Abstract
Background
Metabolic risk varies within adult body mass index (BMI) categories; however, the development of BMI-specific metabolic risk from childhood is unknown.
Methods
The sample included 895 adults (20–38 years of age; 43% male, 34% black) from the Bogalusa Heart Study (1995–2002), who had been measured as children (5–18 years of age) in 1981–1982. Adult metabolic risk was assessed using two definitions: Cardiometabolic risk factor clustering (RFC) included two or more abnormal risk factors [blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides, and fasting glucose] and insulin resistance (IR), comprising the top quartile of the homeostasis model of insulin resistance (HOMA-IR) distribution. Logistic regression, within BMI categories, was used to predict adult metabolic risk from childhood mean arterial pressure (MAP), HDL-C, low-density lipoprotein cholesterol (LDL-C), glucose, and triglycerides. Covariates included childhood age, race, sex, adult BMI, and length of follow-up.
Results
The prevalence of the adult abnormal metabolic risk profile varied by definitions of metabolic risk (normal weight, 5%–9%; overweight, 15%–23%; and obese, 40%–53%). The adult abnormal profile was associated with higher childhood LDL-C [IR, odds ratio (OR), 1.95; 95% confidence interval (CI), 1.06–3.58) and insulin (IR, OR, 1.69; CI, 1.10–2.58) in normal-weight adults; lower childhood HDL-C in overweight adults (RFC, OR, 0.61; CI, 0.40–0.94); and higher childhood MAP (RFC, OR, 1.75; CI, 1.24–2.47) and glucose (IR, OR,1.38; CI, 1.06–1.81) in obese adults.
Conclusions
Some childhood metabolic risk factors are moderately associated with adult BMI-specific metabolic risk profiles. The ability to identify children with high future adult cardiovascular risk may initiate early treatment options.
Introduction
It is well established that obese adults are more likely to develop adverse health conditions than normal-weight individuals. However, there is a subset of obese individuals that have a normal metabolic profile. In contrast, normal-weight individuals with abnormal metabolic profiles have also been identified. There is increasing interest in describing these phenotypes, in determining the time course for their development, and understanding the long-term health risks.
The subset of normal-weight adults with abnormal risk factors is often differentiated from healthy normal-weight individuals by the presence of abdominal obesity and elevated cholesterol, triglycerides, and blood pressure.1,2 Normal-weight males with greater adiposity (fat mass) have a higher prevalence of cardiovascular disease risk factors,3 and normal-weight individuals with an abnormal metabolic risk profile have three times the risk of cardiovascular disease and four times the risk of type 2 diabetes than their healthy normal-weight counterparts.4 Conversely, obese adults with a “healthy” metabolic phenotype tend to have favorable lipid profiles,5 normal insulin sensitivity,6 lower visceral fat depots,7 lower ectopic liver and skeletal muscle fat,8 a slower progression of atherosclerosis as measured by intima media thickness,8 and do not demonstrate an increased risk for either cardiovascular disease or type 2 diabetes when compared to normal-weight healthy individuals.4 These results suggest that there is a complex interaction between body weight status, cardiovascular risk factors, metabolic profile, and an individual's risk for chronic disease.
Recently, the prevalence of body mass index–(BMI) specific metabolic risk profiles was published using a representative sample of U.S. adults, demonstrating that 23.5% of normal-weight adults had an abnormal metabolic risk profile and 31.7% of obese individuals were metabolically healthy.9 These results suggest that these phenotypes are relatively common in the general population.
Although different levels of metabolic risk within BMI categories have been identified, little is known about how these BMI-specific metabolic risk profiles develop and progress from childhood into adulthood. Previous research has established that individual cardiovascular disease risk factor levels are moderately stable from childhood to adulthood.10,11 Clustering of multiple abnormal cardiovascular risk factors can occur during childhood12 and may track from childhood to adulthood.12–14 Thus, the ability to associate specific childhood metabolic risk factors with abnormal BMI-specific metabolic risk profiles in adults may provide an opportunity for early intervention efforts to reduce progression of chronic disease from childhood into adulthood.
A challenge in the existing literature is lack of a standardized definition to describe this interaction between BMI and metabolic risk. Currently, two definitions are employed throughout the research: (1) cardiometabolic risk factor clustering9 and (2) insulin resistance (IR),6–8 or a combination of the two.4,5 Meigs and colleagues compared two BMI-specific metabolic risk definitions in the association of adult BMI-specific risk for the development of type 2 diabetes and cardiovascular disease.4 Although no differences were found between these definitions,4 no other studies have compared definitions within the same sample to examine development from childhood.
The purpose of this study was to (1) determine whether childhood metabolic risk factors are associated with abnormal adult BMI-specific metabolic risk profiles, and (2) compare the associations utilizing two different adult metabolic risk definitions (cardiometabolic risk factor clustering and IR).
