Abstract
We have recently shown that the colon is protected by an inner mucus layer that efficiently separates the bacteria in the outer mucus from the epithelial cells. The inner mucus is impervious for bacteria and built by a network formed by the MUC2 mucin. Lack or defects in this inner mucus layer allow bacteria to reach the epithelia, something that triggers colon inflammation.
Key words: mucin, mucus, bacteria, colon, colitis
The skin of a human being is an easily understood and efficient protective barrier by virtue of its multilayered dead cells. In contrast, the protection afforded by the gut is more complex and less well understood. Not only is the gut surface much larger, but also the supply of water, salt, food and a suitable temperature makes the gut an ideal milieu for microbes. As a consequence of this, the number of commensal bacteria in the adult intestine is estimated to be 1013–1014, a number well exceeding the number of cells comprising the human body.1 These gut microbes provide us with important physiological functions like vitamin production and digestion of indigestible saccharides, but also poses an enormous challenge to the host.1,2 Most of these bacteria are found in the colon, but how we are able to cope with this massive bacterial load remains beyond our understanding. The importance of antibacterial proteins such as lysozyme and peptides like the defensins has been well recognized.3,4 The state of tolerance elicited by the immune system has been widely studied, yet a more detailed and molecular understanding of how intestinal bacteria are tolerated is still lacking.5 The mucus layer has long been recognized as an important ingredient in the gut protection, but has attracted less attention during the last decades. In fact, intestinal mucus is often missing in illustrations depicting gut protection.6 One reason for this may be our relatively poor understanding of how the mucus is organized and its molecular nature. Another reason may be that normal hydrated mucus is transparent and thus not easily observed and envisioned. In fact, looking down on an opened intestine through a dissection microscope reveals the epithelial cell surface, but not the mucus surface ‘high’ above the epithelia unless visualized by overlaying it with charcoal.
In vivo studies on rats by Lena Holm and others using the visualization approach with sprinkled charcoal revealed in the beginning of the new century that there are two mucus layers in colon; an outer loose mucus layer that can be readily sucked off and an inner firmly attached mucus layer that requires scraping to be removed.7,8 More detailed studies in mice revealed a similar organization although these layers were thinner, about 50 µm for the inner and 100 µm for the outer mucus layer.9,10 However, the real surprise came when we discovered that the inner of these two mucus layers was devoid of bacteria and that it acted as an efficient bacterial barrier.10 These studies also revealed that both mucus layers are organized around Muc2 mucin.
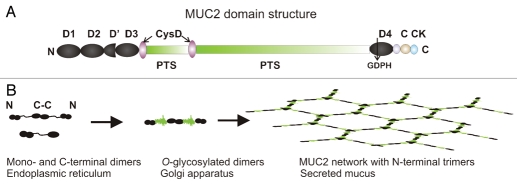
The major mucin produced in the small and large intestine is called MUC2. This mucin belongs to a family of four gel-forming mucins found in humans with an evolutionary history going back to early metazoans.11 Mucins are highly glycosylated glycoproteins that have numerous O-glycans attached to domains rich in the amino acids proline, threonie, and serine (PTS domains). Once the glycans attach to these parts of the molecule they form long and extended structures that can be visualized as a bottlebrush. These structures are called mucin domains. The human MUC2 contains about 5,200 amino acids as estimated from the still not fully sequenced gene (Fig. 1A).12 The two PTS domains are interrupted by two small CysD domains and the whole molecule has large cystein-rich N- and C-termini.12 In these, the dominating domains are the von Willebrand D domain, three in the N-terminus and one in the C-terminus. These domains have been named after the von Willebrand coagulation factor,13 although it is now known that these domains were first utilized during the evolution in mucin-type genes.11
Figure 1.
The MUC2 mucin forms a network in mucus. (A) The domain organization of the MUC2 mucin. (B) The formation of MUC2 mucin in the endoplasmic reticulum, in the Golgi apparatus, and as released from the goblet cells.
