The MUC1 oncoprotein is aberrantly expressed at high levels in most human carcinomas and certain hematologic malignancies.1 Estimates are that, of the 1.4 million tumors diagnosed each year in the US, about 900,000 have overexpression of MUC1. MUC1 has thus become a highly attractive target for the development of new anti-cancer agents, including vaccines, antibodies and small molecules. However, there are presently no approved agents that target MUC1, in part because there has been limited information about how MUC1 contributes to the malignant phenotype. Indeed, MUC1 consists of two subunits, and the more recent focus of research on the MUC1 receptor subunit has provided new insights into (1) how MUC1 induces transformation and is of importance to human cancers, and (2) how to target MUC1 function as an approach for anticancer treatment.
MUC1 is a Heterodimeric Complex
MUC1 is encoded by a single transcript and, following translation, undergoes autocleavage into two subunits that form a stable noncovalent complex.2-4 The MUC1 N-terminal subunit, designated MUC1-N, is the mucin component with variable numbers of tandem repeats that are extensively glycosylated.5,6 MUC1-N associates with the cell surface through binding to the transmembrane MUC1 C-terminal subunit (MUC1-C) (Fig. 1). The MUC1-N and MUC1-C nomenclature is used to denote positioning of the subunits following cleavage from a single polypeptide and to distinguish them from genetic isoforms, which use Greek symbols; for example, ERα and ERβ, the PKC isoenzymes and PDGF receptors.
Figure 1.

Schematic representation of the MUC1 heterodimer positioned at the cell membrane. MUC1-N is the mucin component of the heterodimer that contains the glycosylated variable numbers of tandem repeats. MUC1-N is tethered to the cell surface through binding to the transmembrane MUC1-C subunit (left). Shedding of MUC1-N leaves MUC1-C at the cell membrane as a putative receptor for signaling the presence of extracellular stress to the interior of the cell (right).
Interaction with growth factor receptors
The MUC1 heterodimer is positioned at the apical borders of normal secretory epithelial cells.1 In the response of epithelial cells to stress and the reversible loss of polarity,7 MUC1 can interact with cell surface molecules that otherwise localize to the basolateral borders. In carcinoma cells with irreversible loss of polarity, MUC1 is substantially upregulated, constitutively forms complexes with ErbB1-4,8-10 FGFR3,11 PDGFRβ12 and Met,13 and participates in their downstream signaling pathways. These findings have suggested that a physiologic stress response of epithelial cells to promote repair and survival7 has been exploited in part by carcinoma cells through irreversible interactions between MUC1 and growth factor receptors.
MUC1-C is the signaling subunit
Much of the early work on MUC1 focused on MUC1-N based on the findings that high levels of MUC1-N at the cell surface can block cell-cell and cell-extracellular matrix interactions associated with the malignant phenotype.14-16 MUC1-N is shed into secretions from epithelial cells17 and is detectable in the serum of normal individuals.18 Moreover, MUC1-N is released from the surface of carcinoma cells19 and is found at elevated levels in the circulation of patients with breast cancer.20 The available evidence indicates that release of MUC1-N from the cell surface leaves MUC1-C as a putative receptor for interacting with other cell surface molecules, such as EGFR, and for signaling to the interior of the cell (Fig. 1).21 The overexpression of MUC1 as found in human carcinoma cells is also associated with accumulation of MUC1-C in the cytoplasm1,22-24 and targeting of MUC1-C to the nucleus and mitochondria. The following sections summarize how MUC1-C participates in cell signaling and transformation, and how these findings have provided the basis for designing agents that disrupt MUC1-C function.