Materials and Methods
Study population
The Bogalusa Heart Study is a community-based investigation of the natural history of cardiovascular disease from childhood into adulthood. Eligible participants were drawn from children and adolescents living in Ward 4 of Washington Parish in South East Louisiana, a biracial community (65% white, 35% black) of approximately 20,000 people. Several cross-sectional surveys have been conducted between 1973 and 2002, and many individuals have participated in multiple surveys. The present study sample includes adults who had been measured in the 1995–1996, 1998–2001, or 2001–2002 surveys. Participants met the following criteria for inclusion into this analysis: (1) Previously measured as a child in 1981–1982, (2) no missing data for the metabolic variables of interest at either time points, (3) childhood age 5–17.9 years, 4) adult age ≥18 years. Participants with nonfasting blood samples were removed (n = 86) as were pregnant females (n = 33). If multiple screenings were found for adults, the most recent data were used to maximize follow-up time between childhood and adulthood. The final sample included 895 individuals: 281 adults (20–32 years) were measured in 1995–96, 129 adults (22–36 years) were measured in 1998–2001, and 485 adults (26–38 years) were measured in 2001–2002.
General examination
Height and weight were measured in duplicate to the nearest 0.1 cm and 0.1 kg, respectively. BMI was calculated as the mean weight in kilograms divided by the mean height in meters squared (kg/m2). Waist circumference was measured midway between the lowest rib and the superior border of the iliac crest, with a flexible tape in triplicate and averaged. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) (4th Korotkoff heart sound) were measured on the right arm using mercury sphygmomanometers as the average of six readings from two randomly assigned nurses. Race was self-assessed by participants.
Laboratory analyses
All blood samples were collected after a 12-h fast. Prior to the 1988–1991 survey, blood lipids were measured by the Lipid Research Clinic protocol on an Auto Analyzer II instrument (Technicon Instrument Corp, Tarrytown, NY).15 After 1988–1991, the instrumentation for lipid assessment changed to a VP instrument (Abbott Laboratories, North Chicago, IL) using enzymatic procedures16,17 as required by the Centers for Disease Control and Prevention (Atlanta, GA). All measures were conducted within the Core Lipid Laboratory in New Orleans, Louisiana. Serum triglyceride concentrations were measured with enzymatic procedures, and serum low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured using a heparin–calcium precipitation in combination with an agar–agarose gel electrophoresis.18 From 1978 to 1991, plasma glucose was measured using the glucose oxidase method on a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA) and then using an enzymatic procedure. A commercial radioimmunoassay kit measured plasma immunoreactive insulin levels (Phadebas, Pharmacia Diagnostics, Piscataway, NJ). For quality control, an additional independent blind duplicate sample was collected from a 10% subsample; intraclass correlation coefficients ranged from 0.97 to 0.99 for triglycerides, 0.86 to 0.98 for LDL-C, 0.92 to 0.98 for HDL-C, 0.86 to 0.98 for glucose, and 0.94 to 0.98 for insulin.
Adults were classified as normal weight (BMI <25 kg/m2), overweight (BMI 25.0–29.9), and obese (BMI ≥30 kg/m2) following the recommendations of the World Health Organization (WHO).19 Stratification for high cardiovascular risk was assessed using two definitions: (1) cardiometabolic risk factor clustering and (2) IR. For the cardiometabolic risk factor clustering definition, elevated triglycerides (≥150 mg/dL), high blood pressure (SBP ≥130, or DBP ≥85, or on hypertension medication), elevated fasting glucose (≥100 mg/dL, or on antidiabetic medication), and low HDL-C (men <40 mg/dL; women <50 mg/dL, or on lipid-lowering medication), were identified with the American Health Association/National Heart, Lung and Blood Institute (AHA/NHLBI) guidelines for abnormal cardiometabolic risk factors.20 Adult waist circumference was not used in the definition of risk due to multi–colinearity with adult BMI. Metabolically normal (normal) was defined as having 0 or 1 abnormal risk factors, and metabolically abnormal (abnormal) was defined as having 2 or more abnormal risk factors. For the insulin resistance definition of metabolic risk, the homeostasis model for IR (HOMA-IR) was calculated by {[fasting glucose (mmol/L) · fasting insulin (μU/mL)]/22.5}.21 In the insulin resistance definition, “abnormal” was defined as a HOMA-IR value equal or greater than the lower limit of the top quartile of HOMA-IR distribution values in sample, as suggested by WHO,22 or any individual on antidiabetic medication (top quartile for HOMA-IR ≥3.2).
