Abstract
Deliberate establishment of donor-specific immunologic tolerance is considered to be the “Holy Grail” in transplantation medicine, but clinical tolerance protocols for routine organ transplantation are still an unmet need. Mixed hematopoietic chimerism is an attractive tolerance strategy with considerable potential. Recent pilot trials provide proof-of-principle that mixed chimerism can induce tolerance in renal transplant recipients. Routine clinical translation, however, is impeded by the side effects of the cytotoxic recipient conditioning necessary for the transient engraftment of HLA-mismatched BM. In murine studies recently published in The American Journal of Transplantation, we demonstrated that the therapeutic application of polyclonal recipient regulatory T cells (Tregs) leads to engraftment of practicable doses of fully allogeneic BM and to donor-specific tolerance without any cytotoxic conditioning, thereby eliminating a major impediment for the clinical translation of the mixed chimerism strategy in the experimental setting. The background and the implications of these findings are discussed.
Key words: transplantation, tolerance, mixed chimerism, regulatory T cells (Tregs), bone marrow transplantation
Limitations of Current Mixed Chimerism Protocols
Mixed hematopoietic chimerism is achieved through transplantation of donor hematopoietic stem cells (HSC) after appropriate recipient conditioning. The robustness of this approach in the experimental setting1 and its effectiveness in recent clinical pilot trials2–4 underscore its potential. In one of these studies, operational tolerance (i.e., long-term stable graft function without chronic immunosuppression) was achieved in four of five patients simultaneously transplanted with renal and bone marrow (BM) grafts from a haplo-identical living donor.2 While the intentional establishment of clinical tolerance across HLA barriers is arguably a groundbreaking success, safety concerns preclude routine application of the employed BM transplantation (BMT) protocol. Capillary leak syndrome and profound leukopenia due to the extensive cytotoxic conditioning (which involves T-cell and B-cell depletion on top of myelosuppressive drug treatment) are toxicities widely regarded as unacceptable in organ transplant recipients. Thus, despite its proven effectiveness, the mixed chimerism approach has not made it into routine clinical practice due to unresolved safety concerns. Non-cytotoxic mixed chimerism regimens as a potential solution to this problem are therefore an important research goal.
Feasible non-cytotoxic mixed chimerism protocols have, however, remained elusive so far. Numerous attempts by several groups—including our own—have previously failed to achieve engraftment of conventional doses of BM in a non-cytoreductive setting as neither extensive in vivo T cell depletion nor costimulation blockade were sufficiently effective.5–7 Instead the administration of unrealistic ‘mega’ doses of BM was required to achieve irradiation-free mixed chimerism.7,8
Treg Therapy—Potential and Limitations
Recently, the therapeutic exploitation of regulatory T cells (Tregs) has attracted a lot of attention which is largely based on their well established importance in maintaining self tolerance.9,10 Treg therapy has potent effects in autoimmune models.11–14 With regard to transplantation, efficacy of Treg therapy has been demonstrated employing lymphopenic hosts,15,16 Tregs engineered to express a transgenic TCR17,18 and models crossing minor17 or single major18 histocompatibility barriers.19 Importantly, however, no reports have been published to date that would demonstrate that Tregs on their own are capable of inducing skin graft tolerance across full MHC barriers in otherwise unmanipulated recipients with a polyclonal T-cell repertoire. In view of the numerous tolerance models developed over the last decades that have worked in mice but have nevertheless failed in large animal/clinical studies, the extent of clinical hope invested in a tolerogenic therapy that has so far failed to induce robust tolerance in mice is somewhat surprising.
Combining Treg Therapy with the Mixed Chimerism Strategy
Thus, mixed chimerism leads to robust tolerance, but current protocols are too toxic for widespread translation. Treg therapy, on the other hand, is appealing, but insufficiently potent to establish tolerance on its own. We recently joined these two strategies with the aim of developing a tolerance protocol that is both effective and safe.