MUC1-C is an Oncogen
MUC1-C consists of a 58 amino acid extracellular domain, a 28 amino acid transmembrane domain and a 72 amino acid cytoplasmic domain (Fig. 2). The MUC1-C extracellular domain (MUC1-C/ED) is glycosylated on Asn-36, which then functions as a galectin-3 ligand binding site (Fig. 2A).21 Galectin-3, in turn, functions as a bridge to physically associate MUC1-C with EGFR and other cell surface molecules21 (Fig. 2B). Moreover, galectin-3 participates in intracellular MUC1-C signaling in a miR-322-dependent regulatory loop.21 The MUC1-C cytoplasmic domain (designated MUC1-CD) interacts with β-catenin, the major effector of the canonical Wnt signaling pathway (Fig. 3).25,26 This finding provided the first evidence that MUC1-C interacts with a signaling pathway that is associated with transformation and provided the basis for demonstrating that MUC1 and, specifically, MUC1-CD induces anchorage-independent growth and tumorigenicity.27,28 Subsequent work showed that MUC1-CD interacts with multiple kinases, transcription factors and chaperones associated with malignancies (Fig. 3), and some of the findings have been summarized recently here in Cancer Biology & Therapy29 and in Science Signaling.30
Figure 2.
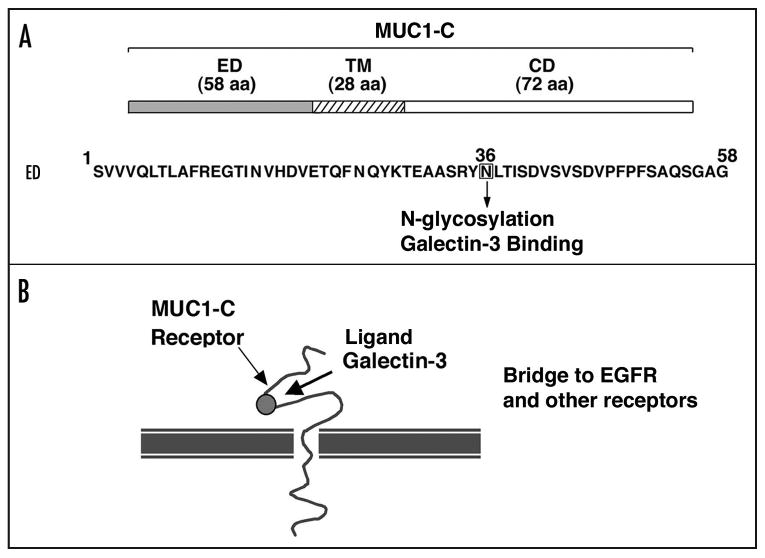
Structure of the MUC1-C subunit. (A) MUC1-C includes a 58 amino acid extracellular domain, a 28 amino acid transmembrane domain and a 72 amino acid cytoplasmic domain. Amino acid sequence of the MUC1-C extracellular domain is shown with highlighting of N-36 that undergoes glycosylation and functions as a galectin-3 binding site. (B) Schematic representation of the MUC1-C receptor with galectin-3 functioning as a bridge to EGFR and other cell surface molecules.
Figure 3.

Amino acid sequence of the MUC1-C cytoplasmic domain. The kinases that interact with the cytoplasmic domain and their phosphorylation sites are highlighted. Effectors that interact with the cytoplasmic domain are also highlighted at their respective binding regions.
Nuclear targeting of MUC1-C
The interaction between MUC1-C and β-catenin was confirmed in human breast,31 pancreatic,12,32 colorectal33 and gastric34 carcinomas, and in MUC1 transgenic mouse models of mammary tumorigenesis.35 The functional significance of the interaction was further supported by the finding that MUC1-C associates with β-catenin in the nucleus and coactivates Wnt target gene transcription (Fig. 4).9,27,28,36-38 The effects of MUC1-C on Tcf/β-catenin-mediated transcription have been attributed to stabilization of β-catenin28 and to MUC1-C occupancy of Wnt target gene promoters as an integral part of the Tcf/β-catenin transcription complex (unpublished data). Promoter occupancy by MUC1-C is, however, not restricted to Tcf/β-catenin complexes based on reports that MUC1-C directly interacts with p53 and ERα, and occupies promoters of their target genes (Fig. 4).39,40
Figure 4.

