Abstract
The specter of intentional release of pathogenic microbes and their toxins is a real threat. This article reviews the literature on adhesins of biothreat agents, their interactions with oligosaccharides and the potential for anti-adhesion compounds as an alternative to conventional therapeutics. The minimal binding structure of ricin has been well characterised and offers the best candidate for successful anti-adhesion therapy based on the Galβ1-4GlcNAc structure. The botulinum toxin serotypes A–F bind to a low number of gangliosides (GT1b, GQ1b, GD1a and GD1b) hence it should be possible to determine the minimal structure for binding. The minimal disaccharide sequence of GalNAcβ1-4Gal found in the gangliosides asialo-GM1 and asialo-GM2 is required for adhesion for many respiratory pathogens. Although a number of adhesins have been identified in bacterial biothreat agents such as Yersinia pestis, Bacillus anthracis, Francisella tularensis, Brucella species and Burkholderia pseudomallei, specific information regarding their in vivo expression during pneumonic infection is lacking. Limited oligosaccharide inhibition studies indicate the potential of GalNAcβ1-4Gal, GalNAcβ-3Gal and the hydrophobic compound, para-nitrophenol as starting points for the rational design of generic anti-adhesion compounds. A cocktail of multivalent oligosaccharides based on the minimal binding structures of identified adhesins would offer the best candidates for anti-adhesion therapy.
Key words: anti-adhesion, oligosaccharide, attachment, respiratory tract, biothreat agents
Concepts of Receptor Mimicry
The use of bacteria, viruses and toxins to intentionally cause infection is a realistic threat. The predominant causes for concern are the rapidity of infection and difficulty in diagnosis and treating the pneumonic disease.1,2 These concerns were highlighted by the inhalational infection of postal workers in the US by Bacillus anthracis endospores.3 The range of biothreat agents that pose a threat requires the utilisation of a range of different conventional therapeutics. Bacteria and viruses require treatment with antibiotics and antiviral compounds respectively, whilst toxins require treatment with specific antisera. There are various issues concerning the use and availability of conventional treatments for biothreat agents. Resistance to antibiotics may be naturally acquired or engineered. Antiviral compounds are limited in availability due to increased toxicity issues arising from the commonality of targets between the host cell and virus. Furthermore, vaccines are unavailable or some way off licensure.4,5 Therefore, it is prudent to research novel therapeutics to combat the spectre of intentional release of biothreat agents.
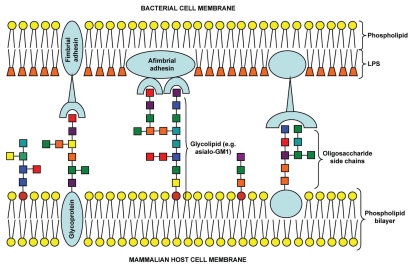
The initial step during infection for any pathogen (or toxin) involves interaction with host cells, generally via the binding of adhesin(s) with carbohydrate receptors on the cell surface via multivalent interactions (Fig. 1).6 Such interactions prevent microbial removal by the physical clearance mechanisms of the host epithelial surfaces e.g., airflow or mucociliary clearance in the respiratory tract.7 These carbohydrate receptors take the form of relatively short chains of saccharide units known as oligosaccharides. The diversity of monosaccharide units, linkages and branching patterns increases the complexity of oligosaccharides enabling fulfilment of a range of biological functions. The oligosaccharide chains are linked to proteins or lipids forming glycoproteins or glycolipids that are inserted into the host cytoplasmic membrane with the oligosaccharide chains facing the external milieu. A range of glycoconjugates located on intact or abraded host cell surfaces may be used by pathogens for attachment (Table 1). Carbohydrate recognition is important in functionality of the innate immune system. Free oligosaccharides present in body fluids such as mucus and breast milk have been proposed to prevent attachment to epithelial cell surfaces.7–9 Furthermore, a number of proteins involved in innate immunity such as man-nose-binding lectin, surfactant proteins A and D, dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN), liver/lymph node-specific intercellular adhesion molecule 3-grabbing nonintegrin (L-SIGN) and the ficolins possess carbohydrate recognition domains (CRDs) specific for oligosaccharide chains present on capsules, LPS and glycoproteins of microbial pathogens.10–12 Utilization of oligosaccharides to inhibit bacterial attachment has proved successful for a number of pathogens both in vitro and in vivo, including Streptococcus pneumoniae, Helicobacter pylori, Pseudomonas aeruginosa and Legionella pneumophila.13–22 Microbial attachment offers an attractive point of intervention in the process of infection.
Figure 1.
Schematic of multivalent oligosaccharide-mediated attachment between bacterial and mammalian host cell membranes.Colored squares represent different monosaccharide units.
Table 1.
Inexhaustive list of glycoconjugate structures used to identify binding moieties of micro-organisms that bind to the respiratory tract
| Glycoconjugate | Structure |
| GD1a | NeuAcα2-3Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer |
| GD1b | Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer |
| GT1b | NeuAcα2-3Galβ1-3GalNAcβ1-4(NeuAcα2-8NeuAcα2-3)Galβ1-4Glcβ1-1Cer |
| GQ1b | NeuAcα2-8NeuAcα2-3Galβ1-3GalNAcβ1-4(NeuAcα2-8NeuAcα2-3Gal)Galβ1-4Glcβ1-1Cer |
| GM1 | Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer |
| Asialo-GM1 | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer |
| Asialo-GM2 | GalNAcβ1-4Galβ1-4Glcβ1-1Cer |
| GM2 | GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer |
| GM3 | NeuAcα2-3Galβ1-4Glcβ1-1Cer |
| Trihexosylceramide | Galα1-4Galβ1-4Glcβ1-1Cer |
| Digalactosylceramide (Gb3) | Galα1-4Galβ1-1Cer |
| Paragloboside (Lacto-N-neotetraosylceramide) | Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer |
| Sialosylparagloboside | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer |
| Globoside | GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer |
| Sulfatide | Gal(3SO4)β1-1Cer |
| Lactosylsulfatide | Gal(3SO4)β1-4Glcβ1-1Cer |
| Lactosylceramide | Galβ1-4Glcβ1-1Cer |
| Glycophorin | NeuAcα2-3Galβ1-3GalNAc-protein |
| Lactotriaosylceramide | GlcNAcβ1-3Galβ1-4Glcβ1-1Cer |
| Lactotetraosylceramide | Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ1-1Cer |
| Lewis A antigen | Galβ1-3(Fucα1-4)GlcNAc |
| Lewis B antigen | Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ1-3Galβ1-4Glc |
| Lewis X antigen | Galβ1-4(Fucα1-3)GlcNAc |
| Heparin | (IdoAβ1-4GlcNS)n (GlcAβ1-4GlcNAc)n (GlcAβ1-4GlcNS)n (IdoASβ1-4GlcNS)n repeating variable disaccharide units; sulphation is variable |
| Chondroitin sulphate | (GlcAβ1-3GalNAcβ1-4)n sulphation is variable in position and quantity |
Cer, ceramide; Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; GlcNS, N-sulphated glucosamine; IdoA, iduronic acid; IdoAS, sulphated iduronic acid; NeuAc, N-acetylneuraminic acid (sialic acid).
A number of methods have been used to determine binding moieties of micro-organisms and toxins including thin-layer chromatography (TLC), solid-phase competitive binding assays, lectin binding and surface plasmon resonance (SPR).21,23–29 The specificity of adhesin-oligosaccharide interactions has an influence on determining a pathogen's host range and tropism for various cell types.30 The majority of pathogens and toxins used as biothreat agents would be disseminated by aerosol and hence initially deposit in the respiratory tract. Therefore, successful therapeutic use of oligosaccharides against such pathogens would require inhibition of adhesion in this anatomical region. This commentary aims to review the current situation of oligosaccharide-pathogen interactions with specific focus on pathogens of concern from a biothreat perspective and assess the potential of oligosaccharide mimics to be developed as novel anti-adhesion therapeutics.
