Abstract
Cell trafficking during pregnancy may result in durable microchimerism, both fetal microchimerism in the mother and maternal microchimerism in her children. Whether microchimerism is continuously replenished has not been well-described. To address this question, we isolated granulocytes, cells with relatively short half-lives, from peripheral blood of healthy women. CD66b-positive cells were isolated by fluorescence activated cell sorting and a panel of polymorphism-specific quantitative pCR assays was employed to investigate fetal and maternal microchimerism. Overall 33% (10/30) of study subjects had at least one source of microchimerism in CD66b+ cells. Interestingly, maternal microchimerism was more common than fetal microchimerism, 40% vs. 15%, respectively (p = 0.05) and was present at higher levels (p = 0.03). The identification of maternal and fetal origin CD66b+ cells is strong evidence for an active microchimeric hematopoietic stem and progenitor cell niche. Furthermore, microchimeric CD66b+ cells could have an impact on innate and adaptive immune responses.
Key words: fetal microchimerism, maternal microchimerism, granulocytes, allogeneic, hematopoietic stem cells
Introduction
The acquisition during and maintenance after pregnancy by a woman of a small number of cells from her child (‘fetal microchimerism’) is now understood to be a common event.1 Similarly, a fetus during development acquires cells from the mother (‘maternal microchimerism’) and can maintain such cells long after birth.2 Both maternal and fetal microchimerism have been reported in the peripheral blood of women within cellular subsets including T-, B-, NK- and myeloid-lineage cells.3 In addition, fetal microchimerism has been identified in hematopoietic cell transplantation grafts derived from parous women.4 Evidence of fetal microchimerism within a woman's mesenchymal stem cell population is also available.5 However, whether there are hematopoietic stem and progenitor cell niches that are microchimeric and constitutively productive is not clear.
To begin to address this uncertainty, we evaluated microchimerism in CD66b sorted granulocytes. CD66b+ granulocytes are neutrophils, eosinophils and basophils and comprise 50–70%, 1–4% and <1%, respectively, of circulating leukocytes. These mature cells are rapidly cleared from the circulation by macrophages by apoptosis in the absence of survival factors,6,7 and therefore are associated with a relatively short half-life of 4–9 hours.8
Results
The mean age of the 30 study subjects was 45, range 26 to 74. The mean parity was 2, range 0 to 5, and the mean time since last birth among the 28 women who were parous was 14 years, range 1 to 49 years. Overall, CD66b+ microchimerism of either fetal or maternal origin was identified in 10 of 30 (33%) women. The purities of all but one CD66b enriched sort were 99% to 100%, mean purity 99.7% (one was 98%). Proportions of CD66b+ maternal and fetal microchimerism are shown in Table 1. Twelve women were informative for testing for both fetal and maternal Mc; 15 for fetal Mc only and three for maternal Mc only. Only one woman tested for fetal and maternal microchimerism had positive results for both.
Table 1.
CD66b+ maternal or fetal microchimerism in women
| CD66b+ microchimerism | Proportion positive | p | Cells tested* (mean) | Concentration** (median, range) | p |
| Maternal | 6/15 | - | 1.12 × 105 gEq | 0 gEq (0–3.05) | - |
| Fetal (by type of assay) | |||||
| HLA and DYS14 | 4/27 | 0.05 | 1.08 × 105 gEq | 0 gEq (0–0.5) | 0.03 |
| HLA | 1/15 | 0.05 | 0.94 × 105 gEq | 0 gEq (0–0.04) | 0.01 |
*Genome equivalent (gEq) total number of cells of the study subject that were tested; **gEq number of microchimeric cells per 105 cells of the study subject.
Overall, CD66b+ microchimerism of maternal origin (6 of 15, 40%) was more common than that observed from fetal origin (4 of 27, 15%), p = 0.05. One of the assays used to measure fetal microchimerism targets a Y-chromosome specific sequence that could also derive from other sources including another male child, a miscarriage, elective termination or theoretically from an older male sibling, therefore this analysis was adjusted for assay type (HLA-specific or DYS14). In addition, analysis was conducted restricting fetal microchimerism results to tests that employed HLA-polymorphism specific assays (as used for maternal microchimerism testing). Results were similar in this analysis; more women tested positive for maternal microchimerism (40%) than for fetal (1 of 15, 7%), p = 0.05.