Intracellular targeting of MUC1-C to the nucleus and mitochondria. In transformed cells, MUC1 expression is upregulated and repositioned from the apical membrane to over the entire cell surface. In addition, MUC1-C accumulates in the cytosol and is transported to the nucleus by importin β, where it interacts with diverse transcription factors. Alternatively, MUC1-C is targeted to the mitochondrial outer membrane by HSP70/HSP90 complexes.
Proteins with a classical nuclear localization signal (NLS) are imported into the nucleus by binding to importin α and β.41,42 The cargo-importin α/β complex interacts with nucleoporins and is transported through the nuclear pore by a Ran GTPase-dependent mechanism. MUC1-C does not have the classical bipartite NLS with two clusters of basic amino acids separated by 10–12 amino acids or a monopartite NLS with a single cluster of four to five basic amino acids. However, like certain other proteins without classical NLSs,43 MUC1-C is transported to the nuclear pore by associating with importin β (Fig. 4).44 In turn, MUC1-C binds directly to the nucleoporin, Nup62, which is located on both the cytoplasmic and nuclear faces of nuclear pore complexes.44 These studies also demonstrated that MUC1-C forms oligomers that are dependent on a CQC motif in MUC1-CD and that oligomerization of MUC1-C is necessary for nuclear import.44 Thus, mutation of the CQC motif to AQA blocks nuclear targeting of MUC1-C and attenuates MUC1-C-mediated transcriptional coactivation.44 These findings provided support for the potential importance of the CQC motif in the function of MUC1-C as an oncoprotein.
Mitochondrial targeting of MUC1-C
The overexpression of MUC1 as found in human tumors is associated with localization of MUC1-C to mitochondria (Fig. 4).45 Treatment of cells with heregulin (HRG), an activator of the ErbB2 pathway, further increases the mitochondrial targeting of MUC1-C,45 a finding that relates to the association of MUC1 with the ErbB1-4 receptors.8,9 Activation of FGFR3 has also been associated with targeting of MUC1-C to mitochondria.11 The functional significance of mitochondrial MUC1-C localization is supported by the demonstration that MUC1 attenuates DNA damage-induced release of mitochondrial apoptogenic factors and the apoptotic response.45 Damage to epithelial cells is associated with activation of the HRG/ErbB pathway as a mechanism for repair and survival;7 thus, localization of MUC1-C to mitochondria is likely an event of physiologic importance that has been exploited by cancer cells.
The mitochondrial targeting of MUC1-C could be the result of release of MUC1-C directly from the cell membrane or redirection at the level of the endoplasmic reticulum.46 The newly liberated MUC1-C would be in the cytosol with an exposed hydrophobic transmembrane domain46 and thereby available for binding to chaperones that deliver unfolded proteins to mitochondria.47 Indeed, like other preproteins without classical N-terminal mitochondrial targeting sequences,48 MUC1-C associates with the molecular chaperones HSP70 and HSP90 (Fig. 4).11,49 MUC1-CD binds directly to HSP70.49 Moreover, HRG induces c-Src phosphorylation of MUC1-CD and, in turn, stimulates binding of MUC1-C to HSP90.49 Complexes of HSP70 and HSP90 function in the transport of preproteins to the mitochondrial surface by delivery to the receptor Tom70 and in turn their import.48 Analysis of purified mitochondria indicated that MUC1-C integrates into the mitochondrial outer membrane (MOM), where it blocks release of apoptogenic proteins, potentially by interfering with localization of proapoptotic Bcl-2 subfamily members (Fig. 4).11,45,49
Dominant-Negative Function of MUC1-C Mutants
There are no reports to date of tumors that express MUC1 with mutations in the MUC1-C cytoplasmic domain. For example, analysis of over 100 human breast carcinomas by sequencing the region encoding MUC1-CD revealed no evidence for mutations (unpublished data). These findings have suggested that human malignancies that exploit MUC1 function do so by overexpressing the wild-type protein. Indeed, the available evidence indicates that certain mutants of MUC1-CD function as dominant-negatives that abrogate the malignant phenotype. To assess the effects of MUC1 mutants, human HCT116 colon cancer cells, which are null for endogenous MUC1, have been transfected to stably express wild-type or mutant MUC1. The available results indicate that MUC1-CD contains at least three sites that are of importance for transformation (Fig. 5).