The primary translation product of MUC2 mucin in the endoplasmic reticulum is about 0.6 MDa including its N-linked glycans. Directly after its formation, it is dimerized by intermolecular disulfide bonds in the cystein-knot (CK) domains found in the MUC2 far C-terminal end (Fig. 1B).14,15 The folding of the MUC2 mucin protein is difficult and seems to require help from additional disulphide bond isomerases as the AGR2 protein.16 Our experience is also that certain cells derived from the intestinal tract are considerably more efficient in expressing recombinant and truncated forms of the MUC2 mucin. This supports the emerging concept of mucin folding as a special and important issue for the formation of a functional mucus protective layer.17
When the MUC2 mucin dimer is accepted by the endoplasmic reticulum quality control system, it passes into the Golgi apparatus, where O-glycosylation of the numerous serines and threonines of the PTS domain takes place. The mass of the dimers then increases up to 5 MDa.15,18 After sorting into the vesicles of the regulated secretory pathway, the N-terminal part of MUC2 polymerizes by an intermolecular process triggered by the low pH of these granulae. Several lines of evidence, including visualization by electro microscopy, suggests that the N-termini form trimeric structures.19 This is probably important as it gives MUC2 a complex net-like structure that is prone to form sheets as illustrated in Figure 1B. The MUC2 mucin is stored in a very condensed form in typical goblet cell mucin granulae (Fig. 2).
Figure 2.
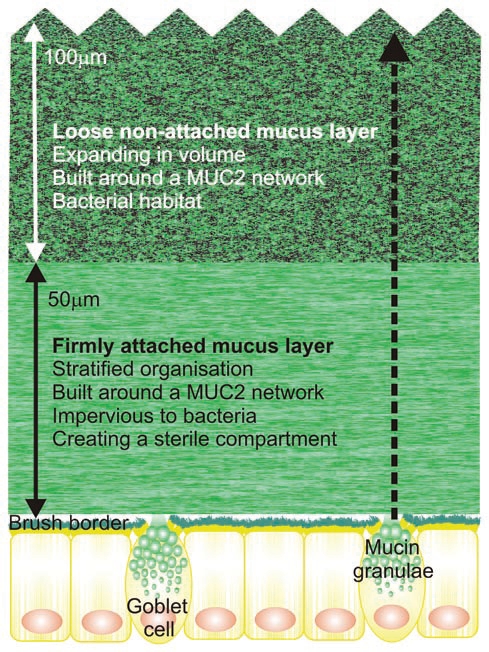
The mucus layers protect the colon epithelium. The inner firmly attached mucus layer is continuously renewed by the goblet cells and transformed from the inner firm mucus layer to the outer loose mucus layer as shown by the dotted arrow. The distances given are for mouse.
The mucin granulae are emptied upon stimulation using machinery likely similar to the one recently reported for tracheal goblet cells.20 When the granulae are emptied, the MUC2 mucin is unfolded and expanded probably a hundred-fold in volume in a process that we have only recently begun to understand.21 While we do not know how this process works in the colon, our pictures of Carnoy stained tissue sections suggest that the secreted and expanded Muc2 mucin spreads on the epithelial surface under the existing mucus layer creating a structurally stratified layer closest to the epithelium. Immunostaining of Muc2 indicates that the expanded mucin organizes itself into sheets that are assembled into the inner mucus layer (Fig. 2). The organization in lamellas is probably facilitated by the net-like structure predicted from the assembly of the mucin. The mucus is composed of the goblet cell vesicle content, but also secreted molecules from the enterocytes as well as ions and water. As the turnover of the epithelial cells of the intestine is fast, there are also a substantial number of detached cells in the mucus. This is illustrated by the mucin proteome which shows a large number of intracellular proteins.22 These proteomic studies reveal that the main structural component of the mucus network is Muc2 mucin, but that there are a number of other components known to be derived from the goblet cells, for example Clca3, Fcgbp and Agr2.10,22 Another prominent component is of course the secreted immunoglobulin IgA.23
It should be pointed out that only the water-free version of the Carnoy fixative can be used if the mucus layers are to be preserved. The commonly used paraformaldehyde fixation dehydrates the mucus that is then lost upon staining. The mucus layers are normally not observed and it is thus understandable that the organization of the mucus layers has been ignored for such a long time.
