Abstract
The cell nucleus communicates with the rest of the cell via nucleo/cytoplasmic transport of proteins and RNA through the nuclear pores. Direct mechanical links between the nucleus and the cytoplasm have recently emerged in the form of LINC (Linkers of the nucleoskeleton to the cytoskeleton) protein complexes. A LINC complex consists of four components. At its core are an inner nuclear membrane (INM) transmembrane protein and an outer nuclear membrane (ONM) transmembrane protein which physically interact with each other in the lumen of the NE. The INM LINC component interacts on the nucleoplasmic side with either the lamina or with an INM-associated protein. The ONM LINC component on the other hand contacts on the cytoplasmatic side a component of the cytoskeleton. This review highlights the components of LINC complexes and their emerging roles in mechanotransduction, nuclear migration, chromosome positioning, signaling, meiosis, cytoskeletal organization and human disease.
Key words: LINC, lamins A/C, LMNA, SUN, nesprins, EDMD
Introduction
In 1994, mutations in a newly discovered gene, EMD, coding for a protein called emerin, were shown to be responsible for the X-linked Emery-Dreifuss muscular dystrophy (XL-EDMD),1 a disease characterized by early contractures of the elbows, Achilles tendons and spine, slowly-progressive muscle wasting and cardiac conduction defects.2 Since emerin contained a C-terminus hydrophobic helix and because mutations responsible for several muscular dystrophies had previously been found in genes coding for sarcolemmal proteins, this transmembrane protein was expected to be another component of a plasma membrane protein complex physically linking the extracellular matrix to the cytoskeleton.1,3 Surprisingly, emerin turned out to be located in the inner membrane of the nuclear envelope. The unexpected location of the disease-causing protein was puzzling and raised the question of how mutations in a ubiquitously expressed nuclear envelope component could lead to heart and skeletal muscle defects.4,5
Since then, several nuclear envelope components have been linked to various tissue-specific human diseases. Most prominently, mutations in the LMNA gene, encoding the intermediate filament proteins lamins A and C, have been associated with a wide variety of tissue-specific human diseases affecting cardiac and skeletal muscle, neurons or adipocytes or other tissues. These diseases are collectively referred to as “laminopathies.”6 The functions of lamins A and C are not completely understood but they play important roles in general nuclear morphology,7 DNA replication,8 RNA transcription,8 cell cycle regulation,9 cell differentiation and apoptosis.10 These observations suggest that the pathophysiological mechanism(s) of laminopathies may be linked to nuclear-specific processes. However the mechanisms by which lamin mutations disrupt these various specific pathways are largely unknown.
Several non-exclusive hypotheses have been proposed to explain how mutations in nuclear envelope components may lead to human disease. The “gene regulation hypothesis” proposes that the interactions between tissue-specific transcription factors or chromatin and the nuclear lamina are altered by defective nuclear envelope components leading to gene misexpression, whereas the “structural hypothesis” suggests that lamin mutations lead to increased nuclear fragility and eventual nuclear disruption in response to mechanical stress.11 Another hypothesis suggests that defects in the nuclear lamina result in aberrant signaling events and negatively affect nuclear functions.12,13
Recent findings point to an entirely different possibility. The discovery that mutations in cytoskeleton components have similar EDMD pathological phenotype as mutations in emerin and lamins suggested the existence of direct physical interactions between the nuclear lamina and the cytoskeleton. Such physical connections between the nuclear interior and the cytoskeleton have now been confirmed and characterized. They are established by the LINC complex (linkers of the nucleoskeleton to the cytoskeleton). In this review, we highlight the emerging important physiological functions of LINC complexes and we discuss how mutations in LINC components may lead to human diseases by affecting the cellular organization and function not just in the nucleus but throughout the cell.
The Nuclear Envelope and the Lamina
The nuclear envelope (NE) is composed of two membranes, the inner and the outer nuclear membranes (INM and ONM, respectively) (Fig. 1). These membranes constitute a physical barrier between the cytoplasm and the nucleoplasm in eukaryotic cells. The ONM is continuous with the endoplasmic reticulum (ER) and shares many of its proteins, whereas the INM additionally contains numerous specific integral membrane proteins including LAP2 (lamina-associated polypeptide 2), Emerin and MAN1, the founding members of the LEM-domain family of proteins.15,39,40 The INM is lined with a ∼10 nm thick protein network called the nuclear lamina consisting largely of the A- and B-type lamin intermediate filament proteins. A- and B-type lamins contain a globular N-terminal domain separated from a larger C-terminal globular domain by a central helical rod domain. Lamins form parallel coiled-coiled dimers, which then assemble into head-to-tail dimers, tetrameric protofilaments and finally higher order networks covering the INM surface.16 A-type lamins include lamins A, C, AΔ10 and C2, which are alternatively spliced products of the same precursor gene, LMNA, which is developmentally regulated and expressed in differentiated cells and tissues, but absent from embryonic stem cells.17–22 B-type lamins are constitutively expressed within all embryonic and somatic tissues23 and are either products of the LMNB1 gene, encoding lamin B1,24 or LMNB2, encoding lamins B2 and B3.18,25
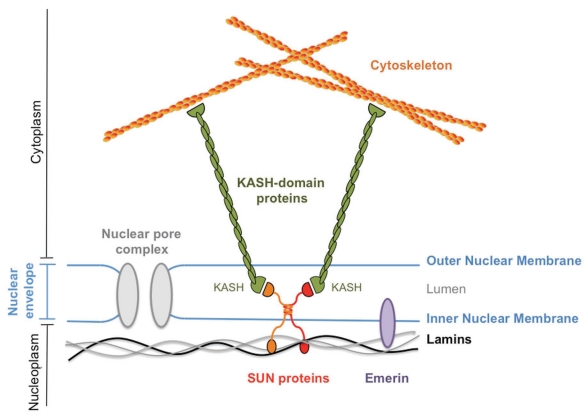
Figure 1.
Prototypical LINC complex. A LINC complex contains four general components. At its core are a SUN-domain transmembrane protein located in the INM and a KASH-domain protein located in the ONM which physically interact with each other in the lumen of the NE. On the nucleoplasmic side, SUN-domain proteins (red and orange) interact with the lamina (black and grey) and with INM-associated proteins such as emerin (purple). On the cytoplasmatic side, the KASH-domain protein (green) contacts a component of the cytoskeleton. LINC complexes constitute physical bridges between the nuclear lamina and the cytoskeleton.
A- and B-type lamins interact directly with several transmembrane INM proteins such as the lamin B receptor (LBR),26 lamin-associated peptides 1 and 2 (LAP1 and -2),27 MAN1,28 and emerin.1 Emerin is a 254 amino acid serine-rich protein containing regions of homology with thymopoietins and a transmembrane domain at its C-terminus.1,29 Emerin is encoded by the EMD gene (also called STA) located on the X-chromosome and is thought to be ubiquitously expressed.1 Emerin is an integral membrane protein located at the INM4,30 where it interacts with nuclear A- and B-type lamins (Fig. 1).31,32 Emerin staining was also reported at the intercalated disks in heart and cultured cardiomyocytes, providing an attractive explanation for the cardiac conduction defects in EDMD,33 however, this observation was not supported by more extensive studies.5,34 Most disease- inducing mutations are nonsense mutations giving rise to truncated and/or unstable emerin proteins, that are degraded or mis localized to the cytoplasm.4,30,35–38
The LINC Complexes: Versatile Connectors of the Nuclear Lamina with the Cytoskeleton
LINC complexes physically connect the nuclear interior with the cytoskeleton (Fig. 1). A LINC complex contains four general components. At its core are an INM transmembrane protein and an ONM transmembrane protein which physically interact with each other in the lumen of the NE. The INM LINC component interacts on the nucleoplasmic side with either the lamina or with an INM-associated protein. The ONM LINC component on the other hand contacts on the cytoplasmatic side a component of the cytoskeleton (Fig. 1).
INM components of LINC complexes.