Figure 5.
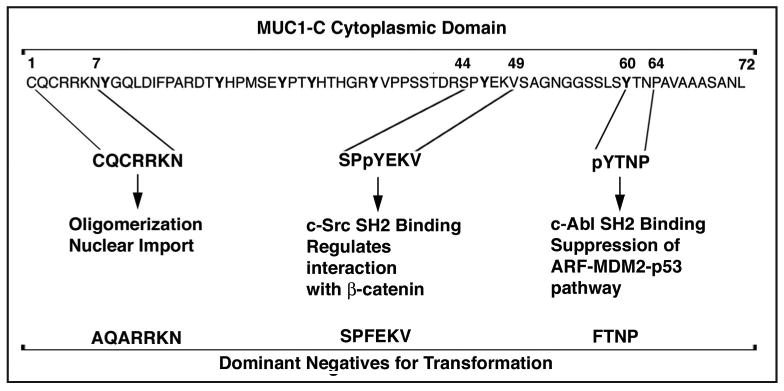
Mutants of the MUC1-C cytoplasmic domain that function as dominant-negatives for transformation. Stable expression of MUC1 with the indicated mutations in HCT116 colon cancer cells abrogates anchorage-independent growth and tumorigenicity, supporting their role as dominant-negatives. Expression of MUC1 with the AQA mutations blocks oligomerization and nuclear import of MUC1-C. Mutation of Tyr-46 to F blocks c-Src phosphorylation and thereby binding of the c-Src SH2 domain. Mutation of Tyr-60 to F blocks c-Abl phosphorylation, binding of the c-Abl SH2 domain and suppression of the ARF-MDM2-p53 pathway.
CQCRRKN motif
The CQCRRKN motif is unique among mammalian proteins (Fig. 5). As noted above, the cysteines are necessary for oligomerization of MUC1-C and targeting of MUC1-C to the nucleus.44 Oligomerization of MUC1-C may also be of importance for interactions between the cytoplasmic domain and its diverse binding partners. Therefore, mutation of CQC to AQA may disrupt functions of MUC1-C that are in addition to nuclear localization and regulation of gene transcription. For example, expression of the MUC1(AQA) mutant attenuates activation of the β-catenin and NFκB p65 pathways (unpublished data). Moreover, MUC1(AQA) blocks anchorage-independent growth of HCT116 cancer cells, indicating that this mutant functions as a dominant-negative for transformation and, as shown below, the CQC motif is a potential site for targeting MUC1-mediated oncogenesis.
TDRSPYEKV motif
The TDRSPYEKV motif (amino acids 41–49; Fig. 5) is phosphorylated on Thr by PKCδ,50 on Ser by GSK3β26 and JNK (unpublished data) and on Tyr by EGFR8 and the Src family kinases.36,38,51 The TDSPY motif regulates the interaction between MUC1-C and β-catenin in that phosphorylation on Thr or Ser decreases their binding26,50 and phosphorylation on Tyr increases the formation of these complexes.8,36,38,51 The phosphorylated SPY motif can also potentially function as a site for isomerization by Pin1.52 Expression of MUC1 with a T41A or a S44A mutation attenuates anchorage-independent growth of HCT116 cells (and unpublished data).50 Moreover, HCT116 cell anchorage-independent growth and tumorigenicity is abrogated by stable expression of MUC1 with the Y46F mutation.37 In another model, stable expression of MUC1-CD, but not MUC1-CD(Y46F), induces transformation of rodent fibroblasts,28 although here the assays detect the effect of the Y46F mutant on the MUC1 transforming function rather than assessing a dominant-negative effect.
