 levels in the presence of a continuing stimulus. These observations reinforce the concept of the additive/cumulative nature of the stress imprint. During recovery from heat stress, the higher HSP70 levels persisted for 2 h and declined thereafter (Figs 1B and 3B). After 3 h of recovery, HSP70 was almost undetectable. However, after a further 15 min HSP70 reappeared (data not shown). Temporal aspects of HSP70 levels after withdrawal of shock have not been studied in detail before the present work, although fluctuations in HSP levels have been observed in response to sub-lethal stress (Tomanek and Sanford 2003). The above-mentioned aspects of plant stress response, such as saturation of the stimulus and persistence of the stress effect, may be viewed as the full engagement of the signalling network (saturation and persistence) and the slower switching off of the same signalling elements when further stimulus fails to arrive. The two different stresses (As and heat) were shown to synergize, keeping the HSP70 levels higher and more stable during recovery compared with heat alone (Figs 2B and 4B). On the other hand, on Day 1 of heat shock of As-exposed plants, HSP70 failed to increase again, indicating saturation of the stimulus through additive/synergistic effects of the combined stresses. Different forms of stress (other than heat stress) are known to induce HSP70 in plants (Sugino et al. 1999; Alvim et al. 2001); however, synergism between two diverse forms of stress in inducing HSP70 in plants is novel. It is likely that the signalling network downstream of As and heat stress, and upstream of HSP70 over-expression has elements in common.
Conclusions and forward look
We find that in stressed rice seedlings, HSP70, a cellular chaperone gene, is over-expressed at the mRNA and protein levels in response to As and heat shock. The effect is accumulative over time and thus dependent upon the duration of the stress. Pre-exposure of seedlings to As strongly enhanced HSP70 protein levels in seedlings heat shocked for up to 3 h at 45 °C. During 3 h recovery, the expression of HSP70 was higher in plants pre-exposed to As. This indicates that synergistic cross-tolerance operates between the responses to these stresses.
Intra-cellular imprints of stress are revealed by over-expression of a stress gene that is manifest as a time-related accumulation of gene product during stress exposure. Pre-exposure to one stress is seen as creating the ability to respond optimally to a subsequent stress. Depending on factors such as exposure time and strength of the stress, the response can be additive for the same stress or a different stress, indicating commonality of plant stress signalling pathways. Future work could usefully examine the upstream signalling networks operative in the cross-tolerance of heat and As stress.
Sources of funding
Financial support was from the Centre for Applied Mathematics and Computational Science, Saha Institute of Nuclear Physics, India.
Contributions by the authors
R.B. and S.R. conceived and designed the experiments. A.G. performed the experiments. A.G. and S.R. analysed the data. A.G. and S.R. prepared the manuscript.
Conflicts of interest statement
None declared.
Acknowledgements
We thank Professor Bikas K. Chakrabarti, Centre for Applied Mathematics and Computational Science, Saha Institute of Nuclear Physics, Kolkata, India, for generous encouragement and useful discussions. We also acknowledge Dr Bijan Adhikari from the Chinsura Rice Research Centre, Hoogly, India, for his cooperation and for providing rice seeds.