Specific Anti-Adhesion Targeting of the Respiratory Tract
The intentional use of biothreat agents is likely to involve dissemination by aerosol resulting in inhalation and pneumonic presentation of the disease. Deposition of inhalable droplets carrying pathogens within the anatomical regions of the mammalian respiratory tract (nasopharyngeal, tracheobronchial and pulmonary) is dependent on droplet size.31 Therefore, depending on the droplet size generated during intentional or natural dissemination, deposition and hence attachment may occur from the nasal passages to the alveoli. During pneumonic infection the opportunity for therapeutic intervention with anti-adhesion compounds occurs between the molecular determinants of attachment, phagocytosis and engulfment expressed on the surfaces of the pathogen adhesin(s) (or toxin) and the host respiratory tract epithelial cells and immune cells (i.e., alveolar macrophages).
A wide variety of oligosaccharide structures are expressed on the respiratory tract epithelium and the mucins associated with the mucus bilayer.32,33 Furthermore, the physiological status of the host cell epithelium affects the types and quantities of surface-expressed oligosaccharides. Cellular differentiation during injury and repair of respiratory epithelial cells leads to an increase in gangliosides (e.g., asialo-GM1) and exposure of the proteoglycan constituents of the extracellular matrix (e.g., collagen, fibronectin, laminin, heparan sulphate and chondroitin sulphate).34–37 Expression of neuraminidases and other glycosidases by pathogens (e.g., P. aeruginosa, S. pneumoniae and influenza virus) will result in desialylation of sialic acid residues on glycolipids and glycoproteins and subsequent presentation of receptors for attachment.38 The quantity of GM1, GM2, asialo-GM1 and asialo-GM2 expressed varied according to the particular respiratory epithelial cell lines used in adhesion assays.39 All these variances in host cell oligosaccharide profiles will affect the relative binding of pathogens and hence their tropism for particular cell and tissue types. This also represents a disadvantage of using animal models because they may not express the same tissue glycoconjugate profiles as humans, hence in vivo trials may not be representative, particularly if the pathogen is host-specific to humans.8 Successful anti-adhesion therapy for biothreat agents requires consideration of the particular adhesin-ligand interactions that must be inhibited within the specific physiology and anatomy of the respiratory tract. Furthermore, in the case of bacteria, adhesin expression is regulated by parameters such as temperature. Therefore specific tissue tropism may not always occur due to lack of expression and phenomenon such as phase variation (phenotypic switching).6
A number of respiratory pathogens have been shown to attach to oligosaccharide structures (Table 2). The minimal disaccharide sequence of GalNAcβ1-4Gal found in the gangliosides asialo-GM1 and asialo-GM2 is required for adhesion for many of the respiratory pathogens examined.13,21,25–27,29,40,41,46,47,57 The broad spectrum of pathogens that use this base disaccharide unit would appear to cast it as a candidate worthy of exploration as a novel generic anti-adhesion therapeutic. Furthermore, pathogens express oligosaccharide structures themselves in the form of capsules, LPS and glycoproteins that may play a role in attachment to the surface of host epithelial and immune cells.69
Table 2.
List of pathogens known to cause infection by the respiratory tract in humans and some of the glycoconjugate structures they are known to bind
| Microorganism | Binding moieties | Reference(s) |
| Bacillus anthracis | GalNAcβ1-4Gal, GalNAcβ1-3Gal, Galβ1-4GlcNAc, dextran sulphate | 29 |
| Bordetella pertussis | Galβ1-4Glc, GalNAcβ 1-4Gal Chondroitin sulphate, heparan sulphate, dextran sulphate Gal(3SO4)β1-1Cer, LewisA, LewisB, LewisX | 6, 40–42 |
| Brucella abortus/B. melitensis | Heparin sulphate, Sialylated glycoconjugates Fibronectin, vitronectin | 43 |
| Burkholderia cepacia | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer GalNAcβ1-4Galβ1-4Glcβ1-1Cer Galβ1-4Galβ1-4Glcβ1-1Cer, Dextran | 25, 26, 44, 45 |
| Burkholderia pseudomallei | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer GalNAcβ1-4Galβ1-4Glcβ1-1Cer | 27, 46 |
| Chlamydia pneumoniae | GalNAcβ1-4Galβ1-4Glc | 47 |
| Chlamydia trachomatis | GalNAcβ1-4Galβ1-4Glc, heparin, heparan sulphate, laminin, vitronectin, fibronectin, collagen I, collagen IV | 6, 47 |
| Haemophilus influenzae | GalNAcβ 1-4Galβ1-4Glcβ1-1Cer Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer Dextran, Sialylated glycolipids, LewisA, fibronectin, laminin, collagen I, collagen III | 6, 25, 26, 48–51 |
| Haemophilus parainfluenzae | GalNAcβ1-4Galβ1-4Glcβ1-1Cer Galβ1-3GalNAcβ 1-4Galβ1-4Glcβ1-1Cer | 26 |
| Human parainfluenza virus type 1 | NeuAcα2-3Galβ1-4GlcNAc | 52 |
| Human parainfluenza virus type 3 | NeuGcα2-3Galβ1-4GlcNAc, NeuAcα2-6Galβ1-4GlcNAc, NeuAcα2-3Galβ1-4GlcNAc | 52 |
| Influenza A | NeuAc(α2-6)Gal, Dextran sulphate | 53, 54 |
| Influenza B | NeuAc(α2-3)Gal | 53 |
| Influenza C | N-acetyl-9-O-NeuAc(α2-3)Gal | 55 |
| Klebsiella pneumoniae | GalNAcβ1-4Galβ1-4Glcα1-1Cer, Mannose | 26 |
| Legionella pneumophila | Galβ1-3GalNAcβ1-4Galβ1-4Glcα1-1Cer GalNAcβ1-4Galβ1-4Glcα1-1Cer, Heparin | 21, 22 |
| Moraxella cattarhalis | Gal(3SO4)β1-1Cer Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer GalNAcβ1-4Galβ1-4Glcβ1-1Cer | 56, 57 |
| Mycoplasma pneumoniae | NeuAcα2-3Galβ1-3GalNAc, Gal(3SO4)β1-1Cer, Dextran sulphate | 58, 59 |
| Mycobacterium tuberculosis | Heparin, mannose | 6 |
| Neisseria meningitidis | LewisA, fibronectin, gangliosides | 6, 60 |
| Pseudomonas aeruginosa | GalNAcβ1-4Galβ1-4Glcβ1-1Cer, Galα1-4Gal Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer Galβ1-4GlcNAc, Galβ1-4Glcβ1-1Cer, sialyl-LewisX Dextran, Heparin, Heparan sulphate, fibronectin, laminin, collagen I, collagen II, cholesterol | 6, 18, 20, 25, 26, 49, 61, 62 |
| Respiratory syncytial virus | Dextran sulphate, Heparin, Chondrotin sulphate B | 63 |
| Staphylococcus aureus | GalNAcβ1-4Galβ1-4Glcβ1-1Cer, Dextran, LewisA, heparin, heparan sulphate, vitronectin, fibronectin, collagen I, laminin | 6, 25, 26, 49, 51 |
| Streptococcus pneumoniae | GalNAcβ1-4Galβ1-4Glcβ1-1Cer Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer NeuAcα2-3Galβ1-4Glc, NeuAcα2-6Galβ1-4Glc Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer Galβ1-4Glcβ1-1Cer, laminin, vitronectin, collagen IV | 6, 13, 25, 26, 64 |
| Streptococcus pyogenes | Fibronectin, vitronectin | 6 |
| Yersinia pestis | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer GalNAcβ1-4Galβ1-4Glcβ1-1Cer, Galβ1-4Glcβ1-1Cer, collagen, laminin | 65–68 |
In vivo Considerations with the Application of Anti-Adhesion Therapy
Clinical trials using anti-adhesion therapeutics.