YTNP motif
The YTNP motif (amino acids 60–64; Fig. 1) is phosphorylated on Tyr by the c-Abl kinase.53 The pYTNP site then functions as a binding motif for the c-Abl SH2 domain (Fig. 5).53 Binding of MUC1-C to c-Abl attenuates phosphorylation of c-Abl on Thr-735 and the interaction between c-Abl and cytosolic 14-3-3. Expression of MUC1 with a Y60F mutation (i) disrupts the interaction between MUC1-C and c-Abl, (ii) relieves the MUC1-induced block of c-Abl phosphorylation on Thr-735, and (iii) attenuates the MUC1 anti-apoptotic function.53 These results support a model in which MUC1-C sequesters c-Abl in the cytoplasm and thereby inhibits the apoptotic response to genotoxic stress. The MUC1(Y60F) mutant is also a potent inducer of the ARF tumor suppressor.54 MUC1(Y60F) induces transcription of the ARF locus by a c-Abl-dependent mechanism that promotes CUL-4A-mediated export of the nuclear replication protein Cdc6. MUC1(Y60F) induced ARF expression results in the inhibition of MDM2 and upregulation of p53.54 These findings and the demonstration that the MUC1(Y60F) mutant abrogates anchorage-independent growth and tumorigenicity of HCT116 cells indicate that the oncogenic function of MUC1-C is conferred in part by the YTNP site through suppression of the ARF-MDM2-p53 pathway.
Is MUC1-C of Importance to Human Cancers?
MUC1-C induces a tumorigenesis gene signature associated with the outcome of patients with breast and lung cancer
The MUC1-C subunit is targeted to the nucleus and contributes to the regulation of gene expression, at least in part, by interacting with certain transcription factors. However, there is no information regarding the effects of MUC1-C on gene expression patterns. To identify genes that are activated or repressed, 3Y1 fibroblasts stably transfected to express MUC1-CD were analyzed for changes in gene expression associated with transformation in vitro and as tumors in nude mice.55 Genes that were activated and involved in tumorigenesis were applied to the analysis of a breast cancer database. A 35-gene MUC1-induced tumorigenesis signature (MTS) was found to predict significant decreases in disease-free and overall survival.55 Similar results were obtained when the MTS was applied to a database derived from lung cancer patients.55
MUC1-C induces a lipid metabolism gene signature
Further analysis of the MUC1-CD-induced alterations in gene expression identified a second cluster involved in cholesterol and fatty acid synthesis.56 A MUC1-induced Lipid Metabolism Signature (MLMS), consisting of 38 genes, was identified that represented lipid metabolic enzymes and transporters. Included in the MLMS are genes encoding: (1) the sterol regulatory element binding protein 1 (SREBP1), a transcription factor that regulates genes involved in the synthesis of cholesterol, fatty acids and triglycerides; (2) ATP citrate lyase (ACLY), an enzyme that mediates the synthesis of acetyl coenzyme A, the common precursor for the cholesterol and fatty acid pathways; and (3) fatty acid synthase (FASN), an enzyme that is upregulated in diverse human cancers and is linked to the transformed phenotype.57
MLMS predicts response of breast cancer patients to adjuvant tamoxifen treatment
MUC1-C associates with the estrogen receptor α (ER) complex on estrogen-responsive promoters, increases recruitment of coactivators and antagonizes the inhibitory effects of the anti-estrogen tamoxifen.40 Tamoxifen is effective as an adjuvant therapy to prevent breast cancer recurrence and as treatment to extend survival of patients with metastatic disease.58 However, the mechanisms responsible for failures to taxoxifen treatment remain unclear. Notably, analysis of a database from 176 patients with ER+ breast cancer who were treated with tamoxifen in the adjuvant setting demonstrated that disease-free survival is significantly decreased in patients with MLMS+ tumors as compared to those with MLMS- tumors.56 Analysis of a second database from 147 patients with breast cancer who were treated with adjuvant tamoxifen also demonstrated that patients with MLMS+ tumors experience highly significant decreases in disease-free and overall survival.56 These findings thus indicate that activation of the MUC1-induced lipid metabolism signature predicts failure to tamoxifen treatment.
Is MUC1-C a Druggable Target?