These studies revealed that the therapeutic application of Tregs leads to engraftment of conventional doses of fully allogeneic BM and donor-specific transplantation tolerance in a murine protocol devoid of cytotoxic recipient treatment (i.e., no irradiation, cytotoxic drugs/mAbs).20 Polyclonal recipient Tregs (∼4 × 106 B6 Tregs/mouse) were co-transplanted with fully mismatched allogeneic donor BM (∼20 × 106 unseparated Balb/c BM cells) into recipients conditioned solely with short-course costimulation blockade (CTLA4Ig, anti-CD40L) and rapamycin (thus employing a relatively costimulation blockade-resistant21 strain combination crossing major and minor histocompatibility barriers). Durable multi-lineage macrochimerism and long-term acceptance of donor (but not 3rd party) skin was achieved with this ‘Treg-chimerism’ protocol. The three tested (polyclonal) Treg populations (FoxP3-transduced Tregs [Foxp3-Tregs], in vitro activated natural CD4+CD25+ Tregs[nTregs] and TGFβ-induced Tregs[iTregs]) turned out to be similarly effective in this model, indicating that this is a robust effect.
Chimerism developed in most recipients of the Treg-chimerism protocol while recipients of the same regimen without Treg administration universally failed to show chimerism (41/51 long-term multi-lineage chimeras with Tregs vs. 0/34 without Tregs, pooled data, p < 0.001). Chimerism levels—albeit rather low—were similarly high as in a previous model transplanting ten (!) times the dose of allogeneic BM (200 × 106) under costimulation blockade alone.7,22 Notably, secondary BMTs transferring BM harvested from Treg-chimeras revealed that donor HSC had engrafted in the primary recipients.
Treg-chimeras accepted donor skin permanently, while promptly rejecting third-party grafts. Immunohistochemical analysis revealed high frequencies of mast cells and FoxP3 positive cells in the tolerated donor grafts. Of note, these intragraft Tregs did not originate from the therapeutically applied Tregs, which we found to have a limited life-span. Thus, the transferred Tregs seem to act in an ‘infectious tolerance-like’ fashion.23,24 Moreover, peripheral and central deletion of donor-reactive T cells, as assessed by following superantigen-reactive T-cell populations, was evident in Treg-chimeras. Deletion was not complete, however, even late after BMT, providing further evidence for an essential role of non-deletional mechanisms in this protocol.
Introduced about twelve years ago, costimulation blockade allowed the elimination of global T-cell depletion from BMT regimens.25–27 As mentioned above, it did not allow chimerism without irradiation, however, as long as conventional doses of BM were transplanted.7,8 Regulation is an important tolerance mechanism in costimulation blockade-based mixed chimerism protocols,28,29 with the extent of contribution depending on the specifics of the protocol.30 Rapamycin had previously been shown to promote BM engraftment.31,32 Rapamycin, however, again did not allow engraftment of conventional doses of BM without recipient irradiation (together with costimulation blockade).33 The present studies revealed that Treg therapy (together with costimulation blockade) needs to be combined with short course rapamycin in order to achieve chimerism without cytoreduction.20 Treg application alone failed to induce chimerism. Whether rapamycin affects Tregs directly,34 or acts through other immunomodulating mechanisms35 is currently unclear.
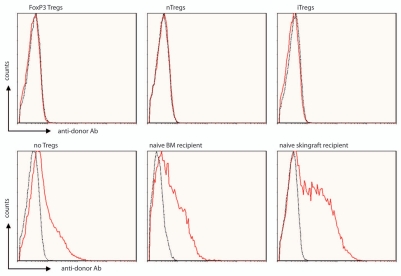
Although tolerance towards the renal allograft was achieved in the aforementioned clinical pilot trial,2 three of four tolerant patients developed de novo donor-specific antibodies with or without intra-graft C4d deposition.36 To date, no evidence for chronic rejection was observed in these patients, but the long-term clinical significance of these findings is unclear. In this regard it is of particular interest that Treg-chimeras completely lacked donor-specific antibodies even after skin grafting.20 In contrast, recipients treated with the same regimen without Tregs developed substantial levels of donor-specific antibodies despite costimulation blockade and rapamycin treatment (Fig. 1).
Figure 1.
Non-cytotoxic Treg-chimerism protocol leads to humoral tolerance. Serum was analyzed for the presence of anti-donor antibodies by flow cytometry. Treg-chimeras (nTregs, iTregs, FoxP3 Tregs, ∼3 months post-BMT, i.e., ∼1–2 months post skin grafting) failed to develop detectable levels of anti-donor antibodies, whereas BMT recipients without Treg treatment (but receiving BM, costimulation blockade and rapamycin) and naïve control mice transplanted with either Balb/c BM or skin (without any treatment) developed substantial antibody levels (6 weeks post transplantation). The reactivity of sera with syngeneic (B6; dotted black line) and donor (Balb/c; red thick line) thymocytes was analyzed by flow cytometry through indirect staining with anti-mouse IgG. Histograms for representative mice are shown.
