Abstract
Recently, we demonstrated that colonic and adipose expression of SAA3 was modulated by the gut microbiota and Toll-like receptor signaling in mice. We observed that SAA3 was expressed by colonic epithelial cells and that its expression was induced in a murine colonocyte cell line following lipopolysaccharide stimulation and nuclear NFκB translocation. In this addendum, we extend this initial study and suggest that SAA3 (1) resembles human SAA1 both in amino acid homology and tissue distribution, (2) appears to have autocrine or paracrine effects rather than endocrine, and (3) binds to bacteria within the gastrointestinal tract. Although speculative, these observations raise the possibility that SAA3 may promote local inflammation in adipose tissue that affects insulin signaling and also function as an antimicrobial agent in the colon.
Key words: gut microbiota, gnotobiology, serum amyloid A, toll-like receptors, metabolic syndrome, antibacterial
Introduction
Humans evolved in a world dominated by microbes and evidence suggests that microrganisms have inhabited metazoan guts for at least 100 million years.1 Unlike foreign infectious microorganisms, these bacteria have evolved extraordinary capabilities that are vital to human health (e.g., the production of vitamins and xenobiotic metabolism). However, not all microbial functions are beneficial: the gut micro-biota can be considered an environmental factor that promotes obesity and may influence insulin resistance.2–6 These phenotypes could be caused by distinct mechanisms affected by the gut microbiota such as increased energy harvest from the diet, altered lipid metabolism, modulation of enteroendocrine cells, and by increasing the inflammatory tone of host tissues.7,8
Microbial diversity is reduced in obese individuals, especially with respect to members of Bacteroidetes,6 a microbial phenotype also observed in patients with inflammatory bowel disease (IBD).9 Together these observations suggest that the gut microbiota is like a finely tuned organ that, when perturbed (i.e., when diversity is reduced or when deleterious microbes become more abundant), may lead to disease. Animal models of inflammatory IBD indicate that intestinal inflammation is dependent on the presence of the intestinal microbiota,10 suggesting that the normal gut microbiota is an important contributor to IBD. To date, there is little information about microbial regulation of host genes within and outside the intestine. In our original report, we demonstrated that colonic and adipose expression of the acute phase reactant serum amyloid A3 (SAA3) was mediated by the gut microbiota and that intact MyD88 signaling was required for this induction in conventionally-raised mice.11
Microbial Regulation of SAA3 Expression in Adipose Tissue
SAA3 is expressed in adipocytes and several other cell types and its expression is increased in adipose tissue of genetically diabetic (db/db) mice compared to wild type controls.12 In our original report, we selected SAA3 after identifying it as a microbially-regulated gene in a small-scale qRT-PCR screen of adipose tissue.11 Since intraperitoneal injections of purified lipopolysaccharide (LPS) increases adipose expression of SAA3,13 we hypothesized that the gut microbiota may induce signaling through Toll-like receptors (Tlrs), which recognizes conserved microbial structures such as LPS.14 Indeed, we found that SAA3 expression was elevated in adipose tissue from wild-type compared with Myd88-/- mice.11 MyD88 is required for downstream signaling of most Tlrs14 but is also required for IL-1 and IL-18 signaling, thus we cannot exclude the possibility that SAA3 expression was induced by microbial-induction of IL-1 and IL-18.15 Moreover, the gut microbiota modulates serum triglyceride and phosphatidylcholine levels, which may further promote proinflammatory signaling via Tlrs. Because MyD88 is required for intracellular signaling upon stimulation of both microbial and endogenous molecules it is difficult to elucidate the mechanistic impact of specific microbial products in vivo.
SAA3 as a Mediator of Metabolic Inflammation in Adipose Tissue—Functional Similarity with Human SAA1
We showed a significant upregulation of SAA3 but not SAA1 and 2 in adipose tissue from CONV-R compared with GF mice. Comparison of the amino acid sequence alignments of SAA isoforms in mice and humans demonstrated that mouse SAA3 is most similar to the human isoform SAA1 (70% amino acid identity).11 SAA1 and SAA2 expression is restricted to the liver, intestine and kidneys whereas SAA3 is more widely expressed in mouse tissues.13,16 In contrast, SAA1 is expressed in human adipocytes and its expression is increased in hypertrophic adipocytes from obese individuals.17 Thus we speculated that mouse SAA3, which is upregulated in the adipose tissue of obese mice,18 might be functionally similar to human SAA1 and perhaps represent a link between low-grade inflammation and metabolic diseases associated with obesity such as insulin resistance. Further elucidation of cell-specific expression of SAA homologs in mice and humans could help to decipher whether different isoforms have overlapping functions in different mammalian species. Development of tissue-specific knockout mouse lines could be an important tool in investigating the specific roles of SAAs in intestinal tissues, macrophages or adipocytes.