The success of anti-adhesion therapy will depend on the pharmacokinetic and pharmacodynamic properties of the compounds within the body. Only a small number of clinical trials have been performed with anti-adhesion oligosaccharides. Clinical trials using 10 or 20 g per day sialyllactose delivered orally to prevent infection by H. pylori proved unsuccessful.70 Similarly, in a phase II clinical trial, intranasal administration of sialyl-3′-lactoneotetraose to children did not reduce the incidence of S. pneumoniae or H. influenzae nasopharyngeal carriage or otitis media.71 The lack of success of clinical trials may be due to a combination of enzymatic degradation and the expression of multiple receptors by the pathogen. In addition, differences occur in the expression of host cell glycoconjugates in adults and infants that may explain the difficulties in the study of Ukkonen et al.71 because the initial screening of were based on epithelial cells obtained from the upper respiratory tract of adults.7 This is supported by the success of local administration of a mixture of monosaccharides (galactose, mannose and N-acetylneuraminic acid) specific for the PA-I and PA-II adhesins of P. aeruginosa in treating otitis externa although recovery was slower than patients receiving gentamicin.6 It is likely that use of multivalent inhibitors or oligosaccharides based on these monosaccharide units would be even more successful.
Selective pressure.
Use of conventional bactericidal or virucidal antimicrobial agents is an aggressive regimen that exerts selective pressure on the pathogens driving the generation of resistant phenotypes. Anti-adhesion therapy would be a safer, gentler therapy, more ecologically sound with the added benefit of reducing selective pressure because the pathogens are prevented from attaching to host cells rather than being killed. The non-infective pathogens would then be removed by the natural physical or immunological clearance mechanisms of the host. Mutation of the adhesin is a plausible scenario during anti-adhesion therapy. However, as the oligosaccharide inhibitor is based on the identical binding event to the pathogens natural adhesion process, any mutation will generally result in reduced binding affinity and hence be less infectious compared to the wild-type.6,7,30 However, the potential exists for altered tropism for tissues that could generate alternative problems. A further benefit is that unlike lytic antibiotics such the β-lactams, anti-adhesion compounds are not bactericidal and would not generate toxic by-products (e.g., LPS) as a result of bacterial death.30 Anti-adhesion therapy may also complement traditional antibiotic therapy by reducing the inherent resistance phenotype associated with attachment to tissues and biofilms.7,8
Receptor redundancy.
It is apparent from the literature that although a few moieties make consistent appearances as the minimal saccharide sequences required for pathogen binding, inhibition is never 100%.6,7,13,15,21,29,39,72 This indicates that multiple receptors binding either the same or different oligosaccharide moieties are required for attachment; and represents the major drawback for anti-adhesion therapy. The issue of multiple receptor-ligand interactions is highlighted by the number of bacterial species in Table 1 that bind a range of glycoconjugates. Even if a high affinity inhibitor can be found or synthesised, it is unlikely that one single compound will inhibit attachment of a single pathogen, let alone a wide range of pathogens. These issues of receptor redundancy are less likely to be an issue for viruses where the number of adhesins present on the surface will be far fewer than on bacteria due to the coding potential of the respective genomic material. Therefore, even for a single pathogen, and certainly for a range of respiratory tract pathogens, a cocktail of inhibitors would be required covering the range of adhesin-ligand interactions that result in attachment of those pathogens. However, this is less likely to be an issue for viruses and toxins where binding is based on one or two adhesin-ligand interactions.
Storage, delivery and administration issues.
Successful use of therapeutic oligosaccharides will rely on efficient storage and delivery. An advantage of oligosaccharides over protein-based vaccines is their inherent physical and chemical properties. Oligosaccharides are highly water-soluble, and stable to heat and alcohols without loss of activity.8 Prophylactic treatment of microbial infections with saccharides, particularly monovalent compounds, may suffer due to degradation by host enzymes under physiological conditions. This could lead to a requirement for excessive and potentially toxic levels to inhibit pathogen attachment.73 Furthermore, the polarity and size of multivalent inhibitors could lead to problems with in vivo use.30 These issues may be alleviated to a degree by the concept of use for the particular therapeutic. For example, topical application of a therapeutic to the skin or epithelium of the respiratory tract is less likely to experience the problems of enzymatic degradation experienced in the gastrointestinal tract through oral delivery or lack of immune tolerance after systemic delivery.
Potential of Anti-Adhesion Compounds Against Biothreat Agents
Biothreat agents may be bacterial, viral and fungal or represented by specific toxins. However, they all share the characteristic that they can elicit disease or toxicity via inhalation.1,2 In the majority of cases, little is known regarding the mechanisms of attachment to the immune cells and epithelium of the respiratory tract. A list of known adhesins of biothreat agents is presented in Tables 3–5.
Table 3.
List of putative adhesins involved in attachment of bacteria and viruses of biothreat concern
| Pathogen/Adhesin | Ligand(s) | Function(s) | Reference(s) |
| Bacillus anthracis (vegetative) | |||
| Poly-γ-D-glutamic acid | Unknown | Capsule—antiphagocytic | 74, 75 |
| EA1 | Unknown | S-layer protein | 74 |
| Sap | Unknown | S-layer protein | 74 |
| BslA | Respiratory and GI epithelial cells | S-layer protein—adhesin | 76 |
| Fibronectin-binding protein | 77 | ||
| Collagen-binding proteins (BA0871, BA5258) | Collagen I | Adhesion | 77, 78 |
| Bacillus anthracis (endospore) | |||
| BclA | Mac-1 integrin | Adhesion, phagocytosis | 79 |
| Francisella tularensis | |||
| Type IV plius (PilA, PilF, PilT) | A549 pneumocytes, macrophages, hepatocytes | Type IV pili, adhesion | 80, 81 |
| FsaP | A549 type II pneumocytes | Adhesin | 82 |
| CR3, CR4, Fc receptor, mannose-binding protein, surfactant protein A | Monocytes, macrophages, dendritic cells | Attachment, invasion | 83–85 |
| Brucella species | |||
| SP41 (UgpB) | Sialic acid | Adhesion, invasion | 86 |
| SP29 | Sialic acid, N-acetylneuraminyl lactose, chondroitin sulphate | Haemagglutination | 87 |
| Venezuelan Equine Encephalitis Virus (VEEV) | |||
| E2 glycoprotein | Laminin, Heparin | 88, 89 | |
| Smallpox | Heparin, chondroitin sulphate | Complement inactivation | 90 |
| Ebola/Marburg virus | |||
| GP1 glycoprotein | DC-SIGN, L-SIGN, Folate receptor-α | Adhesion, invasion | 12, 91, 92 |
GI, gastrointestinal tract
Table 5.