The prototypical INM components of LINC complexes are the SUN proteins. Mammalian SUN1 and SUN2 proteins were first identified by bioinformatic analysis as homologues of C. elegans UNC-84 and later confirmed in screens for NE components.39–41 The SUN domain was identified based on shared homology between Sad1 in Schizosaccharomyces pombe and UNC-84 in Caenorhabditis elegans.41 The SUN domain is evolutionarily highly conserved from plants to mammals: Sad1 in S. pombe;42 Mps3 in S. cerevisiae;43 Unc-84 and SUN1/MTF-1 (Matefin) in C. elegans;41 Klaroid and Giacomo in D. melanogaster44 and OzSAD1 in rice45 suggesting this family of proteins has crucial nuclear and cellular roles. To date, several SUN domain proteins have been identified in mammals including SUN1, SUN2, SUN3, SPAG4 and SPAG4L (also called TSARG4).46 While SUN1 and 2 are widely expressed SUN3 expression seems to be restricted to testes and its localization limited to the ER.47 Similarly, SPAG4 is only expressed in spermatids, pancreas and testes.48
All SUN proteins are conserved type-II INM proteins and contain at least one transmembrane domain and a C-terminal SUN domain localized inside the lumen of the NE (Fig. 1).49,50 SUN1 and SUN2 contain three putative transmembrane domains and are localized in the INM47,51 with their C-terminal SUN domain spanning the periplasmic space between the INM and ONM.51,52 They also contain coiled-coiled domains which have been predicted to form SUN1 and SUN2 homo- and heterodimers,47,53 whereas they can interact directly with A-type lamins via their N-terminal domain (Fig. 1).47,52,54
ONM components of LINC complexes.
The prototypical ONM components of the LINC complexes are the nesprins (Fig. 1). In mammals, nesprins (nuclear envelope spectrin repeat, also called Syne, synaptic nuclear envelope, Myne, myocyte nuclear envelope, or NUANCE) constitute a large family of spectrin repeat (SR) transmembrane proteins.55 Multiple nesprin isoforms, are produced by alternative splicing and transcription initiation of four independent genes.56–59
Nesprin-1 and -2 bind to the actin cytoskeleton via their N-terminal actin-binding domains, whereas their KASH (Klarsicht/ANC-1/Syne homologue) and transmembrane domains mediate their localization to the NE (Fig. 1).52,60 The KASH domains of nesprin-1 and -2 directly bind to SUN domain proteins at the INM stabilizing their interaction with the inner NE.47,52,54 The N- and C-terminal domains are separated by spectrin-repeat domains which, in the largest nesprin-1 and -2 isoforms, are predicted to form helical conformations that extend up to 500 nm from the NE.46
A short nesprin isoform, called nesprin-1a (also Syne-1,61 or Myne-1,62) is preferentially expressed in skeletal and cardiac muscles. Although it contains the KASH and transmembrane domains characteristic of the family, nesprin-1α lacks the actin-binding domain and is located in the INM where it homodimerizes and interacts with both emerin and lamins A/C.62,63 In parallel with SUN proteins, KASH domain proteins are evolutionarily conserved and include ZYG-12,64 UNC-83,65 ANC-1,66 and KDP-1,59 in C. elegans and Klarsicht and Msp-300 in D. melanogaster.67,68
LINC complex variability.
By interacting with both the cytoskeleton components and with the SUN domain proteins at the NE, nesprins physically link the nucleus to the cytoskeleton, whereas the SUN domain proteins bind directly to lamins and interact, directly or indirectly, with INM proteins thus establishing a continuous physical link between the cytoskeleton and the nuclear lamina.49,56 The disruption of SUN-KASH interactions leads to the enlargement of the lumenal space between ONM and INM suggesting these associations regulate the size of NE lumen.47
In Caenorhabditis elegans, where they were initially characterized, the major LINC complexes are composed of ANC-1, a nesprin-1 homologue, UNC-83, a nesprin-related protein, UNC-84, a founding member of the SUN domain proteins, and Ce-lamin (Fig. 2A and B).66,69 Another specialized LINC complex formed by ZYG-12 and SUN-1/MTF-1 (Matefin) has been shown to be crucial for centrosome coupling to the nucleus (Fig. 2C).64 In mammals, the LINC complexes include nesprin proteins in the ONM, SUN domain proteins and emerin in the INM and lamins in the nuclear lamina.47,49
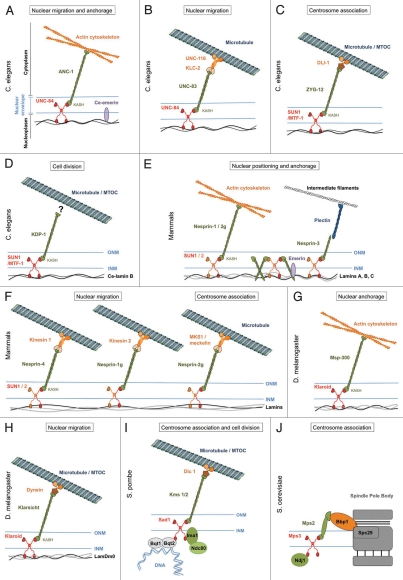
Figure 2.
Diversity of organization and cellular functions of LINC complexes. Examples of LINC complexes from S. pombe (I), S. cerevisiae (J), C. elegans (A to D), D. melanogaster (G and H) and mammals (E and F) and their roles in cell division (D, I and J), nucleus/centrosome association (C, F, I and J) or nuclear migration and anchorage (A, B and E–H).
The large number of protein isoforms and variants of both INM and ONM components of LINC complexes suggests tissue- or developmentally-specific interactions can take place and raises the question of how LINC complexes form. In C. elegans, UNC-84 requires Ce-lamin to be located at the NE but, in contrast, the envelope localization of Ce-lamin, Ce-emerin, Ce-MAN1 and nucleoporins are unaffected by the loss of UNC-84 (Fig. 2A).70 Similarly, in mammals, SUN2 localization in the INM is disrupted in Lmna−/− fibroblasts47 whereas SUN1 localization to the NE is unaffected suggesting that A-type lamins are only necessary to retain SUN2 proteins in the NE. Confirming cell culture observations, SUN2 is mislocalized in synaptic nuclei in Lmna−/− and LmnaH222P/H222P animals, two mouse models of EDMD, whereas SUN1 distribution is not altered in Lmna mutant muscles. However, SUN2 localization is also not affected in extrasynaptic nuclei, suggesting that SUN1 or another NE component compensates for Lmna mutation.71 Instead, SUN1 is implicated in the organization of the nuclear pore complexes72 and requires the presence of nesprins in the ONM.47,52,54
SUN domain proteins are essential for recruitment of KASH domain proteins at the ONM. In C. elegans, both ANC-1 and UNC-83 bind directly to the SUN domain of UNC-84 but fail to be recruited to the ONM in UNC-84 mutant suggesting that UNC-84 is required for their proper ONM localization.65,66,69 Similarly, ZYG-12 requires SUN-1/MTF-1 for its NE localization.64 In mammals, SUN1 or SUN2 inactivation by siRNA leads to the loss of Nesprin-2 at the ONM,47,52 whereas the expression of a recombinant SUN domain of either SUN1 or SUN2 in the ER lumen leads to the displacement of endogenous nesprins from the NE to the ER.47,52,73 This recruitment is mediated by the interaction between SUN and KASH domains as overexpression of the KASH domain of either nesprin-1, -2 or -3 in cells47,52,73 or in animals74 induces the same relocalization of endogenous nesprins. In Lmna−/− and LmnaH222P/H222P muscles, Syne-1 (nesprin-1) is also lost from synaptic nuclei NE following SUN2 mislocalization. However, it is not affected in extrasynaptic nuclei where both SUN1 and SUN2 are normally present.71 Finally, in D. melanogaster, Klaroid is required for the ONM localization of Klarsicht and Msp-300.44,75 These observations illustrate the central role of LINC complexes in connecting the nuclear lamina, the INM and the ONM, making them attractive candidates to explain the tissue specific defects observed in laminopathies.