Microbial Regulation of SAA3 Expression in the Colon
SAA3 expression is associated with increased microbial colonization (i.e., highest intestinal expression is found in the microbe-rich colon) and we observed strong microbial induction of SAA3 in the colon.11 Immunohistochemical analysis confirmed macrophages and epithelial cells were cellular sources of SAA3. Stimulating cultured colonocytes with LPS revealed that microbial components can directly induce SAA3 expression, likely via a TLR4/NFκB-dependent pathway.11 It should be noted that we did not observe SAA3 in serum obtained from the portal vein by performing proteomic analyses, suggesting a local role for SAA3 in the colon (see below). In contrast SAA1, 2 and 4 were observed in all conventionally raised mice assayed (n = 5). Interestingly, SAA1 and 4, but not SAA2 was identified in GF mice. Thus SAA3 may have auto- and/or paracrine functions whereas the others have endocrine functions. Our findings are in agreement with a recent publication that identified SAA1, SAA2 and SAA4 in the peripheral circulation of CONV-R mice.19
Potential Antimicrobial Role of SAA3 in the Intestine
Interestingly, SAA3 may be present in the contents of colonic goblet cells (Fig. 1) and, in fact, interact with bacteria in the gut lumen (Fig. 2). This staining pattern was absent in negative controls where primary antibody treatments were omitted (data not shown). However, goblet cell contents staining positive for SAA3 were only observed in C57Bl/6 mice and not the Swiss-Webster strain (data not shown); this strain difference is currently not understood. Packaging of SAA3 into secretory granules and release into the gut lumen with colonic mucins would provide a cellular mechanism for how SAA3 can interact with the gut microbiota.
Figure 1.
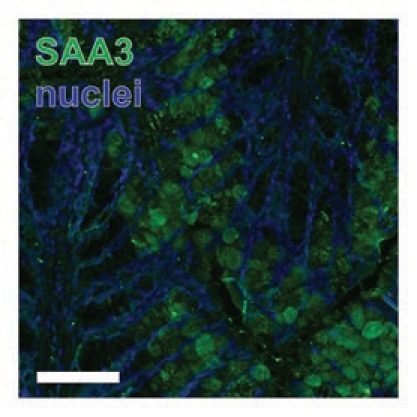
Goblet cells express SAA3. SAA3-positive goblet cells in colon from high-fat fed C57Bl6/J mice. Scale bar = 100 µm.
Figure 2.
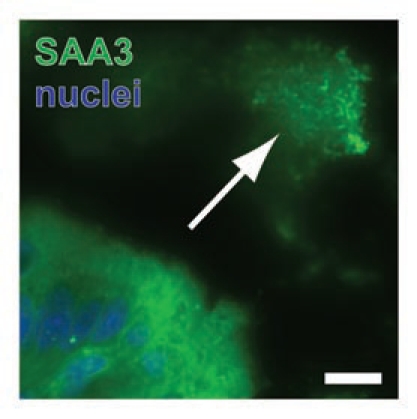
Colonic bacteria bind SAA3. Luminal bacteria located near the colonic epithelium stain positive for SAA3 (arrow). Scale bar = 10 µm.
Direct interaction between SAA1 and OmpA,20 an abundant outer membrane protein found on gram-negative bacteria, suggests that SAAs may function as opsonins in the gut, although this remains to be demonstrated. Moreover, SAA3 has been reported to be secreted by bovine, equine and ovine mammary tissues into colostrums21 and by human mammary epithelial cells in culture.22 Although speculative, SAA3 may play an important role in modulating the microbiota in the gut lumen of infants. For example, treatment of cultured intestinal epithelial cells with a recombinant human SAA3 peptide was shown to induce mucin 3 (MUC3) expression and significantly reduce the binding of enteropathogenic E. coli.23
Conclusion
SAAs are acute phase reactants with ubiquitous functions.24 In our original publication we identified the gut microbiota as an important modulator of SAA3 expression in both adipose and colonic tissues. However, in contrast to other SAAs, SAA3 may have predominantly autocrine and/or paracrine functions and promote inflammation by both activating TLR4,25 and acting as monocyte chemoattractant.26 Here, we also speculate that SAA3 could have antibacterial effects. There are several important questions to answer: do all bacterial strains of the gut microbiota induce SAA3? Could certain microbes suppress SAA3 expression? Is SAA3 antibacterial? Could SAA isoforms function as diagnostic biomarkers or drug targets for metabolic therapies? We have demonstrated potent microbial regulation of SAA3, which also is regulated by Tlr-signaling; however, much work remains to elucidate the function of this molecule.