Oligosaccharide-based receptors for toxins of concern from a biowarfare perspective
| Toxin | Glycoconjugate | Reference(s) |
| BotTx type A | GT1b, GQ1b, GD1a | 106–109 |
| BotTx type B | GT1b, GD1a, GD1b | 106, 110 |
| BotTx type C | GT1b, GD1a, GD1b | 110, 111 |
| BotTx type D | phosphatidylethanolamine | 111 |
| BotTx type E | GT1b, GQ1b, GD1a, unsaturated fatty acids | 112 |
| BotTx type F | GD1a, GD1b, GT1b | 110 |
| BotTx type G | Synaptotagmins I and II only; ganglio-side co-receptors not required | 113 |
| SEB | Digalactosylceramide, Lewisa | 114, 115 |
| Ricin | Asialo-GM1, lactosylceramide, N-acetyllactosamine glycans | 116, 117 |
B. anthracis is the archetypal biothreat agent. Adhesion of inhaled endospores involves interactions between the exterior of the endospore and surface-expressed components of human respiratory tissues. Little is known regarding the mechanisms of attachment for both endospores and vegetative cells. The endospore possesses an external exosporium comprising a hexagonal lattice with protruding filamentous appendages and glycoproteins.118–121 that may mediate attachment and promote internalization into macrophages. After germination, the vegetative cells express a range of putative and known adhesins that may promote attachment to host cells including S-layer proteins (EA1, Sap and BslA), teichoic acid, collagen- and fibronectin-binding proteins and the poly-γ-D-glutamic acid capsule.74,76,77 Inhibition of attachment of vegetative cells of a pXO1 pXO2 double-cured strain to the A549 cell line using oligosaccharides was greatest in the case of GalNacβ1-3Gal, Galβ1-4GlcNAc, GalNAcβ1-4Gal and dextran sulphate (56–71%).29 Adhesion was not mediated by the BslA S-layer protein or capsule, both of which are encoded on the absent pXO1.74,76
F. tularensis clinically presents as a range of manifestations, however the most serious is pneumonic infection. Recently, some cell-surface associated adhesins have been identified. The type IV pili apparatus encoded by the pilF and pilT genes mediated adherence to cultured macrophage, pneumocyte and hepatocyte cell lines.80 FsaP is a surface expressed protein with adhesive properties towards the A549 type II pneumocyte cell line.82 The F. tularensis pilin structural subunit, PilA, was capable of expression of type IV pili within a Neisseria gonorrhoeae mutant lacking the genes for type IV pilus expression. Partial restoration of competence for natural genetic transformation was observed in N. gonorrhaeae mutants expressing the F. tularensis pilA gene.81 The importance of the type IV pili has been demonstrated in vivo with respect to intradermal infection,80 however, the impact of these adhesins in vivo on virulence in pneumonic infection models and their target ligands remain unknown. Furthermore, attachment and invasion of opsonised and non-opsonized F. tularensis to macrophages, dendritic cells and monocytes is mediated by interactions with lectins of the innate immune system including mannose-binding lectin and surfactant protein A.83–85
Brucella species can infect a variety of animal species including sheep and pigs. Transmission to humans can occur by contact with infected animals or their contaminated products such as milk or placental tissues. Brucella species can also be transmitted by the aerosol route producing a primary pneumonia. B. melitensis and B. abortus bind to sialic acid residues on erythrocytes, macrophages and epithelial cells via two surface-expressed proteins that have been identified as adhesins, SP29 and SP41 (UgpB).43,86,87 Inhibition of attachment to erythrocytes was achieved using N-acetylneuraminic acid, N-acetylneuraminyl lactose and the glycosaminoglycan, chondroitin sulphate.87 However, as yet the effect in vivo has not been determined.
Prophylactic administration of anti-adhesion compounds may provide an alternative or supportive therapy to conventional treatments. This review will now focus in more detail on those biothreat agents for whom research into the characteristics of cellular attachment and/or suitability of anti-adhesion therapy has been investigated in some detail, namely Y. pestis, B. pseudomallei, the viral agents, and the toxins, botulinum toxin, ricin and staphylococcal enterotoxin B.
Attachment and inhibition of viruses.
The viral agents Venezuelan equine encephalitis virus (VEEV) and the filoviruses Ebola and Marburg possess glycoprotein spikes that mediate attachment to host cells. The O-linked oligomannose glycans on GP1 of Ebola and Marburg viruses bind to the CRDs of the C-type lectins, DC-SIGN and L-SIGN found on dendritic cells and lymph node cells respectively. L-SIGN is also expressed on the surfaces of endothelial and hepatic cells.12 The CRD of DC-SIGN binds to Man9GlcNAc2 oligosaccharides with 130-fold more affinity than mannose.10 Furthermore, mannose-binding lectin inhibits the interaction of DC-SIGN with dendritic cells by binding to the glycoprotein, GP1, of Ebola and Marburg viruses.122 Ebola and Marburg viruses attach to a range of other cell types using the folate receptor-α. Competitive dose-dependent inhibition can be achieved using monoclonal antibodies or folic acid.91 The potential of oligosaccharides to be used as attachment inhibitors for filoviruses was demonstrated by inhibition of infection of HeLa cells by a glycodendrimer containing thirty-two mannose residues. Infection by a pseudotyped lentivirus expressing Ebola glycoproteins demonstrated dose-dependent inhibition of infection with an IC50 of 337 nM and 1.27 mM obtained for the multivalent oligomannose glycodendrimer and the monosaccharide α-methyl-D-mannopyranoside respectively.123
Orthopoxviruses including variola (smallpox), monkeypox and vaccinia express enzymes that inactivate the complement activation pathways of the host. The smallpox inhibitor of complement enzymes (SPICE) attaches to host cells via glycosaminoglycans and proteoglycans including heparin and chondroitin sulphate increasing inhibitory activity towards complement. The degree of sulphation influenced binding with the greatest occurring to chondroitin sulphate E that contains disulphate disaccharide units. Inhibition of SPICE attachment to host cells by a monoclonal antibody prevented complement inactivation.90 Similarly, oligosaccharide inhibitors based on the interaction between SPICE and its sulphated ligand may offer novel therapeutics for preventing orthopoxvirus induced complement inactivation. The range of tissues infected by variola would indicate that additional adhesins will be expressed to initiate attachment and invasion of host cells.
The alphavirus, VEEV, infects all equine species; however, it can also be transmitted to humans by infected mosquitoes causing encephalitis. VEEV can also be transmitted via the aerosol droplets producing similar disease symptoms with initial flu-like symptoms followed by acute encephalitis in severe cases. The E2 glycoprotein initiates attachment to host cells. Few studies have characterised the receptor specificities of the E2 glycoprotein, however it is known to bind to heparin and laminin-binding protein on the Chinese hamster ovary (CHO) and the C6/36 mosquito cell lines respectively.88,89 Attachment to the BW-J-M murine macrophage cell line was inhibited by the lectins, wheat germ agglutinin, concanavalin A and soybean agglutinin.24 This indicated specificity of VEEV TC-83 (vaccine strain) to terminal residues of N-acetylglucosamine, α-linked mannose and N-acetylgalactosamine respectively.
Oligosaccharides as anti-adhesion compounds against Yersinia pestis.
Y. pestis is the aetiological agent of bubonic plague. It can also cause pneumonic infection by the inhalation of aerosols; an invariably fatal disease with mortality approaching 100% without therapeutic intervention within 48 h.124 A number of adhesins have been identified although the differential expression profiles between the bubonic and pneumonic forms of plague and their ligands have not necessarily been investigated (Table 4). Evidence for Y. pestis attachment to oligosaccharides includes reduced attachment to host cells expressing reduced levels of oligosaccharides due to inhibition of carbohydrate biosynthesis by tunicamycin,39 competitive binding assays29,39,125 and TLC binding assays.65,66 The inhibitory effect of anti-adhesion compounds towards Y. pestis attachment to respiratory epithelial and macrophage cell lines has been investigated.29,39,125 Inhibition differed according to the cell lines examined,39 a situation previously reported for Ps. aeruginosa.126 The greatest effect of the tested anti-adhesion compounds on Y. pestis adhesion was observed towards the RPMI-2650 nasal epithelial cell line.39 The best inhibitor of Y. pestis attachment for each cell line examined was the hydrophobic compound, para-nitrophenol at 73–93% inhibition.29,39 However, some specificity was observed for the disaccharides, GalNAcβ1-4Gal and GalNAcβ1-3Gal with inhibition ranging from 44–84% depending on the cell line.29,39
Table 4.