Statistical analyses
Within each adult BMI category, age-, race-, and sex-adjusted child and adult means for the individual metabolic risk factors were examined between persons classified as abnormal and normal. Similarly, individuals were stratified into BMI categories (normal weight, overweight, and obese) for logistic regression analyses to predict adult metabolic risk (abnormal versus normal). The logistic regressions modeled the risk of abnormal metabolic risk (either cardiometabolic risk factor clustering or insulin resistance) as an adult from childhood measurements of triglycerides, HDL-C, LDL-C, mean arterial blood pressure (MAP) [1/3(SBP − DBP) + DBP], fasting glucose, and insulin. MAP was chosen to include both SBP and DBP into a single variable to represent blood pressure. In all models, childhood BMI, childhood age, race, sex, adult BMI, and length of follow-up were included as covariates. Childhood BMI was added into the model as a covariate to model the effects of the childhood risk factors independent of the effects of childhood BMI.
Two statistical approaches were used to examine predictors of BMI-specific metabolic risk profiles for both definitions of metabolic risk (cardiometabolic risk factor clustering and IR): (1) single risk factor models, in which each childhood metabolic risk factor was examined individually with covariates; (2) multiple risk factor model, in which all childhood metabolic risk factors were entered into the model with covariates, to permit simultaneous adjustment to determine the independent contribution of each predictor. Last, effect sizes in the models were given in standard deviation units to facilitate comparisons among predictors.
Percent agreement between cardiometabolic clustering and IR definitions was calculated from a cross tabulation. (To convert metabolic risk factors to SI units, multiply by 0.0259 for LDL-C, 0.0259 for HDL-C, by 0.0113 for triglycerides, by 0.0555 for glucose, and by 6.945 for insulin.)
Results
The mean age was 11.8 ± 3.4 years in childhood and 29.5 ± 4.8 years in adulthood, with a mean follow-up of 17.8 ± 2.6 years. Approximately 43% of the sample was male and 34% was African American. Table 1 presents these demographic characteristics within the adult BMI categories. Approximately 37% of adults were normal weight, 26% were overweight, and 36% were obese. Table 2 presents the adjusted childhood and adulthood individual metabolic risk factors by BMI category. The individual metabolic risk factors generally increased from the adult normal-weight category through the adult obese category for both the childhood and adulthood physiological values, with the exception of HDL-C, which decreased as expected.
Table 1.
Childhood and Adulthood Demographics by Adult BMI Category
| Normal weight | Overweight | Obese | |
|---|---|---|---|
| N (%) | 333 (37) | 236 (26) | 326 (36) |
| Sex | |||
| Male, n (%) | 118 (35) | 138 (58) | 125 (38) |
| Female, n (%) | 215 (65) | 98 (42) | 201 (62) |
| Race | |||
| White, n (%) | 243 (73) | 154 (65) | 193 (59) |
| Black, n (%) | 90 (27) | 82 (35) | 133 (41) |
| Age (years) | |||
| Childhood | 11.5 ± 3.4 | 12.1 ± 3.3 | 11.8 ± 3.6 |
| Adulthood | 28.8 ± 4.9 | 30.0 ± 4.4 | 29.9 ± 4.9 |
| Length of follow-up (years) | 17.2 ± 2.7 | 17.9 ± 2.6 | 18.2 ± 2.5 |
Table 2.
AdjustedaChildhood and Adulthood Metabolic Risk Factors by Adult BMI Category
| Normal weight | Overweight | Obese | |
|---|---|---|---|
| BMI (kg/m2) | |||
| Childhood | 17.1 ± 0.2 | 18.3 ± 0.2 | 21.7 ± 0.2 |
| Adulthood | 22.1 ± 0.2 | 27.4 ± 0.2 | 36.4 ± 0.2 |
| Waist (cm) | |||
| Childhood | NA | NA | NA |
| Adulthood | 75.9 ± 0.6 | 87.7 ± 0.7 | 108.1 ± 0.6 |
| TGs (mg/dL) | |||
| Childhood | 58.0 ± 1.7 | 62.6 ± 2.0 | 70.4 ± 1.7 |
| Adulthood | 84.1 ± 5.2 | 111.9 ± 5.9 | 139.6 ± 5.1 |
| LDL-C (mg/dL) | |||
| Childhood | 92.0 ± 1.5 | 92.5 ± 1.7 | 96.3 ± 1.4 |
| Adulthood | 109.6 ± 1.9 | 119.6 ± 2.1 | 128.8 ± 1.8 |
| HDL-C (mg/dL) | |||
| Childhood | 63.5 ± 1.1 | 61.6 ± 1.3 | 58.1 ± 1.1 |
| Adulthood | 52.8 ± 0.7 | 48.5 ± 0.8 | 43.6 ± 0.6 |
| MAP (mmHg) | |||
| Childhood | 76.1 ± 0.4 | 77.0 ± 0.5 | 79.3 ± 0.4 |
| Adulthood | 85.9 ± 0.5 | 87.2 ± 0.6 | 93.7 ± 0.5 |
| SBP (mmHg) | |||
| Childhood | 102.4 ± 0.5 | 104.1 ± 0.6 | 107.2 ± 0.5 |
| Adulthood | 110.8 ± 0.6 | 112.5 ± 0.7 | 120.2 ± 0.7 |
| DBP (mmHg) | |||
| Childhood | 63.0 ± 0.4 | 63.6 ± 0.5 | 65.4 ± 0.4 |
| Adulthood | 73.4 ± 0.5 | 74.6 ± 0.6 | 80.5 ± 0.5 |
| Glucose (mg/dL) | |||
| Childhood | 80.3 ± 0.4 | 79.5 ± 0.5 | 80.9 ± 0.4 |
| Adulthood | 80.5 ± 1.3 | 82.7 ± 1.4 | 89.9 ± 1.2 |
| Insulin (μU/mL) | |||
| Childhood | 9.9 ± 0.5 | 10.8 ± 0.5 | 13.3 ± 0.5 |
| Adulthood | 7.8 ± 0.6 | 11.1 ± 0.7 | 19.0 ± 0.6 |
| HOMA-IR | |||
| Childhood | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.7 ± 0.1 |
| Adulthood | 1.6 ± 0.1 | 2.3 ± 0.2 | 4.2 ± 0.1 |
aMeans ± standard error adjusted for sex, race, and age.