Cellular Functions of LINC Complexes
By bridging the nuclear lamina with the cytoskeleton, LINC complexes play key roles in many crucial cellular functions including cellular division, cytoskeleton organization and organelle positioning.
LINC complexes in cell division.
During cellular division, the genome undergoes profound rearrangements involving components of both the NE, such as LAP2α or emerin76 and the cytoskeleton. By physically linking the nuclear lamina to the cytoskeleton, LINC complexes play an active role in both mitosis and meiosis (Fig. 2).
During yeast prophase, shortly after chromosomes condensation, telomeres reposition and cluster in close proximity to the spindle pole body (SPB) forming a “chromosomal bouquet.”77 In S. pombe, Sad1, the SUN protein homologue, has been shown to directly interact with the meiotic-specific bouquet (Bqt) proteins 1 and 2 and to colocalize with chromosomal telomeres (Fig. 2I).78 Sad1 also interacts with Kms1,79 a component of the SPB, itself interacting with Dlc1, a homologue of the 14-kD dynein light chain,80 suggesting that Sad1 links chromosomal telomeres to the SPB and the meiotic cytoskeleton (Fig. 2I).78 In a similar way, the meiotic telomere protein Ndj1 mediates telomere attachment to the NE in S. cerevisiae and interacts with the SUN domain protein Mps3 via their respective N terminal region.81 Mps3 is also required for telomere anchoring at the NE during mitosis (Fig. 2J).82
In C. elegans, SUN1/MTF-1 mutations have been associated with defective homologue pairing.83,84 In the germline, SUN1 is required for the recruitment of KDP-1 (KASH domain protein-1), a third recently identified KASH domain protein.85 Inactivation of either Sun1 or Kdp-1 by RNAi leads to delayed entry into mitosis. Kdp-1 is also required for meiotic and mitotic cell cycle progression suggesting that the SUN1/KDP-1 LINC complex plays a crucial role in cell division (Fig. 2D).85
In mammals, both A-type lamins and SUN proteins appear to play roles in telomere positioning and dynamics. Telomeres positioning at the nuclear periphery occurs in a A-type lamin dependent manner86,87 and loss of A-type lamins alters the nuclear distribution of telomeres and results in telomere shortening, defects in telomeric heterochromatin, and increased genomic instability.88 The interaction between telomeres and the NE may involve LINC complex components since Sun1 colocalizes with telomeres between leptotene and diplotene.89 Furthermore, telomere association with the NE as well as homologue pairing and synapsis are deficient in Sun1−/− mice leading to sterility.89 Sun2 telomeric localization is more controversial since the initial observations in mouse and rat spermatocytes,90 could not be reproduced by others groups89,91 and because Sun2−/− mice do not show any fertility defects.91
Centrosome association with the nucleus.
In interphase, LINC complexes mediate the interactions of cellular organelles with the NE and particularly the attachment of the centrosome to the nucleus. In C. elegans embryos, the KASH domain protein ZYG-12 is recruited to the NE by SUN1/MTF-1 and mediates the coupling between the nucleus and the centrosome.64 Mutations of either ZYG-12 or SUN1/MTF-1 lead to uncoupling of the centrosome and nucleus (Fig. 2C).49,64,84
LINC complexes are also implicated in the interaction between the nucleus and the microtubule-organizing center (MTOC) in yeast (Fig. 2I and J). In S. pombe, the physical connection between the SPB and centromeric chromatin involves the interaction between Sad1 and KASH proteins Kms1 and Kms2 whereas the interaction between Sad1 and the centromeric chromatin is mediated by the INM protein Ima1 and the centromeric complex Ndc80 (Fig. 2I).92 In S. cerevisiae, Mps3 is found in the half bridge, a NE-specialized structure tethering the SPB to the NE.43,93 The interaction between Mps3 and Mps2 is required for the SPB organization. Although Mps2 is not a KASH domain protein homologue, Mps2 binds to the soluble protein Bbp1 which interacts with the core SPB protein Spc29 (Fig. 2J).43,93,94
In mammals, both emerin and A-type lamins have also been shown to play a critical role in the association of the centrosome with the outer NE.95–97 Mouse embryonic fibroblasts from Lmna−/− display an increased distance between the nucleus and the centrosome, and they suffer from deficient centrosome migration and a failure of the centrosome to polarize. Similar defects are observed upon overexpression of A-type lamin mutants in fibroblasts.96,97 Such loss of interaction is mediated by the LINC complexes since nesprin-1 and -2 inactivation leads to similar defects in centrosome migration.98 More particularly, nesprin-2 interacts with meckelin and MKS1, two proteins required for centrosome migration to the apical cell surface during ciliogenesis99 suggesting that nesprins are important for ciliogenesis (Fig. 2F).
Linking the nucleus to the cytoskeleton.
The physical interaction between LINC complexes and cytoskeletal elements, including actin, microtubules and cytoplasmic intermediate filaments, actively influences the cytoskeleton.
One of the main functions of LINC complexes is to transduce mechanicals signals from the plasma membrane to the nucleus. Accordingly, the application of a force to the cytoskeleton or the plasma membrane leads to the deformation of the nucleus and activation of specific mechanosensitive sets of genes.100,101 Importantly, Lmna−/− and Emd−/− fibroblasts exhibit defective mechanotransduction, increased nuclear deformability and impaired viability when subjected to mechanical strain.102–105 In line with a key role of the LINC complex in cytoskeletal-nuclear mechanotransduction, the actin-binding domain of nesprin-2 is crucial for NE morphology of primary dermal fibroblasts and keratinocytes.106 Experimental depolymerization of actin filaments in Cos7 cells induces irregular nuclear shape and a relocalization of nesprin-2 into actin-rich foci (Fig. 2E).58 ANC-1, Msp-300, Nesprin-1 and -2 all bind actin via their N-terminal actin-binding domain (ABD)52,58,66,71 and actin polymerization at the ONM can be inhibited by incubating purified nuclei with anti-nesprin antibodies suggesting that nesprins provide actin-binding sites at the cytoplasmic face of eukaryotic nuclei (Fig. 2A, E and G).107 Nesprin-3, that lacks an ABD, instead binds to the plakin family member plectin which then interacts with the cytoskeletal intermediate filaments (Fig. 2E).57,108 In skeletal and cardiac muscles, nesprins are also localized to the sarcomeric Z-line suggesting that the LINC complexes components participate in the muscle contractile cytoskeleton.55
LINC complexes also appear to contribute to the spatial organization of the perinuclear cytoskeleton as indicated by observations on the organization of the intermediate filament protein desmin. Desmin forms a cytoskeletal scaffold that provides strength and mechanical integrity to myocytes.109 Desmin interacts with a 54 kD lamin B-related-protein110 and is enriched at the neuromuscular junction (NMJ) where it contributes to muscle synaptic nuclei recruitment.111 Desmin−/ muscles present nuclear mispositioning similar to Lmna−/− muscles.71,112,113 Electron microscopy of Lmna−/− cardiomyocytes also shows disorganization and detachment of desmin filaments from the nuclear surface with progressive disruption of the cytoskeletal desmin network114 suggesting that the nuclear lamina contributes to desmin network organization. Lmna−/− NMJs also show a fragmentation of the acetylcholine receptor (AChR) network at the post synaptic membrane, suggesting that the cytoskeleton disorganization extends to the plasma membrane.71 Finally, individuals with mutations in cytoskeletal components as well as LINC components suffer from more severe forms of EDMD.115 Taken together, these observations suggest that the nuclear lamina and LINC components contribute to the organization of the perinuclear cytoskeleton.
LINC complexes in nuclear migration.