The inner mucus layer formed on the surface of the epithelial cells is attached to the epithelial cell surface as shown by the in vivo studies in mice and rat.7,10 This inner mucus layer is constantly renewed by goblet cell secretion and the layered mucus migrates upward as illustrated in Figure 2. Once the mucus layer reaches the top of the inner firm mucus layer it is released from the attached mucus as it is transformed into the loose mucus layer. In the mouse, this takes place when the mucus has reached about 50 µm from the epithelia, but in the rat this distance is around 100 µm. The thickness of this inner mucus layer is surprisingly constant and seems to be controlled by mechanisms that remain undefined. We do not understand how the thickness is controlled or the mechanism for its conversion into a non-attached loose mucus layer.
The loose mucus expands at least four times in volume as observed by the lower concentration of the Muc2 glycoprotein in the loose mucus as compared to the inner firm layer. This expansion in volume is due to proteolytic activities that cleave the Muc2 mucin protein core without disrupting the polymeric network. The reason for this is that the Muc2 mucin polymer is stabilized by the high number of cysteins that form intramolecular disulfide bonds in the N- and C-terminal domains of the Muc2 mucin. Cleavages between such bonds allow the observed volume expansion while the polymeric nature is maintained by the disulfide-bond stabilized network. The enzymes responsible for this expansion are at least partly of host origin as a voluminous loose layer was also observed in germ-free animals.10
The remarkable observation of an inner mucus layer devoid of bacteria opens new questions regarding how this is accomplished. As the two mucus layers are comprised of essentially similar products and that the outer layer is formed from the inner mucus layer make it less likely that the inner mucus layer contain specific antibacterial components. Instead, it is more likely the dense and layered organization of the inner mucus holds the explanation for this. Most likely this mucus layer is sufficiently dense with pores too small to allow bacteria to penetrate. Such a property is reflected in the insolubility of the inner mucus in chaotropic salts (guanidinium chloride) known to disrupt all non-covalent bonds. The only way to solubilize the inner mucus is to reduce (break) the disulfide bonds that maintain the polymeric net-like structure of the Muc2 mucin. Also, proteins other than Muc2 likely contribute to the properties of the inner mucus layer. An important molecule is the Immunoglobulin G Fc Binding Protein, Fcgbp,24 that we have shown to be covalently attached to the Muc2.22 The name of this protein does not accurately reflect its function, which is to cross-link other molecules.22
As the inner mucus layer is an efficient barrier that hinders bacteria from reaching the epithelial cells, what happens if defects are created in this layer? The extreme of course occurs in mice lacking the Muc2 mucin as generated by Anna Velcich.25 In these mice there is no inner mucus layer despite the presence of goblet cells and other components normally present in the mucus layer.10 The reason for the lack of an inner mucus layer is that the polymeric Muc2 mucin provides the three dimensional structure required for organization of the mucus layer. In the absence of Muc2 mucin, the bacteria come in direct contact with the epithelial cells and bacteria are found deep in the crypts and also observed inside epithelial cells.10 Bacteria inside cells or deep in the crypts are not observed in wild type animals. The mice lacking the Muc2 mucin develop loose stools, diarrhea containing blood and fail to thrive.10,26 If these animals are kept for longer times they are also prone to develop colon cancer.25 All these symptoms are similar to those that occur in humans with ulcerative colitis.
The commensal bacteria reside in the outer loose mucus layer. The loose mucus is expanded in volume due to the cleavage of Muc2 mucin, which allows the bacteria to penetrate the mucus. The bacteria can utilize the mucin glycans as energy source as these bacteria produce high levels of carbohydrate degrading enzymes.27,28 The glycans likely act not only as an energy source, but also function as attachment sites for commensal bacteria. Bacteria express adhesion molecules, many of which have specificities for glycan epitopes.29 The binding specificities of these lectins may well contribute to the host selection of commensal flora. That the host selects commensal flora was elegantly demonstrated by Rawls et al. where germ-free mice were found to select for mouse typical bacteria from a flora typical for zebra fish.30 The mechanism for this is not known, but our recent observation of a uniform O-glycosylation of the MUC2 mucin in human colon, in contrast to mucins of other organs, suggests that these specific glycan epitopes could be a mechanism for selecting commensal flora.31
Certain commensal bacteria secrete not only carbohydrate degrading enzymes, but also proteases. An example is the BFT toxin from Bacteriodes fragilis that has been shown to be a proteolytic enzyme.32 Bacterial enzymes that are able to degrade the protein backbone and disrupt the polymeric nature of the Muc2 mucin have not been identified. However, we have shown that the colonic parasite Entamoeba histolytica secretes a cystein protease that is capable of cleaving the human MUC2 mucin at a very specific peptide sequence. This is located at a position where there are no intramolecular disulfide bonds that can maintain an intact MUC2 network. A cleavage at this site disrupts the net-like polymer nature of the MUC2 mucin of the inner mucus layer, allowing the parasite to penetrate, reach the epithelial cells, and invade the host. Such proteolytic enzymes may also be found in some species of the human commensal flora.