For SI conversion factors, multiply by 0.0259 for HDL-C, by 0.0259 for LDL-C, by 0.0113 for triglycerides, by 0.0555 for glucose, and by 6.945 for insulin.
Abbreviations: BMI, body mass index; NA, not available; TGs, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MAP, mean arterial blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; HOMA-IR, homeostasis model for insulin resistance.
BMI-specific metabolic risk
The cardiometabolic risk factor clustering definition resulted in an abnormal risk profile in approximately 9% of normal-weight individuals, 23% of overweight individuals, and 40% of obese individuals (Fig. 1A), whereas the IR definition resulted in approximately 5% of the normal weight, 15% of overweight, and 54% of obese groups classified as abnormal (Fig. 1B). The percent agreement between the cardiometabolic risk factor clustering definition and the IR definition was 47% in normal weight, 47% in overweight, and 51% in obese adults. In other words, between 47% and 51% of the participants classified as abnormal by cardiometabolic risk factor clustering were also classified as abnormal using IR as the criterion.
FIG. 1.
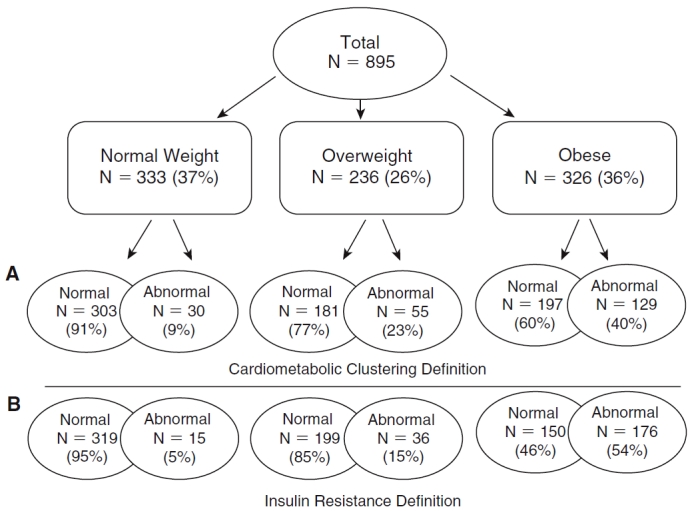
(A) Prevalence of BMI-specific metabolic profiles among Bogalusa Heart Study participants using the cardiometabolic risk factor clustering definition (normal = 0 or 1 irregular risk factor; abnormal = 2 or more irregular risk factors). (B) Prevalence of adult BMI-specific metabolic profiles among Bogalusa Heart Study participants using the insulin resistance definition (abnormal = top 25% of HOMA-IR distribution). Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance.