Unc-84, the SUN protein homologue in C. elegans, was initially identified in a genetic screen for mutants defective in the anchoring of nuclei within the hypodermal syncitium and in the migration of two distal tip cells of the gonad,41 suggesting a role for LINC complexes in nuclear migration and positioning. Moreover, Unc-83 is primarily expressed in nuclei of migratory cells and Unc-83 mutants have nuclear migration defects.65 Furthermore, mutations in ANC-1 affect nuclear anchorage within hypodermal syncitia.66 Interestingly, overexpression of the ANC-1 actin-binding domain results in the same defects suggesting that nuclear migration involves actin (Fig. 2A).66
Microtubule networks and KASH domain proteins can bind directly to molecular motors implicated in nuclear migration. C. elegans Unc-83 interacts with and recruits KLC-2, the kinesin- 1 light chain, at the NE (Fig. 2B). KLC-2 is thought to act as a cargo adaptor for kinesin-1 and mutations in either KLC-2 or UNC-116, the kinesin 1 heavy chain, cause nuclear migration defects similar to Unc-83 mutants (Fig. 2B).116 In C. elegans gonads, ZYG-12-mediated recruitment of the dynein light intermediate chain DLI-1 to the NE is required for microtubule organization and nuclear positioning (Fig. 2C).117
During Drosophila eye development, R cell nuclei migrate to the apical side of the cell. Both Klarsicht and dynactin (glued) mutants display similar nuclear migration defects suggesting nuclear migration involves the interaction between KASH domain proteins and dynein (Fig. 2H).118,119
In mammals, it was suggested that the interaction between Syne-1 (nesprin-1) and the Kif3B subunit of kinesin-2 could play a role in the transport of membrane to the midbody of cells undergoing cytokinesis (Fig. 2F).120 Moreover, Nesprin-4, a recently described KASH domain protein only expressed in secretory epithelia, also interacts directly with the light chain of kinesin-1 (Fig. 2F). Ectopic overexpression of Nesprin-4 in Hela cells leads to the separation of the centrosome and the nucleus by recruiting kinesin-1 at the NE.59
Taken together, these observations demonstrate a crucial role of LINC complexes in nuclear migration.
Nuclear positioning in muscle fibers.
In mammals, the role of LINC complexes in nuclear positioning has been extensively studied in adult muscle fibers. Mature muscle fibers are multinucleated cells containing hundreds of evenly distributed nuclei, called extrasynaptic nuclei, and 3–8 nuclei aggregated just beneath the neuromuscular junction (NMJ) and called synaptic nuclei (Fig. 3).121
Figure 3.
Neuromuscular junctions defects in LINC mutants. In mammals, mature muscle fibers are multinucleated and between 3–8 muscle nuclei are aggregated beneath the muscle/nerve synapse, also called neuromuscular junction (NMJ). in Lmna−/− and LmnaH222P/H222P muscles, mutations in lamins lead to mislocalization of LINC components (SUN2 in red), mispositioning of synaptic nuclei (dark blue) and ineffective NMJs. Similar loss of synaptic nuclei and abnormal neuromuscular junctions have been observed in Syne1:Syne2 and Sun1:Sun2 mouse double mutants (not represented). In Lmna mutants, extrasynaptic nuclei do not seem to be affected, potentially due to the fact that SUN1 (orange), highly enriched in extrasynaptic nuclei, compensates for Lamins A/C mutations.71
Nesprin-1 is highly expressed in skeletal and heart muscles and particularly enriched in synaptic nuclei where it was identified by two-hybrid as an interactor of MuSK (Muscle Specific Kinase), a crucial organizer of the NMJ.61,122 Overexpression of a dominant negative Syne-1 KASH domain in skeletal muscle leads to the loss of synaptic nuclei and a displacement of the endogenous nesprins.74 Homozygous deletion of the KASH domain of Syne-1 induces an even more severe decrease in synaptic nuclei recruitment but also affects extrasynaptic nuclei suggesting that Syne-1 is crucial for the localization of the muscle nuclei at the NMJ but also plays a role in non synaptic muscle nuclei.123 Although the homozygous deletion of Syne-2 does not seem to affect muscle nuclei anchorage, Syne-1:Syne-2 double knock-outs completely fail to recruit synaptic nuclei to the skeletal muscle NMJ.123 As a consequence, Syne-1:Syne2 double knock-out animals die at birth due to severe respiratory insufficiency. Interestingly, transgenic mice overexpressing Syne-1 KASH domain are viable and display no myopathic phenotype74 suggesting that the role of LINC complexes is not limited to nuclear positioning.
Further evidence for a key role of LINC complexes in nuclear positioning in muscle comes from the finding that disruption of both Sun1 and Sun2 in mice leads to abnormal synaptic nuclei positioning.91 Although Sun2−/− mice display no phenotype and Sun1−/− mice only present a modest decrease in the number of synaptic nuclei, Sun1:Sun2 double knock-outs show a drastic loss of synaptic nuclei and die soon after birth.91 Interestingly, Sun1:Sun2 double KO mice can be rescued by overexpression of Sun1 specifically in neurons suggesting that LINC complexes play a role in both the muscle and the nerve at the NMJ.91
The milder phenotypes observed in homozygous deletions of either one of the SUN or nesprin proteins suggest that different combinatorial LINC complexes form in vivo and that SUN/KASH proteins are partially redundant. In support, Sun1 and Sun2 were shown to be differentially expressed in muscle nuclei, with Sun2 being present in all muscle nuclei and particularly enriched in synaptic nuclei in a way similar to Syne-1, whereas Sun1 is predominantly expressed in extrasynaptic nuclei but hardly detectable in synaptic nuclei.71 Confirming that Sun1 localization does not depend on A-type lamins, Sun1 staining is not affected in Lmna−/− and LmnaH222P/H222P muscles.71,124,125 However, Syne-1 and Sun2 were shown to be mislocalized from the NE to the ER in synaptic Lmna−/− and LmnaH222P/H222P nuclei supporting the notion that Sun2 localization is dependent on A-type lamins in synaptic nuclei and suggesting that Syne-1 recruitment at the synaptic nuclei NE is mediated by Sun2. Surprisingly, Syne-1 and Sun2 distribution was not affected in extrasynaptic nuclei suggesting that Sun1 or other extrasynaptic specific NE components can compensate for the absence of lamins. As a consequence of Syne-1 mislocalization, Lmna−/− and LmnaH222P/H222P muscles display a significant loss of synaptic nuclei but also a severe disorganization of the AChR network at the post-synaptic membrane suggesting that LINC complexes also participate in the NMJ cytoskeleton and plasma membrane organization.71
LINC Complexes and Human Diseases
Since the identification of mutations in the human EMD leading to the X-linked form of Emery-Dreifuss muscular dystrophy (XL-EDMD),1,2,29 several genes encoding NE components have been associated with human diseases (Fig. 4). To date, over 300 mutations have been mapped in ten genes encoding NE proteins (Tables 1–3). In LMNA, more than 200 disease mutations have been mapped (www.umd.be/LMNA/) which cause several human diseases including the autosomal-dominant form of Emery-Dreifuss muscular dystrophy (AD-EDMD),126 Dilated cardiomyopathy with conduction system defects disease (DCM-CD);127 Limb-girdle muscular dystrophy 1B (LGMD1B),128 Dunnigan-type familial partial lipodystrophy (FPLD),129 atypical Werner syndrome,130 Charcot-Marie-Tooth syndrome 2B (CMT2B)131 and Hutchinson-Gilford progeria syndrome (HGPS)132,133 (Table 1). Despite the large spectrum of affected tissues and disease phenotypes, a common factor amongst laminopathies is the presence of muscle defects. More than 80% of LMNA mutations lead to cardiac and/or skeletal muscle pathologies and several mouse models reproducing most of the human diseases phenotypes have been generated providing invaluable tools to investigate these diseases.
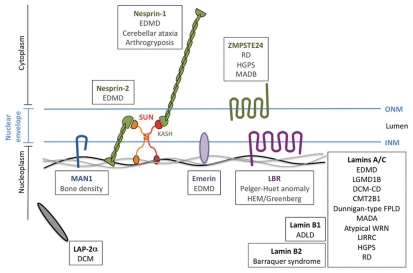
Figure 4.