References
- Alvim FC, Carolino SMB, Cascardo JCM, Nunes CC, Martinez CA, Otoni WC, Fontes EPB. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiology. 2001;126:1042–1054. doi: 10.1104/pp.126.3.1042. doi:10.1104/pp.126.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anawar HM, Akai J, Mostofa KMG, Safiullah S, Tareq SM. Arsenic poisoning in groundwater: health risk and geochemical sources in Bangladesh. Environment International. 2002;27:597–604. doi: 10.1016/s0160-4120(01)00116-7. doi:10.1016/S0160-4120(01)00116-7. [DOI] [PubMed] [Google Scholar]
- Brown TA. Essential molecular biology: a practical approach. 2nd edn. Vol. 1. New York, USA: Oxford University Press; 2000. [Google Scholar]
- Chowdhury TR, Basu GK, Mandal BK, Biswas BK, Samanta G, Chowdhury UK, Chanda CR, Lodh D, Roy SR, Saha KC, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. Arsenic poisoning in the Ganges delta. Nature. 1999;401:545–546. doi: 10.1038/44056. doi: [DOI] [PubMed] [Google Scholar]
- Chowdhury UK, Biswas BK, Roy Chowdhury T, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environmental Health Perspectives. 2000;108:393–397. doi: 10.1289/ehp.00108393. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. doi:10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. doi:10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Galis I, Gaquerel E, Pandey SP, Baldwin IT. Molecular mechanisms underlying plant memory in JA-mediated defense responses. Plant, Cell & Environment. 2009;32:617–627. doi: 10.1111/j.1365-3040.2008.01862.x. doi:10.1111/j.1365-3040.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Gu Z, Wang J, Huang J, Zhang H. Cloning and characterization of a novel rice gene family encoding putative dual-specificity protein kinases, involved in plant responses to abiotic and biotic stresses. Plant Science. 2005;169:470–477. doi:10.1016/j.plantsci.2005.03.005. [Google Scholar]
- Hahn GM, Li GC. Thermotolerance, thermoresistance and thermosensitization. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. Stress proteins in biology and medicine. Berlin, Germany: Cold Spring Harbor Press; 1990. pp. 79–100. [Google Scholar]
- Hong SW, Lee U, Vierling E. Arabidopsis hot mutants define multiple function required for acclimation to high temperatures. Plant Physiology. 2003;132:757–767. doi: 10.1104/pp.102.017145. doi:10.1104/pp.102.017145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Qian XC, Wang GQ, Gu YL, Wang SZ, Cheng ZH, Xiao BY, Gang JM, Wu JY, Kan MY. Syndrome of endemic arsenism and fluorosis: a clinical study. Chinese Medical Journal. 1992;105:586–590. doi: [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annual Review of Genetics. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. doi:10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagents. Journal of Biological Chemistry. 1951;193:265–275. doi: [PubMed] [Google Scholar]
- Nickson R, McArthur J, Burgess W, Ahmed KM, Ravencroft P, Rahman M. Arsenic poisoning in Bangladesh groundwater. Nature. 1998;395:338. doi: 10.1038/26387. doi:10.1038/26387. [DOI] [PubMed] [Google Scholar]
- Qureshi MI, Qadir S, Zolla L. Proteomics-based dissection of stress responsive pathway in plants. Journal of Plant Physiology. 2007;164:1239–1260. doi: 10.1016/j.jplph.2007.01.013. doi:10.1016/j.jplph.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Schöffl F, Prandl R, Reindl A. Molecular responses to heat stress. In: Shinozaki K, Yamaguchi Shinozaki K, editors. Molecular responses to cold, drought, heat and salt stress in higher plants. Austin, TX: R. G. Landes & Co.; 1999. pp. 81–98. [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bulletin of the World Health Organization. 2000;78:1093–1103. doi: [PMC free article] [PubMed] [Google Scholar]
- Stork W, Diezel C, Halitschke R, Gális I, Baldwin IT. An ecological analysis of the herbivory-elicited JA burst and its metabolism: plant memory processes and predictions of the moving target model. PLoS ONE. 2009;4:e4697. doi: 10.1371/journal.pone.0004697. doi:10.1371/journal.pone.0004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino M, Hibino T, Tanaka Y, Nii N, Takabe T. Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytice acquires resistance to salt stress in transgenic tobacco plants. Plant Science. 1999;146:81–88. doi:10.1016/S0168-9452(99)00086-2. [Google Scholar]
- Timperio AM, Egidi MG, Zolla L. Proteomics applied on plant abiotic stresses: role of plant heat shock proteins (HSP) Journal of Proteomics. 2008;71:391–411. doi: 10.1016/j.jprot.2008.07.005. doi:10.1016/j.jprot.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat-Shock Protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (Genus: Tegula) Biological Bulletin. 2003;205:276–284. doi: 10.2307/1543291. doi:10.2307/1543291. [DOI] [PubMed] [Google Scholar]
- Trewavas A. Aspects of plant intelligence. Annals of Botany. 2003;92:1–20. doi: 10.1093/aob/mcg101. doi:10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Plant intelligence. Naturwissenschaften. 2005;92:401–413. doi: 10.1007/s00114-005-0014-9. doi:10.1007/s00114-005-0014-9. [DOI] [PubMed] [Google Scholar]
- Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Current Opinion in Biotechnology. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. doi:10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environmental and Experimental Botany. 2007;61:199–223. doi:10.1016/j.envexpbot.2007.05.011. [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends in Plant Science. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. doi:10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]