Known and putative Yersinia pestis structures involved in or affecting adhesion
| Adhesin | Ligand(s) | Function(s) | Reference(s) |
| F1 antigen (Caf1) | Unknown | Antiphagocytic, inhibit adhesion | 93, 94 |
| Plasminogen activator (Pla) | Laminin, collagen IV, heparan sulphate, lactosylceramide, globoside | Attachment, proteolytic activation of plasminogen to plasmin | 66–68, 95 |
| pH 6 antigen (PsaA) | Phosphatidylcholine, lactosylceramide, asialo-GM1, asialo-GM2, apolipoprotein B | Attachment, antiphagocytic, Fc receptor for IgG | 65, 96–98 |
| Ail (OmpX) | HEp-2 | Adhesion, invasion, serum resistance, Yop delivery | 99, 100 |
| LPS | DC-SIGN (CD209) | Adhesion and invasion of dendritic cells and alveolar macrophages | 101 |
| YapC | Unknown | Attachment, autoaggregation, biofilm formation | 102 |
| YapE | A549 type II pneumocytes, HEp-2 cells | Attachment, dissemination from lymph nodes in bubonic plague | 103 |
| TadABCDEG | Non-specific adhesion | Putative hydrophobic adhesin | 104 |
| HecA homologs (two present in genome) | Unknown | FHA-like afimbrial adhesin; homologous to HecA of Erwinia chrysanthemi | 105 |
DC-SIGN, dendritic cell-specific intercellular adhesion molecule 3-grabbing noni ntegrin; FHA, filamentous haemagglutinin; LPS, lipopolysaccharide
The GalNAcβ1-4Gal and GalNAcβ1-3Gal moieties are present in asialo-GM1 and asialo-GM2 demonstrated to bind PsaA (pH 6 antigen) of Y. pestis.65 PsaA forms a fimbrial structure under low pH conditions and mammalian body temperature additionally permitting binding to phosphatidylcholine on A549 cells and surfactant, the lipid moiety of plasma apolipoprotein B and Fc receptors for IgG isotypes.96–98 PsaA-mediated binding to respiratory tract epithelial cells inhibits intracellular uptake,94 however PsaA is not required for attachment to macrophage cell lines.127
Additional adhesin(s) other than PsaA are involved in attachment to respiratory epithelial cells as indicated by the ability of a ΔcafΔpsa double mutant to adhere and invade cell lines.94 Indeed, two homologs of HecA have been identified as putative adhesins in the Y. pestis genome105 and the tad gene cluster that confers nonspecific adherence in Actinobacillus actinomycetemcomitans is conserved in the Y. pestis genome.104 The YapC autotransporter has recently been described as an adhesin, although its ligand is as yet undetermined.102 The autotransporter adhesin, YapE, is required for colonisation of lymph nodes and subsequent dissemination in the bubonic form. The effect in the pneumonic infection is unknown; however it has been demonstrated that YapE mediates adhesion to A549 type II pneumocytes, implying a function in pulmonary disease.103 The LPS of Y. pestis interacts with the lectin, DC-SIGN, located on the surface of professional antigen-presenting cells such as dendritic cells and alveolar macrophages promoting phagocytosis and subsequent transportation to lymph nodes.101
The plasminogen activator (Pla) of Y. pestis is a multifunctional surface expressed protein. Pla enzymatically converts plasminogen into plasmin inducing degradation of fibrin and the extracellular matrix promoting the dissemination of Y. pestis from the initial site of infection. Evidence has been reported for Pla as an adhesin; Y. pestis KIM lacking pPCP encoding Pla demonstrated reduced binding to certain glycoconjugate components of the cell membrane, basement membrane and extra-cellular matrix. Binding to laminin was five times greater than that observed with heparan sulphate whilst little attachment was observed to chondroitin sulphate, fibronectin or collagens.67 Binding was also observed to the glycolipids, lactosylceramide and globoside; collagen binding was specific for collagen IV.66 Pla interacts specifically with galactose moieties as indicated by reduced binding to collagen IV and host cells after galactosidase treatment.66 Despite the fact that a number of direct interactions between host cells and Y. pestis are known, currently the specific roles of the expressed proteins in relation to the clinical spectrum of diseases are unknown, particularly with respect to pneumonic plague.
Adhesion of Burkholderia pseudomallei to respiratory cell lines.
B. pseudomallei is naturally resistant to a range of conventional antibiotics due to a combination of multidrug efflux pumps, mutations in enzyme targets, and its intracellular niche. It is the causative agent of melioidosis that encompasses a wide clinical disease spectrum of which one of the manifestations is pulmonary infection. B. pseudomallei has a wide tissue tropism demonstrated by the ability to attach to a range of epithelial cell types derived from alveolar, bronchial, nasal, laryngeal, oral, conjunctival and cervical tissues.29,128 Attachment is dependent on growth temperature, with increased adherence at 37°C compared to 30°C.128
B. pseudomallei can attach to human pharyngeal epithelial cells129 mediated by asialo-GM1 and to a lesser extent asialoGM2.46 Furthermore, it was determined that acid phosphatase derived from B. pseudomallei possessed the ability to bind to asialo-GM1 and asialo-GM2.27 B. pseudomallei attachment to the human A549 alveolar pneumocyte type II cell line was inhibited by 89–95% using a range of oligosaccharides including the moieties GalNacβ1-4Gal and GalNAcβ1-3Gal present in asialoGM1 and asialo-GM2.29 Type IV pili encoded by the structural pilA gene have been identified in B. pseudomallei that contributes to attachment and virulence.130,131 Interestingly, pilA transcription varies between strains and was associated with differences in microcolony formation and adhesion in a temperature-dependent manner.131 However, to date the specificity of the type IV pilus adhesin has not been determined.
Oligosaccharides as binding targets for toxins.
A number of biologically derived toxins are considered concerning from a biothreat perspective. These include botulinum toxin (BotTx), staphylococcal enterotoxin B (SEB) and ricin; all have been demonstrated to bind to glycoconjugates (Table 5). BotTx, produced by Clostridium botulinum, is one of the deadliest toxins known. BotTx inhibits neurotransmitter release preventing normal neuronal action. Seven different immunological types are produced (BotTx A–G); each requiring to bind to a complex of proteinaceous (e.g., synaptotagmins) and ganglioside or phospholipid receptors located in the neuronal presynaptic membrane to elicit their action. Evidence indicates that the different serotypes of BotTx interact with serotype specific components on the neuronal cell membrane (Table 5). It appears that the presence of ganglioside binding is required for BotTx activity because binding to the protein receptor alone does not elicit toxicity except for BotTx type G.106,109,113 Furthermore, GT1b has been shown to inhibit the activity of BotTx in a manner that is independent of serotype.132 The affinity of BotTx binding to gangliosides differs, for example, BotTx type A binds preferentially to GQ1b and GT1b, with binding also observed to GD1a and no binding to GM1.107–109
Derived from the castor beans of Ricinis communis, the ricin toxin exhibits toxicity upon inhalation or ingestion. Composed of two subunits, the A subunit inhibits protein synthesis once access to the cytoplasm of a target cell is gained, whilst the B subunit possesses three galactose binding sites eliciting attachment to glycoconjugates containing galactose residues.133–135 Ricin displays a preference for Galβ1-4GlcNAc residues with high affinity for highly branched glycans containing this structure.116 The presence of the Galβ1-4Glc linkage appears important as evidenced by the significantly weaker binding of ricin to a synthetic galactosyl lipid compared to a lactosyl lipid.28 Indeed, the specificities of the two major binding sites of the ricin B chain have been characterised with respect to the electrostatic and Van der Waal interaction energies for a range of monosaccharides and disaccharides. The greatest interaction energies for binding sites 1 and 2 were obtained for Galβ1-4Glc and Glcβ1-4Gal respectively.136 A biantennary oligosaccharide with two terminal Galβ1-4GlcNAc moieties binds to the ricin B chain with a higher association constant than the disaccharides due to bivalency. Increased affinity was due to interactions of the terminal bivalent galactose residues with the binding sites and the additional influence of hydrophobic interactions and hydrogen bonds of saccharide residues further away from the terminus.137 On a molar basis, a poly-L-lysinebased dendrimer containing terminal galactose units (∼1,000 units per mole) was ∼3,300 times better at inhibiting ricin binding to immobilised asialofetuin compared to the galactose monosaccharide; however when the number of galactose units per mole was considered the multivalent dendrimer was only ∼3.3 times more potent than the monosaccharide.138
SEB produces severe diarrhoea if ingested, however it can also be aerosolized and produce respiratory distress.2 Neutral glycosphingolipids derived from human kidney proximal tubule cells demonstrated dose-dependent inhibition of SEB to the same cell line.139 Further analysis revealed the receptor as digalactosylceramide.114 Neuraminidase treatment of fertilized trout egg membranes reduced SEB binding.140 This indicated a preference for glyconjugates containing sialic acid, although the specific nature of the glycoconjugate involved remained unidentified. In addition, SEB binds to human monocytes via the Lewisa antigen.115 Although not one binding moiety has been identified that is recognised by all of the toxins, the potential exists to utilize the specific oligosaccharide structures as therapeutics either individually or as a mixture to provide generic coverage.