Nuclear envelope components implicated in human diseases. Mutations in nuclear envelope components have been associated with several human diseases, collectively referred as “envelopathies”. Mutated components can be part of the nuclear lamina [LAP-2α (lamin-associated protein 2a—dark grey) and A- and B-type lamins (black and grey lines)], the inner nuclear membrane [MAN1 (blue), emerin (purple) and LBR (lamin B receptor—dark purple)] or the outer nuclear membrane [Nesprin-1 and -2 (green) and ZMPSTE24 (dark green)]. The details of each of these diseases are provided in Tables 1, 2 and 3.
Table 1.
Human pathologies related to mutations in genes coding for nuclear lamina components
| Human pathology | OMIM* | Major phenotypes | Mutated gene | Key references |
| Autosomal dominant Emery-Dreifuss muscular dystrophy | #181350 | Early joint contractures, slowly progressive muscle weakness and wasting and cardiac involvement | LMNA, encoding lamins A and C | Bonne 1999; Bonne 2000 |
| Autosomal recessive Emery-Dreifuss muscular dystrophy | #604929 | Early joint contractures, slowly progressive muscle weakness and wasting and cardiac involvement | LMNA, encoding lamins A and C | Bonne 2000 |
| Autosomal dominant limb girdle muscular dystrophy 1B | #159001 | Progressive muscle weakness affecting both upper arms and legs | LMNA, encoding lamins A and C | Muchir 2000 |
| Autosomal dominant dilated cardiomyopathy with conduction system defect 1A | #115200 | Conduction system defects including sinus bradycardia, atrioventricular conduction block, or atrial arrhythmias. Variable skeletal muscle involvement. | LMNA, encoding lamins A and C | Fatkin 1999 |
| Autosomal recessive Charcot-Marie-Tooth disorder type 2B1 | #605588 | Axonal peripheral neuropathy characterized by distal muscle weakness and atrophy, mild sensory loss and normal or near-normal nerve conduction velocities | LMNA, encoding lamins A and C | De Sandre-Giovanni 2002 |
| Autosomal dominant Dunnigan-type familial partial lypodystrophy | #151660 | Loss of subcutaneous fat in the lower limbs and the gluteal region. Increase in subcutaneous fat in the face, neck and upper trunk. Insulin resistance and related metabolic complications, such as glucose intolerance, diabetes, dyslipidemia and hepatic steatosis. | LMNA, encoding lamins A and C | Cao and Hegele 2000; Shackleton 2000 |
| Autosomal dominant lipoatrophy with diabetes, hepatic steatosis, hypertrophic cardiomyopathy and leukemelanodermic papules | #608056 | Acquired lipoatrophy with insulin-resistant diabetes, hypertriglyceridemia, hepatic steatosis, hypertrophic cardiomyopathy with valvular involvement and disseminated whitish papules | LMNA, encoding lamins A and C | Caux 2003 |
| Autosomal recessive mandibuloacral dysplasia | #248370 | Postnatal growth retardation, craniofacial anomalies, skeletal malformations and mottled cutaneous pigmentation | LMNA, encoding lamins A and C | Novelli 2002 |
| Autosomal dominant Hutchinson-Gilford progeria syndrome | #176670 | Short stature, alopecia, absence of subcutaneous fat, stiffness of joints, bone changes, musculosketal abnormalities, diabetes type II, severe atherosclerosis | LMNA, encoding lamins A and C | Eriksson 2003; De Sandre-Giovanni 2003 |
| Autosomal dominant atypical Werners's syndrome | #277700 | Loss and graying of hair, hoarseness, and scleroderma-like skin changes, followed by bilateral ocular cataracts, type 2 diabetes mellitus, hypogonadism, skin ulcers and osteoporosis | LMNA, encoding lamins A and C | Chen 2003 |
| Autosomal dominant restrictive dermopathy lethal | #275210 | Intrauterine growth retardation, tight and rigid skin with prominent superficial vessels, bone mineralization defects, dysplastic clavicles, arthrogryposis and early neonatal death | LMNA, encoding lamins A and C | Navarro 2004 |
| LMNA-related congenital muscular dystrophy | Myopathy phenotype detected as early as 1 y of age. Much more rapid course than eDMD. Deficit of head support or axial muscle weakness. | LMNA, encoding lamins A and C | Quijana-roy 2008 | |
| Autosomal dominant leukodystrophy | #169500 | Progressive neurological disorder characterized by symmetrical widespread myelin loss in the central nervous system | LMNB1, encoding lamin B1 | Padiath 2006 |
| Barraquer-Simons syndrome, heterozygous mutations | #608709 | Acquired partial lipodystrophy | LMNB2, encoding lamin B2 | Hegele 2006 |
| Dilated cardiomyopathy | #188380 | Increase in both left ventricular systolic and diastolic diameter and decrease in ejection fraction | LAP2a, encoding Lamin associated protein 2a | Taylor 2005 |
OMIM (Online Mendelian Inheritance in Man) is accessible at: http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM
Table 3.
Human pathologies related to mutations in genes coding for outer nuclear membrane components
| Human pathology | OMIM* | Major phenotypes | Mutated gene | Key references |
| Autosomal dominant restrictive dermopathy lethal | #275210 | Intrauterine growth retardation, tight and rigid skin with prominent superficial vessels, bone mineralization defects, dysplastic clavicles, arthrogryposis and early neonatal death | ZMPSTE24, encoding Zmpste24 | Navarro 2004 |
| Autosomal dominant Hutchinson-Gilford progeria syndrome | #176670 | Short stature, alopecia, absence of subcutaneous fat, stiffness of joints, bone changes, musculosketal abnormalities, diabetes type II, severe atherosclerosis | ZMPSTE24, encoding Zmpste24 | Denecke 2006 |
| Mandibuloacral dysplasia | #608612 | Progeroid syndrome, characterized by mandibular hypoplasia, acroosteolysis affecting distal phalanges and clavicles, delayed closure of the cranial sutures, atrophic skin and lipodystrophy | ZMPSTE24, encoding Zmpste24 | Miyoshi 2008 |
| Autosomal dominant Emery-Dreifuss muscular dystrophy | #181350 | Early joint contractures, slowly progressive muscle weakness and wasting and cardiac involvement | SYNE1, encoding Nesprin-1 | Zhang 2007 |
| Autosomal recessive cerebellar ataxia | #610743 | Significant dysarthria and cerebellar ataxia. Can also include dysmetria, brisk lower-extremity tendon reflexes, and minor abnormalities in ocular saccades and pursuit. | SYNE1, encoding Nesprin-1 | Gros-Louis 2007 |
| Autosomal recessive arthrogryposis | Congenital joint contractures. Bilateral clubfoot, decreased fetal movements, delay in motor milestones, and progressive motor decline. | SYNE1, encoding Nesprin-1 | Attali 2009 | |
| Autosomal dominant Emery-Dreifuss muscular dystrophy | #181350 | Early joint contractures, slowly progressive muscle weakness and wasting and cardiac involvement. | SYNE2, encoding Nesprin-2 | Zhang 2007 |
OMIM (Online Mendelian Inheritance in Man) is accessible at: http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM
Mice lacking the Lmna gene, and thus not expressing either lamin A nor lamin C, die 6–7 weeks after birth due to severe muscular dystrophy and cardiomyopathy, resembling autosomal dominant Emery-Dreifuss muscular dystrophy (AD-EDMD).125 As observed in human EDMD biopsies, Lmna−/− muscle nuclei display hypercondensed chromatin, nuclear fragmentation, nuclear invaginations and intranuclear filaments.134 LmnaH222P/H222P mice carry a missense mutation which was originally identified in a family with AD-EDMD.124 Adult H222P homozygous male mice exhibit several symptoms reminiscent of AD-EDMD patients including reduced locomotion with abnormal stiff walking posture, cardiac fibrosis, chamber dilatation and hypokinesia with conduction defects and typically die by 9 months of age.124
In these two AD-EDMD mouse models, the absence or mutation of A-type lamins leads to the mislocalization of SUN2 and Syne-1 proteins specifically in synaptic nuclei, the mispositioning of synaptic nuclei and the structural disorganization of the NMJ.71 As a consequence, Lmna−/− and LmnaH222P/H222P mice show innervation defects including misexpression of electrical activity-dependent genes and altered epigenetic chromatin modifications.71 Similar hallmarks of functional denervation were observed in AD-EDMD patients demonstrating that defects in neuromuscular junctions are part of the disease mechanism in AD-EDMD.71 These observations suggest a model in which the presence of specific combination of NE components in the LINC complexes determines which nuclei and tissues are affected in the disease. In skeletal muscles, A-type lamin mutations specifically affect LINC complexes organization in synaptic nuclei leading not only to a synaptic nuclei mispositioning but also to the disorganization of the NMJ cytoskeleton and muscle denervation.