Acknowledgements
We thank Carina Arvidsson, Caroline Wennberg, Gunnel Östergren-Lundén, and Jenny Felin for superb technical assistance, Elisabeth Carlshon and Petra Linde for mass spectrometry analysis, and Philipp Scherer for the generous gift of SAA3 antibody. This work was supported by the Swedish Research Council, Swedish Foundation for Strategic Research, Petrus and Augusta Hedlund Foundation, The Novo Nordisk Foundation, Harald Jeansson Foundation, Torsten and Ragnar Söderbergs foundations, and a LUA-ALF grant from Västra Götalandsregionen. CSR is the recipient of a postdoctoral fellowship from the Wenner-Gren Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/10514
References
- 1.Poinar GO., Jr Description of an early Cretaceous termite (Isoptera: Kalotermitidae) and its associated intestinal protozoa, with comments on their co-evolution. Parasit Vectors. 2009;2:12. doi: 10.1186/1756-3305-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäckhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.09.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt C, Reigstad CS, Backhed F. Intestinal microbiota during infancy and its implications for obesity. J Pediatr Gastroenterol Nutr. 2009;48:249–256. doi: 10.1097/mpg.0b013e318183187c. [DOI] [PubMed] [Google Scholar]
- 9.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reigstad CS, Lunden GO, Felin J, Backhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS ONE. 2009;4:5842. doi: 10.1371/journal.pone.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–42083. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- 13.Meek RL, Benditt EP. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986;164:2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 15.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 16.Benditt EP, Meek RL. Expression of the third member of the serum amyloid A gene family in mouse adipocytes. J Exp Med. 1989;169:1841–1846. doi: 10.1084/jem.169.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. Faseb J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 18.Scheja L, Heese B, Zitzer H, Michael MD, Siesky AM, Pospisil H, et al. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res. 2008;2008:230837. doi: 10.1155/2008/230837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiba T, Han CY, Vaisar T, Shimokado K, Kargi A, Chen MH, et al. Serum amyloid A3 (SAA3), an adipose tissue-derived inflammatory protein, does not contribute directly to increased SAA levels in HDL of obese mice. J Lip Res. 2009;50:1353–1362. doi: 10.1194/jlr.M900089-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hari-Dass R, Shah C, Meyer DJ, Raynes JG. Serum amyloid A protein binds to outer membrane protein A of gram-negative bacteria. J Biol Chem. 2005;280:18562–18567. doi: 10.1074/jbc.M500490200. [DOI] [PubMed] [Google Scholar]
- 21.McDonald TL, Larson MA, Mack DR, Weber A. Elevated extrahepatic expression and secretion of mammary-associated serum amyloid A 3 (M-SAA3) into colostrum. Vet Immunol Immunopathol. 2001;83:203–211. doi: 10.1016/s0165-2427(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 22.Larson MA, Wei SH, Weber A, Weber AT, McDonald TL. Induction of human mammary-associated serum amyloid A3 expression by prolactin or lipopolysaccharide. Biochem Biophys Res Commun. 2003;301:1030–1037. doi: 10.1016/s0006-291x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner GE, O'Flaherty S, Casey PG, Weber A, McDonald TL, Cronin M, et al. Evaluation of colostrum-derived human mammary-associated serum amyloid A3 (M-SAA3) protein and peptide derivatives for the prevention of enteric infection: in vitro and in murine models of intestinal disease. FEMS Immunol Med Microbiol. 2009;55:404–413. doi: 10.1111/j.1574-695X.2009.00539.x. [DOI] [PubMed] [Google Scholar]
- 24.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 25.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 26.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–2273. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]