Rational Design of High Affinity Oligosaccharide Compounds for Increased Inhibition
The binding of monovalent oligosaccharides to their receptors is a low affinity interaction in the range of 103 to 106 M-11. This is perhaps unsurprising; the situation in nature between a pathogen adhesin and its ligand on a mammalian cell surface is much stronger due to the presence of multiple receptor-ligand interactions producing the so-called ‘Velcro effect.’8 This is highlighted by the 500-fold increase in the potency of sialylated oligosaccharides to inhibit adhesion of S. pneumoniae to cultured cells when covalently attached to human serum albumin creating multivalent interactions.64 Research has focussed on attempts to increase binding affinity and hence increase the potential of oligosaccharides to be used as anti-adhesion compounds. The production of multivalent oligosaccharide compounds5,30,141,142 or structural modification of the base oligosaccharide19,125 are two methods that have the potential to increase binding affinity for an adhesin.
The rational design of oligosaccharide inhibitors will require three-dimensional knowledge (i.e., crystal structure) of the specific adhesins that are temporally expressed during infection of the respiratory tract and the minimal saccharide moieties to which they bind. This may differ at an anatomical level depending on whether deposition occurs in the nasopharyngeal, tracheobronchial or pulmonary regions of the respiratory tract. Even within these anatomical regions, differences in adhesion profiles may occur at the cellular level due to interactions with specific cell types. Multivalent inhibitors can be created by attaching oligosaccharides to a proteinaceous scaffold such as human serum albumin15 or generating large glycodendrimer structures with terminal saccharide moieties;5,30,138 this abrogates the advantage of low molecular weight oligosaccharides by increasing immunogenicity of the glycoconjugate and the potential for an adverse immune response.8,30 This issue has been circumvented by designing dendrimer scaffolds from polyamido amine (PAMAM) with biocompatibility for in vivo use.5
Difficulties exist in the chemical synthesis of oligosaccharides due to the necessity to sequentially protect and deprotect the reactive hydroxyl groups located on the saccharide units to be linked. Scientific and technological progress in carbohydrate research has sped up the synthetic process for saccharide chains up to trisaccharides and further advances are expected in the future.5,30 Currently milligram to gram quantities may be synthesized relatively easily,141,143 however, large scale synthesis of the relevant oligosaccharides is required to generate the quantities needed to treat a population under dosing regimens that are likely to last a number of days.
Multivalent inhibitors.
The value of multivalency in the design of adhesion inhibitors has been demonstrated in a number of studies. Increasing the valency of a-mannosyl clusters significantly increased inhibition of erythrocyte haemagglutination via type 1 fimbriae of E. coli. However, increasing valency past a cluster of three did not increase inhibition.141 The potency of the GalNAcβ1-4Gal moiety present in asialo-GM1 and asialoGM2 for F1C-fimbriated E. coli and Pseudomonas aeruginosa was significantly increased in divalent and tetravalent inhibitors.143 Indeed, the potency varied with the spacer length between the two GalNAcβ1-4Gal moieties present on divalent inhibitors with shorter linker arms being more potent.143 This indicates a further consideration for rational design of anti-adhesion inhibitors. Similarly, multivalent inhibitors have been rationally designed against toxins. The Shiga-like toxin of E. coli O157:H7 and Shigella dysenteriae contains a single enzymatic A subunit attached to the homopentameric B subunit that recognizes the glycolipid Gb3 located on host cells. Each B subunit contains three binding sites equating to a total of fifteen binding sites for Gb3 in the toxin.144,145 However, site-directed mutagenesis of the B subunits revealed that the first two binding sites of each subunit are most important for binding. This permitted construction of a five-armed ‘STARFISH’ inhibitor. Each arm possessed two tethered Gala1-4Galβ1-4Glc sac charides allowing decameric presentation to the toxin resulting in a 106-fold increase in inhibition compared to the monovalent trisaccharide.142 Hence, due to their higher binding affinities, multivalent oligosaccharide inhibitors can be used at lower concentrations to achieve similar levels of inhibition of attachment as their monovalent counterparts.5 This may increase the therapeutic potential of oligosaccharides in vivo.
Structural modification of saccharide moieties.
The binding affinity of an adhesin-oligosaccharide interaction can be altered by structural modification of the oligosaccharide, for example halogenation, sulphation, methylation, acetylation or benzylation. This approach requires knowledge of the minimal saccharide sequence required for attachment by changing the size or charge of the base unit and affecting the ‘goodness of fit’ between adhesin and ligand. For example, the binding specificity of the pilus adhesin of P. aeruginosa PAK for asialo-GM1 was analyzed by modification of specific hydroxyl and acetamido groups on the disaccharide, GalNAcβ1-4Gal. The hydroxyl groups were substituted to O-methyl or O-propyl groups whilst the acetamido group was changed to a propionamido group. An analog substituted with 2-O-propyl had a 9.9-fold increased binding affinity for the pili.19 Increasing hydrophobicity by substitution of the C2 hydroxyl group of galactose with different length side chains may generate better inhibitors with increased binding affinity enabling therapeutic intervention of P. aeruginosa infections in vivo.
Charge often plays a critical role in the interaction of adhesins with their oligosaccharide moieties. The role of charge in the adhesin-oligosaccharide interactions of Chlamydia trachomatis has been demonstrated using sulphated polyanions. Compounds with increased negative charge due to the possession of more than one sulphate group per disaccharide unit were the best inhibitors.146 Derivatives of dextran possessing various percentages of carboxymethyl, benzylamide or sulphonate groups differed in their ability to inhibit attachment of a range of viruses including Respiratory Syncytial Virus (RSV), Herpes Simplex viruses (HSV) 1 and 2, Human Cytomegalovirus (HCMV) and Human Immunodeficiency virus (HIV). The composition profoundly affected inhibitory activity. Activity against HSV was dependent on the dextran containing a high percentage of benzylamide and sulphonate substitutions. In contrast, the activity of the derivatised dextrans against RSV, HCMV and HIV was not influenced by the chemical composition of the substituted groups but appeared to be due to physical distribution of the groups along the polymeric glucose backbone of dextran.63
Modified variants of a base disaccharide, galactosucrose, demonstrated the utility of this approach for Y. pestis. In particular, compounds that were highly negatively charged due to substitution of the galactosucrose hydroxyl groups with chlorosulphate or chlorine groups demonstrated significantly better levels of inhibition compared to galactosucrose.125 The best galactosucrose variant averaged 65% inhibition across the A549, BEAS2-B and RPMI-2650 cell lines due to substitution of a hydroxyl with a hydrophobic benzyl group.125 It appears that charge and hydrophobic interactions could play an important role in adhesinoligosaccharide interactions for Y. pestis. It would be interesting to assess the situation with a base saccharide more closely resembling a known ligand used by Y. pestis for attachment, such as GalNAcβ1-4Gal. These examples provide evidence that rational structural modification of the minimal saccharide structures required for adhesin binding could increase binding affinity and offer candidates for anti-adhesion therapy.
Alternative ‘Novel’ Anti-Adhesion Compounds
Hydrophobic interactions.