The aberrant overexpression of MUC1 in diverse human malignancies, the interaction between the MUC1-C subunit and multiple effectors associated with transformation, and the demonstration that MUC1-CD is sufficient to induce the malignant phenotype have indicated that MUC1-C is a potential target for cancer treatment. In addition, the recent findings that MUC1-CD induces gene signatures that are predictive of outcome for patients with breast and lung cancer have provided further support for the importance of MUC1-C as a therapeutic target. However, MUC1-C has no kinase or enzymatic function that would allow for targeting a catalytic site. Therefore, one potential strategy is to disrupt MUC1-CD interactions with specific effectors that are linked to transformation. However, disrupting oncogenic protein-protein interactions has posed challenges that require targeting flat and extended protein surfaces.59 So, then what is the evidence that MUC1-C is a potentially druggable target?
MUC1-CD peptide decoys as potential agents
The MUC1-C cytoplasmic domain contains a VSAGNGGSSLSY motif that interacts with β-catenin25 and disruption of that interaction with a mutation at the Tyr-46 site inhibits transformation.37 These findings suggested that a potential approach for targeting MUC1-C is through disruption of the MUC1-C interaction with β-catenin. In this regard, a decoy GGSSLSY peptide was shown to block binding of MUC1-C and β-catenin.25 More recent studies with a MUC1-CD-derived peptide encompassing the Tyr-46 site and the downstream β-catenin binding motif (PMIP; YEKVSAGNGGSSLS) has been used as a decoy to block the interaction with β-catenin.60 The YEKV motif, which functions as a substrate for EGFR and c-Src,8,51 may also provide a decoy for phosphorylation of endogenous MUC1-C at Tyr-46 and thereby attenuate the interaction between MUC1-C and β-catenin. The PMIP decoy partially inhibits growth of human BT20 breast cancer cells in culture.60 PMIP was also effective in partially inhibiting growth of human MDA-MB-231 breast tumor xenografts in SCID mice, indicating that disrupting the MUC1-C interaction with β-catenin and/or EGFR can slow proliferation.60 However, interaction of the decoy PMIP peptide with β-catenin and/or EGFR could also affect cellular proliferation by mechanisms independent of MUC1, that is by disrupting their interactions with yet other effectors. Another decoy MUC1 peptide has been used to block the interaction between MUC1-C and Grb2, and thereby disrupt MUC1-C signaling to the SOS/Ras pathway.61
Direct targeting of the MUC1-C cytoplasmic domain
A potentially more specific approach is to develop agents that interact directly with MUC1-C and block its function. In this context, the MUC1-C cytoplasmic domain contains a CQC motif that is necessary for oligomerization.44 Moreover, MUC1-C oligomerization is required for its nuclear localization and interaction with diverse effectors.44 A MUC1 peptide inhibitor (GO-201) has been synthesized that binds to the MUC1-C cytoplasmic domain through interaction with the CQC motif and blocks MUC1-C oligomerization.62 Importantly, treatment of ZR-75-1, MCF-7 and MDA-MB-231 breast cancer cells with GO-201 in vitro is associated with induction of late apoptotic/necrotic cell death. Consistent with the demonstration that MUC1 protects cells against disruption of redox balance,63,64 direct targeting of MUC1-C was associated with increases in ROS and activation of the DNA damage response. Of significance is whether GO-201 induces death by a mechanism dependent on MUC1 expression or if it is a non-specific cytotoxin. Indeed, the finding that GO-201 has no effect on MUC1-negative cells indicates that this agent is selective for carcinoma cells that may be addicted to MUC1 for maintaining the malignant phenotype.62 Another question of importance was whether GO-201 could be delivered in vivo with an effective therapeutic index. In models of ZR-75-1, MCF-7 and MDA-MB-231 tumor xenografts growing in nude mice, administration of GO-201 was well tolerated and associated with complete regressions that were prolonged after completing treatment.62 These findings thus provide proof-of-principle that MUC1-C is a druggable target and that blocking MUC1-C function is effective in inducing death of human breast cancer cells.