Cardiometabolic risk factor clustering definition
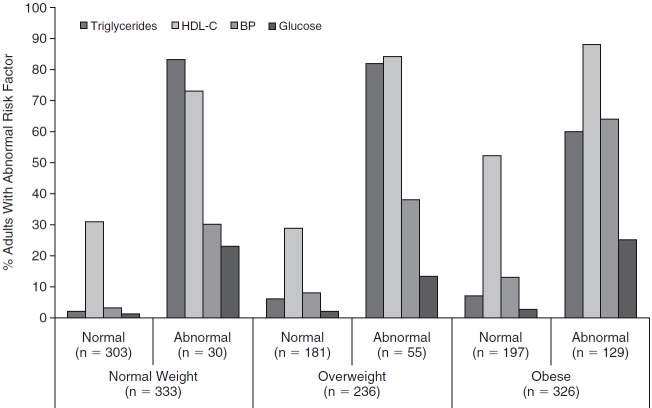
The most common abnormal metabolic risk factors among all adults were elevated triglycerides for normal-weight adults and low HDL-C for both overweight and obese adults (Fig. 2). Among normal-weight adults, no childhood metabolic risk factors were associated with having an abnormal metabolic risk profile in either the single risk factor or the multiple risk factor models (Table 3). Among overweight adults, higher levels of childhood LDL-C [single risk factor model: odds ratio (OR) per standard deviation (SD), 1.49; 95% confidence interval (CI), 1.07–2.07] and lower levels of HDL-C (single risk factor model, OR per SD, 0.58; 95% CI, 0.41–0.84; multiple risk factor model, OR per SD, 0.61; 95% CI, 0.40–0.94) were associated with having an abnormal adult metabolic risk profile (Table 3). Therefore, among overweight adults, for each 1 SD higher childhood LDL-C or lower HDL-C, there is a 49% increase and 39%–42% increase, respectively, in the odds of becoming an abnormal overweight adult. In obese adults, the abnormal metabolic risk profile was associated with having higher childhood LDL (single risk factor model, OR per SD, 1.34; 95% CI, 1.02–1.76), lower HDL (single risk factor model, OR per SD, 0.75; 95% CI, 0.57–0.99), higher childhood MAP (single risk factor model, OR per SD, 1.74; 95% CI, 1.25–2.42; multiple risk factor models, OR per SD, 1.75; 95% CI, 1.24–2.47), and higher childhood insulin (single risk factor model, OR per SD, 1.33; 95% CI, 1.01–1.74) (Table 3). Thus, in the multiple risk factor model, among obese adults, a 1 SD higher childhood MAP was associated with a 75% higher odds of having an abnormal metabolic risk profile.
FIG. 2.
Percentage of adults within abnormal or normal BMI-specific metabolic profiles with abnormal metabolic risk factors. Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; BP, blood pressure.
Table 3.
Results of Logistic Regression Predicting Abnormal Adult BMI-Specific Metabolic Profiles From Childhood Metabolic Measures Using the Cardiometabolic Risk Factor Clustering Definition
| |
Single risk factor modela |
Multiple risk factor modelb |
||
|---|---|---|---|---|
| Childhood measure | OR per SD3 (CI) | P value | OR per SD3 (CI) | P value |
| Normal weightc | ||||
| Triglycerides (mg/dL) | 1.17 (0.78–1.77) | 0.45 | 0.89 (0.53–1.49) | 0.65 |
| LDL-C (mg/dL) | 1.26 (0.86–1.85) | 0.23 | 1.24 (0.80–1.91) | 0.33 |
| HDL-C (mg/dL) | 0.72 (0.48–1.08) | 0.11 | 0.77 (0.48–1.23) | 0.27 |
| MAP (mmHg) | 0.97 (0.58–1.61) | 0.89 | 0.99 (0.58–1.69) | 0.96 |
| Glucose (mg/dL) | 0.82 (0.54–1.25) | 0.36 | 0.82 (0.53–1.26) | 0.36 |
| Insulin (μU/mL) | 1.30 (0.94–1.79) | 0.11 | 1.35 (0.97–1.89) | 0.08 |
| Overweightc | ||||
| Triglycerides (mg/dL) | 1.26 (0.91–1.74) | 0.17 | 0.95 (0.60–1.50) | 0.82 |
| LDL-C (mg/dL) | 1.49 (1.07–2.07) | 0.02 | 1.34 (0.92–1.94) | 0.12 |
| HDL-C (mg/dL) | 0.58 (0.41–0.84) | 0.003 | 0.61 (0.40–0.94) | 0.03 |
| MAP (mmHg) | 1.01 (0.67–1.50) | 0.98 | 1.01 (0.65–1.58) | 0.96 |
| Glucose (mg/dL) | 1.30 (0.92–1.85) | 0.14 | 1.37(0.94–1.98) | 0.10 |
| Insulin (μU/mL) | 0.93 (0.65–1.33) | 0.68 | 0.86 (0.57–1.30) | 0.48 |
| Obesec | ||||
| Triglycerides (mg/dL) | 1.30 (0.99–1.70) | 0.06 | 0.98 (0.70–1.38) | 0.92 |
| LDL-C (mg/dL) | 1.34 (1.02–1.76) | 0.04 | 1.22 (0.89–1.66) | 0.21 |
| HDL-C (mg/dL) | 0.75 (0.57–0.99) | 0.04 | 0.76 (0.56–1.04) | 0.08 |
| MAP (mmHg) | 1.74 (1.25–2.42) | 0.001 | 1.75 (1.24–2.47) | 0.002 |
| Glucose (mg/dL) | 1.27 (0.96–1.68) | 0.09 | 1.16 (0.86–1.56) | 0.32 |
| Insulin (μU/mL) | 1.33 (1.01–1.74) | 0.04 | 1.29 (0.95–1.75) | 0.10 |
Standard deviations: Normal weight—triglycerides 25.8 mg/dL, LDL-C 24.2 mg/dL, HDL-C 18.4 mg/dL, MAP 8.7 mmHg, glucose 7.2 mg/dL, insulin 7.5 μU/mL; overweight—triglycerides 30.4 mg/dL, LDL-C 26.2 mg/dL, HDL-C 20.3 mg/dL, MAP 9.3 mmHg, glucose 7.6 mg/dL, insulin 8.2 μU/mL; obese—triglycerides 34.3 mg/dL, LDL-C 26.4 mg/dL, HDL-C 20.8 mg/dL, MAP 7.9 mmHg, glucose 7.2 mg/dL, insulin 9.5 μU/mL.