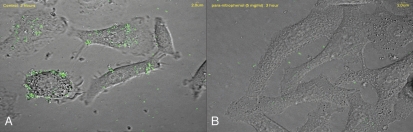
Hydrophobic interactions, such as van der Waal forces, play a critical role in the initial non-specific adhesion to host cells prior to higher affinity specific interactions.147 Hydrophobic interactions also play a role in more specific binding as indicated by the expression of ‘hydrophobins’ on the surface of bacterial cells that interact with hydrophobic moieties.6 The interaction of GalNAcβ1-4Gal with the PAK pilus adhesin of P. aeruginosa was driven by hydrophobic forces.19 The role of hydrophobicity in adhesin-saccharide interactions has been highlighted by the anti-adhesion characteristics of hydrophobic aromatic compounds. High levels of inhibition of E. coli attachment to vaginal epithelial cells were achieved by compounds including para-nitrophenol, para-ansidine, D/L-tyrosine and monosaccharides substituted with aminophenyl or nitrophenol groups.148 Indeed, with the exception of B. anthracis, the most successful compound tested for a range of bacteria that infect the respiratory tract was para-nitrophenol indicating the generic nature of hydrophobic interactions in adhesion (Fig. 2; reviewed in refs. 21, 29 and 39). Indeed, Y. pestis possesses homologs of the tad cluster of A. actinomycetemcomitans that confer hydrophobic binding properties,104 although the functionality in Y. pestis has not been determined.
Figure 2.
Confocal microscopic images of adhesion of fluorescein isothiocyanate (FITC)-stained Legionella pneumophila to the A549 human alveolar type II pneumocyte cell line in the (A) absence and (B) presence of para-nitrophenol (5 mg/ml).
Polymeric compounds.
The polymeric nature of some glycoconjugate structures automatically lend themselves to multivalency if their structures contain saccharide moieties with specificity for microbial adhesins. For example, heparin, heparan sulphate and chondroitin sulphate are components on the extracellular matrix and commonly used by pathogens for host attachment and colonisation. Specific plant-derived polysaccharides inhibited attachment of H. pylori to human gastric mucosa sections. The most active compounds were derived from Okra (acidic rhamnogalacturonan), liquorice root (pectic polymers), blackcurrant seeds (arabinogalactan) and bladderwrack (sulphated fucoidans). Okra extracts also reduced the attachment of Campylobacter jejuni and Porphyromonas gingivalis to chicken colonic mucosa and rat oesophageal tissue respectively.149 Increased activity was related to the acidity of the polysaccharide, promoting electrostatic interactions between the carbohydrate polymer and the microbial adhesins.149 However, the action of such large polymeric saccharides appears to be predominantly non-specific without a dependence on surface charge. Dextran, dextran sulphate, glycogen and mannan inhibit the attachment to cell lines of a range of respiratory pathogens including P. aeruginosa, Group A streptococci, Staphylococcus aureus, L. pneumophila, Haemophilus influenzae and biothreat agents.21,39,49 Dextran is composed of poly(α1–6)glucose chains and binds to both bacteria and the eukaryotic cell lines, implying lack of specificity for microbial adhesins.49
Proanthocyanidins and related plant-derived extracts.
Proanthocyanidins comprise a range of polymeric phenolic chains of flavonoid catechins connected by A- or B-type linkages. Various plant extracts have been demonstrated to contain proanthocyanidinsincludingthose from grape, apple,cinnamon, black currant, green tea, choke berry and cranberry. Certain proanthocyanidins have been linked with diverse health effects including reduced risk to coronary disease, antioxidant, anticancer and anti-adhesion activity.6 Cranberry juice has historical activity in reducing the incidence of urinary tract infections. This activity is due to the presence of A-type proanthocyanidins that interact with the fimbrial adhesins of uropathogenic bacteria such as E. coli preventing access to the Galα1-4Gal linkages present on uroepithelial cells.150 Proanthocyanidins in cranberry juice have also been demonstrated to inhibit attachment dental caries causing oral bacteria.6,7 Polymeric proanthocyanidins derived from the South African geranium, Pelargonium sidioides, significantly reduced adhesion of H. pylori to sections of human gastric mucosa.149 In addition to proanthocyanidins, cranberry juice also contains non-dialysable high molecular weight materials that significantly reduced attachment and infectivity of influenza virus types A and B to Madine-Darby canine kidney (MDCK) cells by inhibition of the sialic acid-specific haemagglutinin.151 The potential of these compounds to inhibit the attachment of respiratory tract pathogens has not been determined.
Conclusion
All pathogens (and toxins), including those of biothreat concern, are required to attach to host cells usually via adhesin-oligosaccharide interactions to invade and cause disease; this lends credence to the potential utilisation of receptor analogues for therapeutic intervention. However, this review has highlighted that relatively little is known regarding the adhesins expressed by many pathogens that may be utilised in biothreat situations. Furthermore, even less is known regarding their expression in the location of the respiratory tract where initial interaction with the host epithelium occurs after inhalation of aerosolised pathogen. Information on the respiratory epithelial glycome of both humans and animal models is urgently required to enable efficient targeting of anti-adhesion therapeutics; furthermore, focus on the entire respiratory epithelial surface where pathogen interactions may occur (i.e., lower and upper respiratory tract) would be beneficial due to the kinetics of particle deposition during inhalation leading to the potential for multiple sites for initial interactions. In clinical practice, the use of anti-adhesion compounds has stalled due to the limited success of clinical trials. However, the future should dictate that advances in the identification of the specific adhesinoligosaccharide interactions between the pathogen and host cells of the respiratory tract using diverse techniques such as molecular biology, computational modelling and x-ray crystallography will enable the directed synthesis of rationally designed oligosaccharides with increased binding affinities. These advances should result in the identification of better lead compounds for anti-adhesion therapy in vivo.
Acknowledgements
R.J. Thomas acknowledges that the Ministry of Defense provided funding for some of the research detailed in this article.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/10049
References
- 1.Atlas RM. Bioterrorism: from threat to reality. Annu Rev Microbiol. 2002;56:167–185. doi: 10.1146/annurev.micro.56.012302.160616. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield RA, Brown BR, Hutchins JB, Iandolo JJ, Jackson R, Slater LN, et al. Microbiological, biological and chemical weapons of warfare and terrorism. Am J Med Sci. 2002;323:326–340. doi: 10.1097/00000441-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimand M, Guiyoule A, Gerbaud G, Rasoamanana B, Chanteau S, Carniel E, et al. Multidrug resistance in Yersinia pestis mediated by transferable plasmid. N Engl J Med. 1997;337:677–80. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 5.Schengrund CL. “Multivalent” saccharides: development of new approaches for inhibiting the effects of glycosphingolipid-binding pathogens. Biochem Pharmacol. 2003;65:699–707. doi: 10.1016/s0006-2952(02)01553-8. [DOI] [PubMed] [Google Scholar]
- 6.Ofek I, Hasty DL, Doyle RJ. In: Bacterial adhesion to animal cells and tissues. First edition. Ofek I, Hasty DL, Doyle RJ, editors. Washington DC: ASM Press; 2003. [Google Scholar]
- 7.Sharon N, Ofek I. Fighting infectious disease with inhibitors of microbial adhesion to host tissues. Crit Rev Food Sci. 2002;42:267–272. doi: 10.1080/10408390209351914. [DOI] [PubMed] [Google Scholar]
- 8.Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. 1996;347:1017–1021. doi: 10.1016/s0140-6736(96)90150-6. [DOI] [PubMed] [Google Scholar]