The importance of the LINC complexes in EDMD has been confirmed by observations in both animal models and human patients. Disruption of the KASH domain of nesprin-1 in mice leads to an EDMD-like phenotype135 and Sun1:Sun2 and Syne-1:Syne-2 double knock-out mice show similar NMJ defects as Lmna−/− and LmnaH222P/H222P mice71,91,123 suggesting that mutations in other LINC components could also lead to human diseases. In support, mutations in SYNE-1 and SYNE-2 have be found in patients with EDMD phenotype but no mutations in EMD or LMNA14 and nesprin-1α and nesprin-2β interactions with the NE are altered in XL-EDMD patients tissues136 suggesting that mutations in emerin may also lead to defects in LINC complexes organization (Table 2). However, to date, no mutations in SUN genes have been identified in EDMD patients.
Table 2.
Human pathologies related to mutations in genes coding for inner nuclear membrane components
| Human pathology | OMIM* | Major phenotypes | Mutated gene | Key references |
| X-Linked Emery-Dreifuss muscular dystrophy | #310300 | Early contractures of tendons, slowly progressive muscle weakness and wasting, conduction defects in heart | EMD, also called STA, gene encoding emerin | Bione 1994 |
| Autosomal recessive Buschke-Ollendorff syndrome | #166700 | Increased bone density | MAN1 (LEMD3), encoding Man1 | Hellemans 2004 |
| Autosomal recessive melorheostosis, familial and with osteopoikilosis | #155950 | Increased bone density | MAN1 (LEMD3), encoding Man1 | Hellemans 2004 |
| Autosomal recessive Pelger-Huet anomaly | #169400 | Abnormal nuclear shape and chromatin organization in blood granulocytes. Varying degrees of developmental delay, epilepsy and skeletal abnormalities. | LBr, encoding Lamin B Receptor | Hoffman 2002 |
| Autosomal recessive Hydrops-ectopic calcification-moth-eaten skeletal dysplasia (HEM)/Greenberg skeletal dysplasia | #215140 | Abnormal nuclear shape and chromatin organization in blood granulocytes. Varying degrees of developmental delay, epilepsy and skeletal abnormalities. | LBR, encoding Lamin B Receptor | Waterham 2003 |
OMIM (Online Mendelian Inheritance in Man) is accessible at: http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM
Evidence that LINC complex mutations are causal in human diseases comes from recent discoveries. Lmna−/− mice present a reduction in axonal density characterized by the presence of non-myelinated axons resembling a peripheral neuropathy,131 and loss of UNC-83/84 lead to defects in neuronal P-cell and uncoordinated movements.41 Furthermore, it was shown that muscle-restricted inactivation of Lmna by siRNA is sufficient to recapitulate most of the muscle phenotypes in wild-type mice.71 However, the rescue of the Sun1:Sun2 double knock-out mice phenotype by nerve-specific expression of Sun1 suggest that the LINC complexes may also play a role in nerve survival.91
Mutations in LINC components also lead to non-muscle diseases. For example, mutations in human SYNE-1 cause autosomal recessive cerebellar ataxia137 and have been implicated in autosomal recessive arthrogryposis, a disease characterized by multiple joint contractures.138 Heterozygous mutations in lamin B receptor (LBR), a membrane protein that binds lamin B, are responsible for the dominant Helger-Puet anomaly of white cell blood cell nuclear shape; whereas homozygous defects in LBR are linked to bone and cartilage disorders and developmental delays (Tables 2 and 3).139 Finally, nesprin-2 overexpression can compensate some progeria phenotype in LMNAS143F mutant cells.140
Concluding Remarks
Our understanding of the nuclear lamina and its cellular functions has fundamentally changed in the last 15 years since the surprising discovery linking emerin to EDMD. The importance of the nuclear lamina as a platform for genome organization, signaling and gene regulation has been confirmed by its multiple roles in DNA replication, RNA transcription,8 cell cycle regulation,9 cell differentiation and apoptosis.10 Its complexity has been demonstrated by the identification of a large number of NE-associated and interacting proteins.39,40 While long thought to have purely nuclear functions, the identification of LINC complexes that connect the lamina with the cytoskeleton has dramatically expanded the functional role of the lamina, by physically and functionally linking the nuclear interior with the rest of the cell.47 This knowledge has led to the identification of new genes, like SYNE-1 and SYNE-2, as origins and potential therapeutic targets of human diseases.14,137,138,140
Although the importance of LINC complexes tends to reinforce the “structural hypothesis” proposed to explain laminopathies, the transcriptional activation of genes in response to mechanical stimuli and NE component suggests that the structural and gene expression hypothesis are not mutually exclusive and are in all likelihood closely related. The identification of LINC complexes represents a major step forward in our conceptual understanding of the NE and sheds new light on the functional interdependence of the nucleus and the rest of the cell.