For SI conversion factors, multiply by 0.0259 for HDL-C, by 0.0259 for LDL-C, by 0.0113 for triglycerides, by 0.0555 for glucose, and by 6.945 for insulin.
Bold indicates P < 0.05.
aEach model included only one childhood metabolic risk factor as a predictor, with covariates (childhood age, childhood BMI, time for follow-up, adult BMI, race, and sex).
bThe model included all childhood metabolic risk factors as predictors, with covariates.
cAbnormal metabolic risk is predicted for each model within adult BMI categories.
Abbreviations: BMI, body mass index; OR, odds ratio; SD, standard deviation; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MAP, mean arterial blood pressure.
Race and sex were not significantly related to having the abnormal phenotype among either normal-weight or overweight adults. However, for obese individuals, both white race (reference = black, OR, 2.37; 95% CI, 1.30–4.31, P = 0.005) and male sex (reference = female, OR, 4.94; 95% CI, 2.76–8.83, P < 0.0001) were independently and positively associated with an abnormal risk profile in the multiple risk factor model.
IR definition
Among normal-weight adults, for each SD higher childhood LDL-C, there was between 78% and 95% increase in odds of having an abnormal cardiovascular risk profile (single risk factor model P = 0.02; multiple risk factor model P = 0.03). Furthermore, within normal-weight adults, higher fasting insulin in childhood resulted in a 51%–69% increased odds of having an abnormal adult profile (single risk factor model P = 0.04; multiple risk factor model P = 0.02) (Table 4). In overweight adults, no childhood metabolic risk factors were related to the abnormal metabolic risk profile. For obese adults, higher levels of glucose in childhood were associated with 38% increased odds of having an abnormal metabolic risk profile in adulthood (single risk factor model P = 0.01; multiple risk factor model P = 0.02) (Table 4).
Table 4.
Results of Logistic Regression Predicting Abnormal Adult BMI-Specific Metabolic Profiles From Childhood Metabolic Measures Using the Insulin Resistance Definition
| |
Single risk factor modelsa |
Multiple risk factor modelb |
||
|---|---|---|---|---|
| Childhood measure | OR per SD3(CI) | P value | OR per SD3(CI) | P value |
| Normal weightc | ||||
| Triglycerides (mg/dL) | 1.59 (0.91–2.79) | 0.10 | 1.16 (0.55–2.46) | 0.69 |
| LDL-C (mg/dL) | 1.78 (1.09–2.91) | 0.02 | 1.95 (1.06–3.58) | 0.03 |
| HDL-C (mg/dL) | 0.83 (0.49–1.43) | 0.51 | 1.22 (0.65–2.29) | 0.53 |
| MAP (mmHg) | 0.77 (0.38–1.56) | 0.46 | 0.78 (0.36–1.68) | 0.52 |
| Glucose (mg/dL) | 0.99 (0.56–1.74) | 0.97 | 0.94 (0.51–1.73) | 0.84 |
| Over weightc | ||||
| Insulin (μU/mL) | 1.51 (1.02–2.24) | 0.04 | 1.69 (1.10–2.58) | 0.02 |
| Triglycerides (mg/dL) | 0.86 (0.57–1.32) | 0.50 | 0.87 (0.51–1.49) | 0.61 |
| LDL-C (mg/dL) | 0.86 (0.58–1.28) | 0.46 | 0.81 (0.53–1.24) | 0.34 |
| HDL-C (mg/dL) | 0.81 (0.55–1.19) | 0.29 | 0.68 (0.42–1.08) | 0.10 |
| MAP (mmHg) | 0.78 (0.48–1.27) | 0.31 | 0.81 (0.48–1.37) | 0.44 |
| Glucose (mg/dL) | 0.95 (0.65–1.38) | 0.78 | 0.95 (0.63–1.42) | 0.79 |
| Obesec | ||||
| Insulin (μU/mL) | 0.79 (0.50–1.23) | 0.29 | 0.78 (0.49–1.25) | 0.30 |
| Triglycerides (mg/dL) | 1.11 (0.86–1.43) | 0.42 | 0.96 (0.71–1.31) | 0.80 |
| LDL-C (mg/dL) | 1.05 (0.83–1.34) | 0.69 | 1.04 (0.79–1.36) | 0.78 |
| HDL-C (mg/dL) | 0.89 (0.69–1.14) | 0.35 | 0.89 (0.67–1.17) | 0.39 |
| MAP (mmHg) | 0.91 (0.68–1.21) | 0.5 | 0.88 (0.65–1.17) | 0.37 |
| Glucose (mg/dL) | 1.38 (1.07–1.78) | 0.01 | 1.38 (1.06–1.81) | 0.02 |
| Insulin (μU/mL) | 1.24 (0.96–1.61) | 0.10 | 1.18 (0.90–1.54) | 0.24 |
Standard deviations: Normal weight—triglycerides 25.8 mg/dL, LDL-C 24.2 mg/dL, HDL-C 18.4 mg/dL, MAP 8.7 mmHg, glucose 7.2 mg/dL, insulin 7.5 μU/mL; overweight—triglycerides 30.4 mg/dL, LDL-C 26.2 mg/dL, HDL-C 20.3 mg/dL, MAP 9.3 mmHg, glucose 7.6 mg/dL, insulin 8.2 μU/mL; obese—triglycerides 34.3 mg/dL, LDL-C 26.4 mg/dL, HDL-C 20.8 mg/dL, MAP 7.9 mmHg, glucose 7.2 mg/dL, insulin 9.5 μU/mL.