As discussed here, the inner firmly attached mucus layer is very important for protection of the colon epithelia and prevents a direct contact between the enormous load of commensal bacteria and the epithelia. This system is probably instrumental for a balanced and symbiotic relation between host and bacteria.33,34 It is suggested that various types of defects of the inner mucus layer allow more bacteria to penetrate and reach the epithelia. This has already been observed in mouse strains with genetic loss or defects in the Muc2 mucin as well as in molecules that are involved in the formation of the Muc2 mucin polymer.10,16,17,26 Additional mechanisms that generate a defective inner mucus layer can be envisioned, for example bacterial-derived proteases that disrupt the polymeric mucin that forms the mucus skeleton. Models for colonic inflammation based on the recent observations of an inner mucus layer that separates bacteria from the epithelia and disruption of such, discussed herein, could explain some of the pathogenetic phenomena observed in the disease ulcerative colitis.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/10470
References
- 1.Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F, Ley RE, Sonnenburg JL, et al. Host-Bacterial Mutualism in the Human Intestine. Science. 2005;307:1915–1520. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Boman HG. Innate immunity and the normal micro-flora. Immunol Rev. 2009;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 4.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23:115–120. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 5.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. The Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 6.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 7.Atuma C, Strugula V, Allen A, et al. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:922–929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee IA, Havler ME, Dettmar PW, et al. Colonic mucus: secretion and turnover in relation to dietary fibre intake. Proc Nutritional Soc. 2003;62:245–249. doi: 10.1079/pns2003206. [DOI] [PubMed] [Google Scholar]
- 9.Malmberg EK, Noaksson KA, Phillipson M, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:203–210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MEV, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gum JR, Hicks JW, Toribara NW, et al. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 13.Sadler JE. Biochemistry and genetics of von Willebrand factor. Ann Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 14.Asker N, Baeckstrom D, Axelsson MAB, et al. The human MUC2 mucin apoprotein appears to dimerize before O-glycosylation and shares epitopes with the ‘insoluble’ mucin of rat small intestine. Biochem J. 1995;308:873–880. doi: 10.1042/bj3080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asker N, Axelsson MAB, Olofsson SO, et al. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Zhen G, Verhaeghe C, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heazlewood CK, Cook MC, Eri R, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Medicine. 2008;5:54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelsson MAB, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 19.Godl K, Johansson MEV, Karlsson H, et al. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 20.Tuvim MJ, Mospan AR, Burns KA, et al. Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum. J Biol Chem. 2009;284:9781–9787. doi: 10.1074/jbc.M807849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis trans-membrane regulator dependant bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res. 2009;8:3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- 24.Harada N, Iijima S, Kobayashi K, et al. Human IgGFc binding protein (Fcgamma BP) in colonic epithelial cells exhibits mucin-like structure. J Biol Chem. 1997;272:15232–15241. doi: 10.1074/jbc.272.24.15232. [DOI] [PubMed] [Google Scholar]
- 25.Velcich A, Yang WC, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 26.Van der Sluis M, De Koning BAE, De Bruijn ACJM, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenburg JL, Xu J, Leip DD, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Bjursell MK, Himrod J, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 29.Kline KA, Falker S, Dahlberg S, et al. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Rawls JF, Mahowald MA, Ley RE, et al. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmén Larsson JM, Karlsson H, Sjövall H, et al. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 32.Rhee KJ, Wu S, Wu X, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infection and Immunity. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooper LV. Do symbiotic bacteria subvert host immunity? Nature Rev Micro. 2009;7:367–374. doi: 10.1038/nrmicro2114. [DOI] [PubMed] [Google Scholar]
- 34.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]