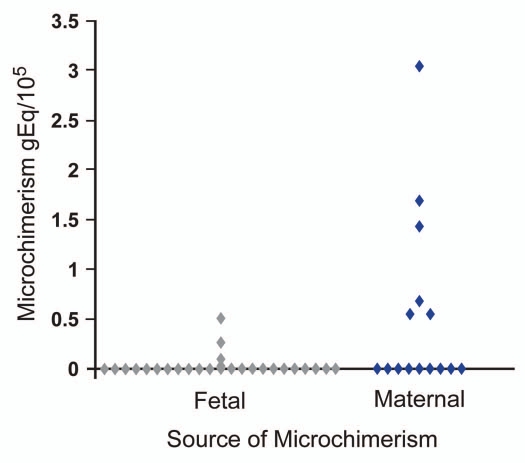
Concentrations of CD66b+ microchimerism were greater when of maternal origin compared to fetal origin (Fig. 1). Considering all results the range of CD66b+ maternal microchimerism concentrations (15 women) was 0 to 3.05 gEq/105 and for fetal microchimerism (27 women) 0 to 0.49 gE/105, p = 0.03. (Medians were 0 for both). The total amounts of CD66b+ cells tested for maternal and fetal microchimerism were very similar, mean 1.12 × 105 and 1.08 × 105 respectively. Analysis results were similar if conducted adjusting for the small difference in total cells tested (p = 0.03). When analysis was considered restricted to tests that employed HLA-specific assays, maternal microchimerism concentrations were also significantly higher than fetal microchimerism concentrations (p = 0.01).
Figure 1.
CD66b+ microchimerism per 105 proband cells. Fetal microchimerism result combines data from HLA-specific and DYS14 assays.
Discussion
Because of the extremely short-half lives of most granulocytes in circulation, the maternal and fetal cells quantified in our studies provide strong evidence for the existence of active microchimeric hematopoietic stem and progenitor cell niches. Prior studies have demonstrated microchimerism in CD14+ cells which include primarily cells of myeloid lineage but also B cells, epithelial and endothelial cells in circulation.3 In the current study, one third of all women tested positive for maternal or fetal microchimerism in granulocytes sorted with the very specific CD66b marker. Larger proportions of women might test positive for even lower quantities of microchimerism if more sensitive assays were available and practically more blood could be sorted and tested for microchimerism.
An intriguing finding was the higher proportion and quantity of maternal than fetal microchimerism. One potential explanation for this observation is that the developing fetal immune system may better accommodate maternal cells because of entrained tolerance.11 Alternatively, the younger hematopoietic stem and progenitor cell niche in a fetus may better enable the relatively older maternal microchimerism to survive in contrast to the young stem cells from the fetus that must establish themselves in the ‘aged’ maternal hematopoietic stem and progenitor cell niche.12 Some evidence is now available that acquisition of new microchimerism (additional fetal, for example) may influence pre-existing microchimerism in Ficoll-isolated peripheral blood mononuclear cells (Gammill HS, et al. Blood, PMID in press) suggesting that grafts may interact. It will be of interest in future studies to perform longitudinal assessments to interrogate the effect of new microchimerism acquisition on granulocytes specifically.
While granulocytes have well-appreciated roles in innate immunity, evidence for function in adaptive processes also now exists (reviewed in ref. 13). For example, in conventional inflammatory responses to infectious agents or tissue injury, allogeneic CD66b+ cells as part of the first responders could inadvertently initiate autoimmune processes, offering one potential explanation for reports of microchimerism associations with autoimmunity.14–16 CD66b+ cells may also have a beneficial role in clearing (pre-)cancerous tissues.17–19 After engulfment of allogeneic neutrophils in tumor tissue, dendritic cells may better present cancer antigens to initiate or prime an adaptive immune response similar to graft-versus-tumor observed after hematopoietic cell transplantation. Finally, based on prior reports of male microchimerism commonly identified in hematopoietic cell grafts from parous women, expansion of ‘contaminating’ microchimeric allogeneic CD66b+ cells could participate in graft-versus-host disease after hematopoietic cell transplantation.4
Materials and Methods
Volunteers and specimens.
Women were invited to participate in the study for whom complete pregnancy history was known and who were informative for fetal microchimerism and/or maternal microchimerism after HLA-genotyping was conducted for all family members. A total of 30 women (probands) were studied, 28 of whom were parous and 2 nulliparous. All study subjects provided informed consent and the study protocol was approved by the Fred Hutchinson Cancer Research Center IRB. Women probands were invited to provide a freshly drawn blood specimen collected into acid citrate dextrose solution vacutainer tubes.
Fluorescence activated cell sorting (FACS) enrichment of CD66b+ cells.
Peripheral blood samples were processed within 2.5–3 hours of phlebotomy for the isolation of granulocytes by FACS. Briefly, first whole blood was lysed to eliminate contamination from red blood cells. Remaining cells were rinsed, stained with the granulocyte marker mouse anti-human CD66b-FITC (Biolegend) and followed by FACS (Aria, BD Biosciences). A small aliquot of each specimen's sort was collected to determine purity. Sorted cells were centrifuged and stored as dry pellets for DNA extraction.