Summary
MUC1 has emerged as an especially attractive target for the development of anticancer agents. However, to date, there are no approved antibodies or small molecules that target MUC1. One reason is that historically much of the work on MUC1 focused on MUC1-N, the shed mucin component, and not MUC1-C, the transmembrane receptor subunit. Nonetheless, recent advances have provided new insights into: (1) the interactions of MUC1-C with diverse effectors, such as β-catenin, receptor tyrosine kinases, c-Src, c-Abl, p53, HSPs and galectin-3 among others, that have been linked to transformation; (2) the function of MUC1-C and specifically the MUC1-C cytoplasmic domain in inducing transformation and the role of dominant-negative mutants in reversing the malignant phenotype; and (3) the activation of gene signatures by MUC1-C that are predictive of clinical outcome in patients with carcinomas. Moreover, the demonstration that direct targeting of MUC1-C function blocks survival and tumorigenicity of human breast carcinoma cells indicates that MUC1-C is a druggable target that is of potential importance to cancer treatment.
Acknowledgments
This work was supported by Grants CA97098, CA42802 and CA100707 awarded by the National Cancer Institute. Dr. Kufe has an ownership interest in Genus Oncology and is a consultant to the company.
References
- 1.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–32. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 2.Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267:6171–7. [PubMed] [Google Scholar]
- 3.Levitin F, Stern O, Weiss M, Gil-Henn C, Ziv R, Prokocimer Z, et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–86. doi: 10.1074/jbc.M506047200. [DOI] [PubMed] [Google Scholar]
- 4.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 2006;13:71–6. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–3. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell JA. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–3. [PubMed] [Google Scholar]
- 7.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–6. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Ren J, Yu WH, Li G, Kuwahara H, Yin L, et al. The EGF receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J Biol Chem. 2001;276:35239–42. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Yu WH, Ren J, Huang L, Kharbanda S, Loda M, et al. Heregulin targets γ-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 protein. Mol Cancer Res. 2003;1:765–75. [PubMed] [Google Scholar]
- 10.Schroeder J, Thompson M, Gardner M, Gendler S. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–64. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–83. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh PK, Wen Y, Swanson BJ, Shanmugam K, Kazlauskas A, Cerny RL, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201–10. doi: 10.1158/0008-5472.CAN-06-4647. [DOI] [PubMed] [Google Scholar]
- 13.Singh PK, Behrens ME, Eggers JP, Cerny RL, Bailey JM, Shanmugam K, et al. Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J Biol Chem. 2008;283 doi: 10.1074/jbc.M805036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligtenberg MJL, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:223–32. [PubMed] [Google Scholar]
- 15.van de Wiel-van Kemenade E, Ligtenberg MJL, de Boer AJ, Buijs F, Vos HL, Melief CJM, et al. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J Immunol. 1993;151:767–76. [PubMed] [Google Scholar]
- 16.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine H, Kufe D. Purification and characterization of a high molecular weight glycoprotein detectable in human milk and breast carcinomas. J Immunol. 1985;135:3610–6. [PubMed] [Google Scholar]
- 18.Hayes DF, Sekine H, Ohno T, Abe M, Keefe K, Kufe DW. Use of a murine monoclonal antibody for detection of circulating DF3 antigen levels in breast cancer patients. J Clin Invest. 1985;75:1671–8. doi: 10.1172/JCI111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe M, Kufe D. Structural analysis of the DF3 human breast carcinoma associated protein. Cancer Res. 1989;49:2834–9. [PubMed] [Google Scholar]
- 20.Hayes D, Zurawski V, Kufe D. Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986;4:1542–50. doi: 10.1200/JCO.1986.4.10.1542. [DOI] [PubMed] [Google Scholar]
- 21.Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perey L, Hayes DF, Maimonis P, Abe M, O'Hara C, Kufe DW. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res. 1992;52:2563–8. [PubMed] [Google Scholar]
- 23.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973–82. doi: 10.1002/1097-0142(20010601)91:11<1973::aid-cncr1222>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Croce MV, Isla-Larrain MT, Rua CE, Rabassa ME, Gendler SJ, Segal-Eiras A. Patterns of MUC1 tissue expression defined by an anti-MUC1 cytoplasmic tail monoclonal antibody in breast cancer. J Histochem Cytochem. 2003;51:781–8. doi: 10.1177/002215540305100609. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem. 1997;272:12492–4. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol. 1998;18:7216–24. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–10. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005;65:10413–22. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 29.Kufe D. Targeting the MUC1 oncoprotein: a tale of two proteins. Cancer Biol Ther. 2008;7:81–4. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 30.Carson D. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–32. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 32.Wen Y, Caffrey T, Wheelock M, Johnson K, Hollingsworth M. Nuclear association of the cytoplasmic tail of MUC1 and β-catenin. J Biol Chem. 2003;278:38029–39. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]