For SI conversion factors, multiply by 0.0259 for HDL-C, by 0.0259 for LDL-C, by 0.0113 for triglycerides, by 0.0555 for glucose, and by 6.945 for insulin.
Bold indicates P < 0.05.
aEach model included only one childhood metabolic risk factor as a predictor, with covariates (childhood age, childhood BMI, time for follow-up, adult BMI, race, and sex).
bThe model included all childhood metabolic risk factors as predictors, with covariates.
cAbnormal metabolic risk is predicted for each model within adult BMI categories.
Abbreviations: BMI, body mass index; OR, odds ratio; SD, standard deviation; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MAP, mean arterial blood pressure.
In normal-weight and obese adults, there was no effect of either sex or race when using the insulin resistance definition for adult metabolic risk. In overweight individuals, however, white adults were less likely to have an abnormal metabolic risk profile according to the insulin resistance definition (reference = black, OR, 0.39; 95% CI, 0.17–0.86, P = 0.02).
Discussion
The results of this study demonstrate that adult BMI-specific metabolic risk is associated with childhood metabolic risk factors, although the results vary across BMI categories and definitions of adult metabolic risk. These results suggest that the development of adult metabolic risk within BMI may begin in childhood; however, specific childhood risk factors that associate with the adult profile depend on the definition of metabolic risk.
In the current study, we found that different metabolic risk factors were associated with the abnormal adult profile depending on the definitions of adult metabolic risk. Within the cardiometabolic risk factor clustering definition, some individual risk factors were significant in the single risk factor model, but then lost significance in the multiple risk factor model. These results suggest that these specific childhood risk factors are not independent predictors of the abnormal profile in overweight and obese adults once the other related risk factors are taken into account. It is also interesting to note that different childhood cardiometabolic risk factors were predictors for the overweight and obese abnormal profiles. This may suggest a different physiological mechanism to explain the development of the adult abnormal profile from childhood.
These results also highlight the finding that only 47%–51% of the same individuals were classified as abnormal by both methods; thus, to some extent the two methods identified different individuals as being at elevated risk. Meigs and colleagues4 compared similar definitions of metabolic risk as the present study (cardiometabolic risk factor clustering and insulin resistance) and found comparable results between the two definitions when assessing risk for development of cardiovascular disease and type 2 diabetes in adults. Our study examined the period of development from childhood to early adulthood, whereas Meigs and colleagues examined the period of development from abnormal risk in adulthood to the manifestation of chronic disease. Thus, it is possible for there to be different physiological mechanisms to explain these distinct periods of development for risk and disease.
The existing research on BMI-specific metabolic risk profiles explores mostly cross-sectional physiological, demographic, and behavioral correlates in adults.4,5,7–9 Our study extends these results to the prediction of adult BMI-specific metabolic risk profiles from childhood risk factors, independent of childhood BMI, providing insight concerning the longitudinal development of these risk profiles. Furthermore, much of the previous research has focused on normal-weight adults with the abnormal profile, or, conversely, the obese adult with a normal profile. While our analyses predicted an abnormal metabolic risk profile in obese adults, these results imply that obese adults with the normal risk profile are associated with lower MAP and lower fasting glucose in childhood. We also examined BMI-specific metabolic risk in overweight adults, in which low HDL-C emerged as an important indicator of abnormal metabolic risk.
It is well established that many lipid, blood pressure, and metabolic abnormalities in adulthood are partly modifiable through positive lifestyle changes. Recommendations for adults focus on the incorporation of increased physical activity and altered dietary intake for improving both lipid profiles,20 elevated blood pressure,23 glucose, and insulin levels.24,25 However, more research is needed to assess whether childhood physical activity level and nutritional habits predict BMI-specific metabolic risk in adulthood and whether modifying these habits in childhood can reduce the progression of an abnormal metabolic risk profile.