Quantitative PCR.
Total genomic DNA from each sort was extracted using a commercially available kit (Promega, Madison, WI USA) and resuspended in water to an approximate final concentration 25 ng/µl. Extracted DNA was then tested employing an appropriate polymorphism-specific quantitative PCR assay.
To be informative for maternal microchimerism required that the proband's mother had a unique HLA allele that differed from the proband's HLA alleles and also differed from the HLA alleles of any children of the proband (so that maternal microchimerism could be distinguished from fetal microchimerism). To be informative for fetal microchimerism required that the fetal paternally-inherited HLA allele differed from both of the proband's HLA alleles and also differed from both HLA alleles of the proband's mother; however, fetal microchimerism could also be tested without this requirement provided the child was a male. Testing was conducted with either an HLA-sequence specific or a Y-chromosome specific quantitative assay. The approach and methods for HLA polymorphism-specific quantitative PCR have previously been described.9 The Y chromosome-specific quantitative PCR assay targets the sequence DYS14 and has also previously been described.10 Each quantitative PCR assay was reviewed in a blinded manner. Results were expressed as the genome equivalent number of microchimeric cells per 100,000 cells equivalents of the subject tested (gEq/105).
Data review and statistical analysis.
Logistic regression analysis was used to compare proportions of women positive for fetal versus maternal microchimerism. Concentrations of fetal versus maternal microchimerism were compared via linear regression on the ranks of microchimerism concentrations. Adjustment for possible correlation between repeated measures from the same subject was conducted in the regression analyses via generalized estimating equations. Models were adjusted for confounding variables, defined by a discrepancy of 10% or more in the estimated coefficient of interest between the multivariable model including the factor and the model without it. The total number of cell equivalents tested, parity and time from last birth were considered but not found to be confounders in the analysis. Analyses were performed using SAS software version 9 (SAS Institute, Inc., Cary, NC). Although HLA-polymorphism specific assays and the DYS14 assay for male DNA were developed to the same sensitivity level the DYS14 PCR assay could be contributed to by other sources including prior miscarriage or elective termination or theoretically cells from an older male sibling. For this reason we also analyzed results limited to testing conducted with HLA-specific quantitative PCR.
Acknowledgements
We thank Dawn Stief for her efforts in subject recruitment and Ara Walline for performing DYS14 quantitative PCR. We also thank the study participants for their time, interest and contributions. This work was supported by NIH grants AI45659 and AI41721.
Abbreviations
- FACS
fluorescence-activated cell sorting
- gEq
genome equivalents
- HLA
human leukocyte antigen
- PCR
polymerase chain reaction
Footnotes
Previously published online: http://www.landesbioscience.com/journals/chimerism/article/13098
References
- 1.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loubiere LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA, et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 2006;86:1185–1192. doi: 10.1038/labinvest.3700471. [DOI] [PubMed] [Google Scholar]
- 4.Adams KM, Lambert NC, Heimfeld S, Tylee TS, Pang JM, Erickson TD, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102:3845–3847. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 5.O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 6.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–393. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 8.Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies. II. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (Dfp) J Clin Invest. 1960;39:1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, et al. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906–914. doi: 10.1002/art.20200. [DOI] [PubMed] [Google Scholar]
- 10.Lambert NC, Lo YM, Erickson TD, Tylee TS, Guthrie KA, Furst DE, et al. Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood. 2002;100:2845–2851. doi: 10.1182/blood-2002-01-0295. [DOI] [PubMed] [Google Scholar]
- 11.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463:495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- 13.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 14.Kremer Hovinga IC, Koopmans M, Baelde HJ, de Heer E, Bruijn JA, Bajema IM. Tissue chimerism in systemic lupus erythematosus is related to injury. Ann Rheum Dis. 2007;66:1568–1573. doi: 10.1136/ard.2007.070516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaizumi M, Pritsker A, Unger P, Davies TF. Intrathyroidal fetal microchimerism in pregnancy and postpartum. Endocrinology. 2002;143:247–253. doi: 10.1210/endo.143.1.8563. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 17.Tomihara K, Guo M, Shin T, Sun X, Ludwig SM, Brumlik MJ, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b+Gr-1+ cells. J Immunol. 2010;184:6151–6160. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 18.Terasawa M, Nagata K, Kobayashi Y. Neutrophils and monocytes transport tumor cell antigens from the peritoneal cavity to secondary lymphoid tissues. Biochem Biophys Res Commun. 2008;377:589–594. doi: 10.1016/j.bbrc.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Buonocore S, Haddou NO, Moore F, Florquin S, Paulart F, Heirman C, et al. Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J Leukoc Biol. 2008;84:713–720. doi: 10.1189/jlb.0108075. [DOI] [PubMed] [Google Scholar]