Abbreviations
- RNA
ribonucleic acid
- LINC
linkers of the nucleoskeleton to the cytoskeleton
- EDMD
Emery-Dreifuss muscular dystrophy
- DNA
deoxyribonucleic acid
- NE
nuclear envelope
- INM
inner nuclear membrane
- ONM
outer nuclear membrane
- SPB
spindle pole body
- MTOC
microtubule-organizing center
- ABD
actin-binding domain
- NMJ
neuromuscular junction
- AChR
acetylcholine receptor
- DCM-CD
dilated cardiomyopathy with conduction system defects
- LGMD1B
limb-girdle muscular dystrophy 1B
- FPLD
Dunnigan-type familial partial lipodystrophy
- CMT2B
Charcot-Marie-Tooth syndrome 2B
- HGPS
Hutchinson-Gilford progeria syndrome
- LBR
lamin B receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/10530
References
- 1.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:39–41. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 2.Emery AE, Dreifuss FE. Unusual type of benign x-linked muscular dystrophy. J Neurol Neurosurg Psychiatry. 1966;29:338–342. doi: 10.1136/jnnp.29.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum Mol Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- 5.Yorifuji H, Tadano Y, Tsuchiya Y, Ogawa M, Goto K, Umetani A, et al. Emerin, deficiency of which causes Emery-Dreifuss muscular dystrophy, is localized at the inner nuclear membrane. Neurogenetics. 1997;1:135–140. doi: 10.1007/s100480050020. [DOI] [PubMed] [Google Scholar]
- 6.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. Bioessays. 2008;30:226–236. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- 8.Chakalova L, Debrand E, Mitchell JA, Osborne CS, Fraser P. Replication and transcription: shaping the landscape of the genome. Nat Rev Genet. 2005;6:669–677. doi: 10.1038/nrg1673. [DOI] [PubMed] [Google Scholar]
- 9.Zastrow MS, Vlcek S, Wilson KL. Proteins that bind A-type lamins: integrating isolated clues. J Cell Sci. 2004;117:979–987. doi: 10.1242/jcs.01102. [DOI] [PubMed] [Google Scholar]
- 10.Morris GE. Nuclear proteins and cell death in inherited neuromuscular disease. Neuromuscul Disord. 2000;10:217–227. doi: 10.1016/s0960-8966(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 11.Burke B, Stewart CL. Life at the edge: the nuclear envelope and human disease. Nat Rev Mol Cell Biol. 2002;3:575–585. doi: 10.1038/nrm879. [DOI] [PubMed] [Google Scholar]
- 12.Muchir A, Pavlidis P, Bonne G, Hayashi YK, Worman HJ. Activation of MAPK in hearts of EMD null mice: similarities between mouse models of X-linked and autosomal dominant Emery Dreifuss muscular dystrophy. Hum Mol Genet. 2007;16:1884–1895. doi: 10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- 13.Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, et al. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 15.Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, et al. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386:1392–1402. doi: 10.1016/j.jmb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, et al. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–3947. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biamonti G, Giacca M, Perini G, Contreas G, Zentilin L, Weighardt F, et al. The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol Cell Biol. 1992;12:3499–3506. doi: 10.1128/mcb.12.8.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 20.Furukawa K, Inagaki H, Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp Cell Res. 1994;212:426–430. doi: 10.1006/excr.1994.1164. [DOI] [PubMed] [Google Scholar]
- 21.Machiels BM, Zorenc AH, Endert JM, Kuijpers HJ, van Eys GJ, Ramaekers FC, et al. An alternative splicing product of the lamin A/C gene lacks exon 10. J Biol Chem. 1996;271:9249–9253. doi: 10.1074/jbc.271.16.9249. [DOI] [PubMed] [Google Scholar]
- 22.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 23.Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, et al. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–517. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- 24.Lin F, Worman HJ. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995;27:230–236. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993;12:97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worman HJ, Yuan J, Blobel G, Georgatos SD. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci USA. 1988;85:8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993;73:1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- 28.Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, et al. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- 29.Bione S, Small K, Aksmanovic VM, D'Urso M, Ciccodicola A, Merlini L, et al. Identification of new mutations in the Emery-Dreifuss muscular dystrophy gene and evidence for genetic heterogeneity of the disease. Hum Mol Genet. 1995;4:1859–1863. doi: 10.1093/hmg/4.10.1859. [DOI] [PubMed] [Google Scholar]
- 30.Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, et al. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat Genet. 1996;12:254–259. doi: 10.1038/ng0396-254. [DOI] [PubMed] [Google Scholar]
- 31.Manilal S, Nguyen TM, Morris GE. Colocalization of emerin and lamins in interphase nuclei and changes during mitosis. Biochem Biophys Res Commun. 1998;249:643–647. doi: 10.1006/bbrc.1998.9209. [DOI] [PubMed] [Google Scholar]
- 32.Clements L, Manilal S, Love DR, Morris GE. Direct interaction between emerin and lamin A. Biochem Biophys Res Commun. 2000;267:709–714. doi: 10.1006/bbrc.1999.2023. [DOI] [PubMed] [Google Scholar]
- 33.Cartegni L, di Barletta MR, Barresi R, Squarzoni S, Sabatelli P, Maraldi N, et al. Heart-specific localization of emerin: new insights into Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 1997;6:2257–2264. doi: 10.1093/hmg/6.13.2257. [DOI] [PubMed] [Google Scholar]
- 34.Manilal S, Sewry CA, Pereboev A, Man N, Gobbi P, Hawkes S, et al. Distribution of emerin and lamins in the heart and implications for Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 1999;8:353–359. doi: 10.1093/hmg/8.2.353. [DOI] [PubMed] [Google Scholar]
- 35.Ellis JA, Craxton M, Yates JR, Kendrick-Jones J. Aberrant intracellular targeting and cell cycle-dependent phosphorylation of emerin contribute to the Emery-Dreifuss muscular dystrophy phenotype. J Cell Sci. 1998;111:781–792. doi: 10.1242/jcs.111.6.781. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, Wehnert M, et al. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- 37.Ellis JA, Yates JR, Kendrick-Jones J, Brown CA. Changes at P183 of emerin weaken its protein-protein interactions resulting in X-linked Emery-Dreifuss muscular dystrophy. Hum Genet. 1999;104:262–268. doi: 10.1007/s004390050946. [DOI] [PubMed] [Google Scholar]
- 38.Manilal S, Recan D, Sewry CA, Hoeltzenbein M, Llense S, Leturcq F, et al. Mutations in Emery-Dreifuss muscular dystrophy and their effects on emerin protein expression. Hum Mol Genet. 1998;7:855–864. doi: 10.1093/hmg/7.5.855. [DOI] [PubMed] [Google Scholar]
- 39.Dreger M, Bengtsson L, Schoneberg T, Otto H, Hucho F. Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci USA. 2001;98:11943–11948. doi: 10.1073/pnas.211201898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 41.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 42.Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaspersen SL, Martin AE, Glazko G, Giddings TH, Jr, Morgan G, Mushegian A, et al. The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J Cell Biol. 2006;174:665–675. doi: 10.1083/jcb.200601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly. 2007;1:75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- 45.Moriguchi K, Suzuki T, Ito Y, Yamazaki Y, Niwa Y, Kurata N. Functional isolation of novel nuclear proteins showing a variety of subnuclear localizations. Plant Cell. 2005;17:389–403. doi: 10.1105/tpc.104.028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starr DA, Fischer JA. KASH 'n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- 47.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol. 1999;211:109–123. doi: 10.1006/dbio.1999.9297. [DOI] [PubMed] [Google Scholar]
- 49.Tzur YB, Wilson KL, Gruenbaum Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol. 2006;7:782–788. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- 50.Worman HJ, Gundersen GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–69. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279:25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 52.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, et al. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Du X, Cai Z, Greene MI. Characterization of the structures involved in localization of the SUN proteins to the nuclear envelope and the centrosome. DNA Cell Biol. 2006;25:554–562. doi: 10.1089/dna.2006.25.554. [DOI] [PubMed] [Google Scholar]
- 54.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, et al. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG. The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics. 2002;80:473–481. [PubMed] [Google Scholar]
- 56.Warren DT, Zhang Q, Weissberg PL, Shanahan CM. Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev Mol Med. 2005;7:1–15. doi: 10.1017/S1462399405009294. [DOI] [PubMed] [Google Scholar]
- 57.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 59.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci USA. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, et al. Nesprin-2 is a multiisomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 61.Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- 62.Mislow JM, Kim MS, Davis DB, McNally EM. Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C. J Cell Sci. 2002;115:61–70. doi: 10.1242/jcs.115.1.61. [DOI] [PubMed] [Google Scholar]
- 63.Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, et al. Nesprin-1alpha selfassociates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- 64.Malone CJ, Misner L, Le Bot N, Tsai MC, Campbell JM, Ahringer J, et al. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell. 2003;115:825–836. doi: 10.1016/s0092-8674(03)00985-1. [DOI] [PubMed] [Google Scholar]
- 65.Starr DA, Hermann GJ, Malone CJ, Fixsen W, Priess JR, Horvitz HR, et al. unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development. 2001;128:5039–5050. doi: 10.1242/dev.128.24.5039. [DOI] [PubMed] [Google Scholar]