- 33.Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–6. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 34.Udhayakumar G, Jayanthi V, Devaraj N, Devaraj H. Interaction of MUC1 with beta-catenin modulates the Wnt target gene cyclinD1 in H. pylori-induced gastric cancer. Mol Carcinog. 2007;46:807–17. doi: 10.1002/mc.20311. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–47. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Chen W, Ren J, Yu W, Li Q, Yoshida K, et al. DF3/MUC1 signaling in multiple myeloma cells is regulated by interleukin-7. Cancer Biol Ther. 2003;2:187–93. doi: 10.4161/cbt.2.2.282. [DOI] [PubMed] [Google Scholar]
- 37.Huang L, Ren J, Chen D, Li Y, Kharbanda S, Kufe D. MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther. 2003;2:702–6. [PubMed] [Google Scholar]
- 38.Li Q, Ren J, Kufe D. Interaction of human MUC1 and β-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem Biophys Res Commun. 2004;315:471–6. doi: 10.1016/j.bbrc.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–78. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor α. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Weis K. Regulating access to the genome: nucleoplasmic transport throughout the cell cycle. Cel. 2003;112:441–51. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 42.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 43.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–17. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 44.Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–30. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 45.Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anti-cancer agents. Cancer Cell. 2004;5:163–75. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traven A, Huang D, Lithgow T. Protein hijacking: key proteins held captive against their will. Cancer Cell. 2004;5:163–75. doi: 10.1016/s1535-6108(04)00029-7. [DOI] [PubMed] [Google Scholar]
- 47.Voos W. A new connection: chaperones meet a mitochondrial receptor. Mol Cell. 2003;11:1–3. doi: 10.1016/s1097-2765(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 48.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 49.Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25:20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- 50.Ren J, Li Y, Kufe D. Protein kinase C δ regulates function of the DF3/MUC1 carcinoma antigen in β-catenin signaling. J Biol Chem. 2002;277:17616–22. doi: 10.1074/jbc.M200436200. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J Biol Chem. 2001;276:6061–4. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 52.Lu K, Zhou X. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–16. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 53.Raina D, Ahmad R, Kumar S, Ren J, Yoshida K, Kharbanda S, et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J. 2006;25:3774–83. doi: 10.1038/sj.emboj.7601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raina D, Ahmad R, Chen D, Kumar S, Kharbanda S, Kufe D. MUC1 oncoprotein suppresses activation of the ARF-MDM2-p53 pathway. Cancer Biol Ther. 2008;7:1956–67. doi: 10.4161/cbt.7.12.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khodarev N, Pitroda S, Beckett M, MacDermed D, Huang L, et al. Weichselbaum R. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–7. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitroda S, Khodarev N, Beckett M, Kufe D, Weichselbaum R. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106:5837–41. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menendez J, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 58.Jordan V. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 59.Verdine GL, Walensky LD. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. Clin Cancer Res. 2007;13:7264–70. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 60.Bitler B, Menzl I, Huerta C, Sands B, Knowlton W, Chang A, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–9. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–3. [PubMed] [Google Scholar]
- 62.Raina D, Ahmad R, Joshi M, Yin L, Wu Z, Kawano T, et al. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–41. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin L, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278:35458–64. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 64.Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1 alpha activation in a survival response to hypoxia. J Biol Chem. 2007;282:257–66. doi: 10.1074/jbc.M610156200. [DOI] [PubMed] [Google Scholar]