Conclusion
Application of polyclonal recipient Tregs leads to engraftment of conventional doses of fully allogeneic BM in recipients conditioned solely with costimulation blockade and rapamycin. Robust donor-specific transplantation tolerance ensues. Thus, Treg therapy is uniquely effective in allowing the induction of lasting chimerism and tolerance in a stringent mouse model without cytotoxic recipient treatment. This non-cytotoxic mixed chimerism protocol offers hope that the development of non-toxic clinical tolerance protocols will eventually be achievable.
Acknowledgements
Work described herein was supported by the Austrian Science Fund (FWF, F2310 to Thomas Wekerle). We thank Haley Ramsey, MSc., for providing control data for Figure 1.
Footnotes
Previously published online: http://www.landesbioscience.com/journals/chimerism/article/12964
References
- 1.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–370. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118–1135. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 5.Wekerle T, Nikolic B, Pearson DA, Swenson KG, Sykes M. Minimal conditioning required in a murine model of T cell depletion, thymic irradiation and high-dose bone marrow transplantation for the induction of mixed chimerism and tolerance. Transpl Int. 2002;15:248–253. doi: 10.1007/s00147-002-0411-3. [DOI] [PubMed] [Google Scholar]
- 6.Sykes M, Szot GL, Swenson K, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nature Med. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 7.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 8.Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, et al. Administration of anti-CD40 ligand and donor bone marrow leads to hematopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000;165:1–4. doi: 10.4049/jimmunol.165.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 11.Jaeckel E, von Boehmer H, Manns M. Antigen-specific Foxp3 transduced T cells can control established Type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 12.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 13.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGFβ-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-β and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 15.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 16.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai JG, Xue SA, Coe D, Addey C, Bartok I, Scott D, et al. Regulatory T cells, derived from naive CD4+CD25− T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation. 2005;79:1310–1316. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 18.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battaglia M. Potential T regulatory cell therapy in transplantation: how far have we come and how far can we go? Transpl Int. 2010;23:761–770. doi: 10.1111/j.1432-2277.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 20.Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transpl. 2010;10:751–762. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams MA, Trambley J, Ha J, Adams AB, Durham MM, Rees P, et al. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165:6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 22.Wekerle T, Blaha P, Langer F, Schmid M, Muehlbacher F. Tolerance through bone marrow transplantation with costimulation blockade. Transpl Immunol. 2002;9:125–133. doi: 10.1016/s0966-3274(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 23.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 24.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PA, Lees CJ, Waldmann H, Noelle RJ, Blazar BR. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood. 2001;98:467–474. doi: 10.1182/blood.v98.2.467. [DOI] [PubMed] [Google Scholar]
- 27.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, et al. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a nonmyeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Bigenzahn S, Blaha P, Koporc Z, Pree I, Selzer E, Bergmeister H, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 29.Domenig C, Sanchez-Fueyo A, Kurtz J, Alexopoulos SP, Mariat C, Sykes M, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol. 2005;175:51–60. doi: 10.4049/jimmunol.175.1.51. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz J, Wekerle T, Sykes M. Tolerance in mixed chimerism—a role for regulatory cells? Trends Immunol. 2004;25:518–523. doi: 10.1016/j.it.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PA, Lees CJ, Wilson JM, Ehrhardt MJ, Campbell MT, Noelle RJ, et al. Combined effects of calcineurin inhibitors or sirolimus with anti-CD40L mAb on alloengraftment under nonmyeloablative conditions. Blood. 2002;100:3400–3407. doi: 10.1182/blood-2002-03-0872. [DOI] [PubMed] [Google Scholar]
- 33.Blaha P, Bigenzahn S, Koporc Z, Sykes M, Muehlbacher F, Wekerle T. Short-term immunosuppression facilitates induction of mixed chimerism and tolerance after bone marrow transplantation without cytoreductive conditioning. Transplantation. 2005;80:237–243. doi: 10.1097/01.tp.0000164510.25625.70. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 35.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcheray F, Wong W, Saidman SL, De Vito J, Girouard TC, Chittenden M, et al. B-cell immunity in the context of T-cell tolerance after combined kidney and bone marrow transplantation in humans. Am J Transplant. 2009;9:2126–2135. doi: 10.1111/j.1600-6143.2009.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]