- 66.Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- 67.Fischer-Vize JA, Mosley KL. Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development. 1994;120:2609–2618. doi: 10.1242/dev.120.9.2609. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg-Hasson Y, Renert-Pasca M, Volk T. A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech Dev. 1996;60:83–94. doi: 10.1016/s0925-4773(96)00602-8. [DOI] [PubMed] [Google Scholar]
- 69.McGee MD, Rillo R, Anderson AS, Starr DA. UNC-83 IS a KASH protein required for nuclear migration and is recruited to the outer nuclear membrane by a physical interaction with the SUN protein UNC-84. Mol Biol Cell. 2006;17:1790–1801. doi: 10.1091/mbc.E05-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee KK, Starr D, Cohen M, Liu J, Han M, Wilson KL, et al. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol Biol Cell. 2002;13:892–901. doi: 10.1091/mbc.01-06-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mejat A, Decostre V, Li J, Renou L, Kesari A, Hantai D, et al. Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J Cell Biol. 2009;184:31–44. doi: 10.1083/jcb.200811035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, et al. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314:1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grady RM, Starr DA, Ackerman GL, Sanes JR, Han M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc Natl Acad Sci USA. 2005;102:4359–4364. doi: 10.1073/pnas.0500711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Technau M, Roth S. The Drosophila KASH domain proteins Msp-300 and Klarsicht and the SUN domain protein klaroid have no essential function during oogenesis. Fly (Austin) 2008:2. doi: 10.4161/fly.6288. [DOI] [PubMed] [Google Scholar]
- 76.Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, et al. LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J Cell Sci. 2004;117:6117–6128. doi: 10.1242/jcs.01529. [DOI] [PubMed] [Google Scholar]
- 77.Scherthan H. A bouquet makes ends meet. Nat Rev Mol Cell Biol. 2001;2:621–627. doi: 10.1038/35085086. [DOI] [PubMed] [Google Scholar]
- 78.Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 79.Shimanuki M, Miki F, Ding DQ, Chikashige Y, Hiraoka Y, Horio T, et al. A novel fission yeast gene, kms1+, is required for the formation of meiotic prophase-specific nuclear architecture. Mol Gen Genet. 1997;254:238–249. doi: 10.1007/s004380050412. [DOI] [PubMed] [Google Scholar]
- 80.Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics. 2004;270:449–461. doi: 10.1007/s00438-003-0938-8. [DOI] [PubMed] [Google Scholar]
- 81.Conrad MN, Lee CY, Chao G, Shinohara M, Kosaka H, Shinohara A, et al. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3 and CSM4 and is modulated by recombination. Cell. 2008;133:1175–1187. doi: 10.1016/j.cell.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 82.Bupp JM, Martin AE, Stensrud ES, Jaspersen SL. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol. 2007;179:845–854. doi: 10.1083/jcb.200706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fridkin A, Penkner A, Jantsch V, Gruenbaum Y. SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell Mol Life Sci. 2009;66:1518–1533. doi: 10.1007/s00018-008-8713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penkner A, Tang L, Novatchkova M, Ladurner M, Fridkin A, Gruenbaum Y, et al. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev Cell. 2007;12:873–885. doi: 10.1016/j.devcel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 85.McGee MD, Stagljar I, Starr DA. KDP-1 is a nuclear envelope KASH protein required for cell cycle progression. J Cell Sci. 2009;122:2895–2905. doi: 10.1242/jcs.051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ottaviani A, Rival-Gervier S, Boussouar A, Foerster AM, Rondier D, Sacconi S, et al. The D4Z4 macrosatellite repeat acts as a CTCF and A-type laminsdependent insulator in facio-scapulo-humeral dystrophy. PLoS Genet. 2009;5:1000394. doi: 10.1371/journal.pgen.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ottaviani A, Schluth-Bolard C, Rival-Gervier S, Boussouar A, Rondier D, Foerster AM, et al. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. EMBO J. 2009;28:2428–2436. doi: 10.1038/emboj.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci USA. 2007;104:7426–7431. doi: 10.1073/pnas.0609198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci USA. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munoz-Centeno MC, McBratney S, Monterrosa A, Byers B, Mann C, Winey M. Saccharomyces cerevisiae MPS2 encodes a membrane protein localized at the spindle pole body and the nuclear envelope. Mol Biol Cell. 1999;10:2393–2406. doi: 10.1091/mbc.10.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schramm C, Elliott S, Shevchenko A, Schiebel E. The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. EMBO J. 2000;19:421–433. doi: 10.1093/emboj/19.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, Stewart CL, et al. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys J. 2008;95:5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SE, Kim JH, Kim NH. Inactivation of MAPK affects centrosome assembly, but not actin filament assembly, in mouse oocytes maturing in vitro. Mol Reprod Dev. 2007;74:904–911. doi: 10.1002/mrd.20695. [DOI] [PubMed] [Google Scholar]
- 98.Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, et al. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci. 2009;122:2716–2726. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 100.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 102.Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 104.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luke Y, Zaim H, Karakesisoglou I, Jaeger VM, Sellin L, Lu W, et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci. 2008;121:1887–1898. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- 107.Munter S, Enninga J, Vazquez-Martinez R, Delbarre E, David-Watine B, Nehrbass U, et al. Actin polymerisation at the cytoplasmic face of eukaryotic nuclei. BMC Cell Biol. 2006;7:23. doi: 10.1186/1471-2121-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J Cell Sci. 2007;120:3384–3394. doi: 10.1242/jcs.014191. [DOI] [PubMed] [Google Scholar]
- 109.Capetanaki Y, Milner DJ, Weitzer G. Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct. 1997;22:103–116. doi: 10.1247/csf.22.103. [DOI] [PubMed] [Google Scholar]
- 110.Cartaud A, Jasmin BJ, Changeux JP, Cartaud J. Direct involvement of a lamin-B-related (54 kDa) protein in the association of intermediate filaments with the postsynaptic membrane of the Torpedo marmorata electrocyte. J Cell Sci. 1995;108:153–160. doi: 10.1242/jcs.108.1.153. [DOI] [PubMed] [Google Scholar]
- 111.Agbulut O, Li Z, Perie S, Ludosky MA, Paulin D, Cartaud J, et al. Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil Cytoskeleton. 2001;49:51–66. doi: 10.1002/cm.1020. [DOI] [PubMed] [Google Scholar]
- 112.Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, et al. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- 113.Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muntoni F, Bonne G, Goldfarb LG, Mercuri E, Piercy RJ, Burke M, et al. Disease severity in dominant Emery Dreifuss is increased by mutations in both emerin and desmin proteins. Brain. 2006;129:1260–1268. doi: 10.1093/brain/awl062. [DOI] [PubMed] [Google Scholar]
- 116.Meyerzon M, Fridolfsson HN, Ly N, McNally FJ, Starr DA. UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development. 2009;136:2725–2733. doi: 10.1242/dev.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou K, Rolls MM, Hall DH, Malone CJ, Hanna-Rose W. A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad. J Cell Biol. 2009;186:229–241. doi: 10.1083/jcb.200902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- 119.Whited JL, Cassell A, Brouillette M, Garrity PA. Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development. 2004;131:4677–4686. doi: 10.1242/dev.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan J, Beck KA. A role for the spectrin superfamily member Syne-1 and kinesin II in cytokinesis. J Cell Sci. 2004;117:619–629. doi: 10.1242/jcs.00892. [DOI] [PubMed] [Google Scholar]
- 121.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 122.Strochlic L, Cartaud A, Cartaud J. The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions. Bioessays. 2005;27:1129–1135. doi: 10.1002/bies.20305. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, et al. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- 124.Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 125.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bonne G, Di Barletta MR, Varnous S, Becan HM, Hammouda EH, Merlini L, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 127.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 128.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 129.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- 130.Bonne G, Levy N. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:1585–1586. doi: 10.1016/S0140-6736(03)14761-7. [DOI] [PubMed] [Google Scholar]
- 131.De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, et al. Homozygous defects in LMNA, encoding lamin A/C nuclearenvelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–736. doi: 10.1086/339274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 133.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mittelbronn M, Sullivan T, Stewart CL, Bornemann A. Myonuclear degeneration in LMNA null mice. Brain Pathol. 2008;18:338–343. doi: 10.1111/j.1750-3639.2008.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wheeler MA, Davies JD, Zhang Q, Emerson LJ, Hunt J, Shanahan CM, et al. Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp Cell Res. 2007;313:2845–2857. doi: 10.1016/j.yexcr.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 137.Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, et al. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet. 2007;39:80–85. doi: 10.1038/ng1927. [DOI] [PubMed] [Google Scholar]
- 138.Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, et al. Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp290. [DOI] [PubMed] [Google Scholar]
- 139.Hoffmann K, Dreger CK, Olins AL, Olins DE, Shultz LD, Lucke B, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly) Nat Genet. 2002;31:410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 140.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, Jaeger VM, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum Mol Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]