There are strengths and limitations to this study that warrant discussion. Future studies should determine the extent to which metabolic risk differs within categories of adiposity such as dual-energy X-ray absorptiometry– (DEXA) derived fat mass. The well-characterized biracial cohort of men and women with standardized clinical measurements available in both childhood and adulthood is also a significant strength of this study. Future studies that employ longer periods of follow-up into older ages are warranted. Although the sample size was large for the overall cohort, the statistical analysis did stratify analyses first into BMI categories, significantly reducing sample sizes and statistical power for each subsequent analysis. Efforts should be made to replicate these results in other cohorts to better generalize the ethnic and sex results to other populations, as well as to characterize these relationships with a longer follow-up into middle age.
In conclusion, adult BMI-specific metabolic risk is associated with childhood metabolic risk factors such as high LDL-C, low HDL-C, and high MAP with the cardiometabolic risk factor clustering definition of risk; and high LDL-C, high glucose, and high insulin with the IR definition. These risk factors in childhood may develop into abnormal metabolic risk in adulthood. Recommendations for improving overall lipid, blood pressure, and metabolic profiles should continue to emphasize positive lifestyle changes in adults. Future research is needed to establish whether positive lifestyle changes, if adopted during childhood, decrease the development and progression of metabolic risk in adulthood.
Acknowledgments
This study was supported by funds from the National Heart, Lung, and Blood Institute grant HL-38844, the National Institute on Aging grant AG-16592, and the National Institute of Child Health and Human Development grant HD-043820.
Contributor Information
Sarah M. Camhi, Ph.D., Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Peter T. Katzmarzyk, Ph.D., Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Stephanie Broyles, Ph.D., Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Sathanur R. Srinivasan, Ph.D., Tulane Center for Cardiovascular Health and Department of Epidemiology, Tulane Health Sciences Center, New Orleans, Louisiana.
Wei Chen, M.D.,Ph.D., Tulane Center for Cardiovascular Health and Department of Epidemiology, Tulane Health Sciences Center, New Orleans, Louisiana.
Claude Bouchard, Ph.D., Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Gerald S. Berenson, Ph.D., Tulane Center for Cardiovascular Health and Department of Epidemiology, Tulane Health Sciences Center, New Orleans, Louisiana.
Author Disclosure Statement
S.M.C., S.B., S.R.S., W.C., and G.S.B. have no conflicts of interest to disclose. P.T.K. is partially funded by the Louisiana Public Facilities Authority Endowed Chair in Nutrition. C.B. is partially funded by the George A. Bray Jr. Endowed Chair in Nutrition.
References
- 1.Conus F, Rabasa-Lhoret R, Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 2.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka S, Togashi K, Rankinen T, Perusse L, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Is adiposity at normal body weight relevant for cardiovascular disease risk? Int J Obes Relat Metab Disord. 2002;26:176–183. doi: 10.1038/sj.ijo.0801880. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 5.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 6.Marini MA, Succurro E, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30:2145–2147. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 7.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 9.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 10.Kemper HC, Snel J, Verschuur R, Storm-van Essen L. Tracking of health and risk indicators of cardiovascular diseases from teenager to adult: Amsterdam Growth and Health Study. Prev Med. 1990;19:642–655. doi: 10.1016/0091-7435(90)90061-n. [DOI] [PubMed] [Google Scholar]
- 11.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–899. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 12.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- 13.Twisk JW, Kemper HC, van Mechelen W, Post GB. Tracking of risk factors for coronary heart disease over a 14-year period: A comparison between lifestyle and biologic risk factors with data from the Amsterdam Growth and Health Study. Am J Epidemiol. 1997;145:888–898. doi: 10.1093/oxfordjournals.aje.a009048. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: The Bogalusa Heart Study. Am J Epidemiol. 2007;166:527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 15.Program LRC; Health NIo. Vol. 1. Washington DC: 1975. Manual of operations: Lipid and lipoprotein anlaysis. [Google Scholar]
- 16.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 17.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 18.Srinivasan SR, Webber LS, Whitaker CF, Berenson GS. Quantification of lipoprotein cholesterol in serum from children with different lipoprotein profiles: heparin-calcium precipitation and ultracentrifugation compared. Clin Chem. 1983;29:481–485. [PubMed] [Google Scholar]
- 19.WHO. Geneva: WHO; Physical Status: The Use and Interpretation of Anthropometry. Technical Report Series 854, 1995. [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health. Bethesda, MD: National Heart, Lung, and Blood Institute, 2004, 04-5230; The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. [PubMed] [Google Scholar]
- 24.Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I, Verity LS. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32:1345–1360. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services; 2008. Physical Activity Guidelines for Americans. [Google Scholar]