- 9.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. J Biol Chem. 2001;276:2939–2945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 11.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez CP, Lasala F, Carrillo J, Muñiz O, Corbí AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cundell DR, Tuomanen EI. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb Pathog. 1994;17:361–374. doi: 10.1006/mpat.1994.1082. [DOI] [PubMed] [Google Scholar]
- 14.van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA, Briles DE. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. PNAS. 2003;100:14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson A, Hultberg A, Sjöström R, Kacskovics I, Breimer ME, Borén T, et al. Carbohydrate-dependent inhibition of Helicobacter pylori colonization using porcine milk. Glycobiol. 2006;6:1–10. doi: 10.1093/glycob/cwj031. [DOI] [PubMed] [Google Scholar]
- 17.Bryan R, Feldman M, Jawetz SC, Rajan S, DiMango E, Tang H, et al. The effects of aerosolized dextran in a mouse model of Pseudomonas aeruginosa pulmonary infection. J Infect Dis. 1999;179:1449–1458. doi: 10.1086/314755. [DOI] [PubMed] [Google Scholar]
- 18.Ramphal R, Carnoy C, Fievre S, Michalski J-C, Houdret N, Lamblin G, et al. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Galβ1-3GlcNAc) or type 2 (Galβ1-4GlcNAc) disaccharide units. Infect Immun. 1991;59:700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer F, Jiao H, Hindsgaul O, Wong WY, Irvin RT. Interaction between the pili of Pseudomonas aeruginosa PAK and its carbohydrate receptor β-D-GalNAc(1→4)β-D-Gal analogs. Can J Microbiol. 1997;44:307–311. [PubMed] [Google Scholar]
- 20.Plotkowski MC, Costa AO, Morandi V, Barbosa HS, Nader HB, de Bentzmann S, et al. Role of heparan sulphate proteoglycans as potential receptors for non-piliated Pseudomonas aeruginosa adherence to non-polarised airway epithelial cells. J Med Microbiol. 2001;50:183–190. doi: 10.1099/0022-1317-50-2-183. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RJ, Brooks T. Oligosaccharide receptor mimics inhibit Legionella pneumophila attachment to human respiratory cell lines. Microb Pathog. 2004;36:83–92. doi: 10.1016/j.micpath.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Ader F, Le Berre R, Fackeure R, Raze D, Menozzi FD, Viget N, et al. In vivo effect of adhesion inhibitor heparin on Legionella pneumophila pathogenesis in a murine pneumonia model. Intensive Care Med. 2008;34:1511–1519. doi: 10.1007/s00134-008-1063-2. [DOI] [PubMed] [Google Scholar]
- 23.Hidari KI, Shimada S, Suzuki Y, Suzuki T. Binding kinetics of influenza viruses to sialic acid-containing carbohydrates. Glycoconj J. 2007;24:583–590. doi: 10.1007/s10719-007-9055-y. [DOI] [PubMed] [Google Scholar]
- 24.Huggins JW, Jahrling PB, Rill W, Linden CD. Characterization of the binding of the TC-83 strain of Venezuelan Equine Encephalitis virus to BW-J-M, a mouse macrophage-like cell line. J Gen Virol. 1983;64:149–157. doi: 10.1099/0022-1317-64-1-149. [DOI] [PubMed] [Google Scholar]
- 25.Krivan HC, Ginsburg V, Roberts DD. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from Cystic Fibrosis patients bind specifically to gangliotetraosylceramide (Asialo GM1) and gangliotriaosylceramide (Asialo GM2) Arch Biochem Biophys. 1988;260:493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- 26.Krivan HC, Roberts DD, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcβ1-4Gal found in some glycolipids. Proc Nat Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai K, Suzuki Y, Kondo E, Maejima Y, Miyamoto D, Suzuki T, Kurata T. Specific binding of Burkholderia pseudomallei cells and their cell-surface acid phosphatase to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2) Southeast Asian J Trop Med Public Health. 1997;28:781–790. [PubMed] [Google Scholar]
- 28.Critchley P, Clarkson GJ. Carbohydrate-protein interactions at interfaces: comparison of the binding of Ricinus communis lectin to two series of synthetic glycolipids using surface plasmon resonance studies. Org Biomol Chem. 2003;1:4148–4159. doi: 10.1039/b306784j. [DOI] [PubMed] [Google Scholar]
- 29.Thomas RJ, Brooks T. Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J Med Microbiol. 2004;53:833–840. doi: 10.1099/jmm.0.45643-0. [DOI] [PubMed] [Google Scholar]
- 30.Pieters RJ. Intervention with bacterial adhesion by multivalent carbohydrates. Med Res Rev. 2007;27:796–816. doi: 10.1002/med.20089. [DOI] [PubMed] [Google Scholar]
- 31.Menache M, Miller F, Raabe O. Particle inhalability curves for humans and small laboratory animals. Ann Occ Hyg. 1995;39:317–328. [PubMed] [Google Scholar]
- 32.Lamblin G, Aubert JP, Périni JM, Klein A, Porchet N, Degand P, et al. Human respiratory mucins. Eur Respir J. 1992;5:247–256. [PubMed] [Google Scholar]
- 33.Klein A, Carnoy J, Wieruszeski M, Strecker G, Strang AM, van Halbeek H, et al. The broad diversity of neutral and sialylated oligosaccharides derived from human salivary mucins. Biochem. 1992;31:6152–6165. doi: 10.1021/bi00141a028. [DOI] [PubMed] [Google Scholar]
- 34.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bentzmann S, Plotkowski C, Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am J Respir Crit Care Med. 1996;154:155–162. doi: 10.1164/ajrccm/154.4_Pt_2.S155. [DOI] [PubMed] [Google Scholar]
- 36.De Bentzmann S, Roger P, Puchelle E. Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur Respir J. 1996;9:2145–2150. doi: 10.1183/09031936.96.09102145. [DOI] [PubMed] [Google Scholar]
- 37.De Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski MC, et al. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bavington C, Page C. Stopping bacterial adhesion: a novel approach to treating infections. Respir. 2005;72:335–344. doi: 10.1159/000086243. [DOI] [PubMed] [Google Scholar]
- 39.Thomas RJ, Brooks T. Attachment of Yersinia pestis to human respiratory cell lines is inhibited by certain oligosaccharides. J Med Microbiol. 2006;55:309–315. doi: 10.1099/jmm.0.46102-0. [DOI] [PubMed] [Google Scholar]
- 40.Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson GC, et al. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated epithelial cells. J Exp Med. 1988;168:267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan MJ, Hannah JH, Leininger E. Adhesion of Bordetella pertussis to sulfatides and to the GalNAcβ1-4Gal sequence found in glycosphingolipids. J Biol Chem. 1991;266:18827–18831. [PubMed] [Google Scholar]
- 42.Guijen CA, Willems RJ, Mooi FR. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect Immun. 1996;64:2657–2665. doi: 10.1128/iai.64.7.2657-2665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casteñeda-Roldán EI, Avelino-Flores F, Dall'Agnol M, Freer E, Cedillo L, Dornand J, et al. Adherence of Brucella to human epithelial cells and macrophages is mediated by sialic acid residues. Cell Microbiol. 2004;6:435–445. doi: 10.1111/j.1462-5822.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 44.Sylvester FA, Sajjan US, Forstner JF. Burkholderia (pseudonym Pseudomonas) cepacia binding to lipid receptors. Infect Immun. 1996;64:1420–1425. doi: 10.1128/iai.64.4.1420-1425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu CH, Wong S, Hancock RE, Speert DP. Adherence of Burkholderia cepacia to respiratory tract epithelial cells and inhibition with dextrans. Microbiol. 2001;147:2651–2658. doi: 10.1099/00221287-147-10-2651. [DOI] [PubMed] [Google Scholar]
- 46.Gori AH, Ahmed K, Martinez G, Masaki H, Watanabe K, Nagatake T. Mediation of attachment of Burkholderia pseudomallei to human pharyngeal epithelial cells by the asialoganglioside GM1-GM2 receptor complex. Am J Trop Med Hyg. 1999;61:473–475. doi: 10.4269/ajtmh.1999.61.473. [DOI] [PubMed] [Google Scholar]
- 47.Krivan HC, Nilsson B, Lingwood CA, Ryu H. Chlamydia trachomatis and Chlamydia pneumoniae bind specifically to phosphatidylethanolamine in HeLa cells and to GalNAcβ1-4Galβ1-4Glc sequences found in asialo-GM1 and asialo-GM2. Biochem Biophys Res Commun. 1991;175:1082–1089. doi: 10.1016/0006-291x(91)91676-4. [DOI] [PubMed] [Google Scholar]
- 48.van Alphen L, Geelen-van den Broek L, Blaas L, van Ham M, Dankert J. Blocking of fimbria-mediated adherence of Haemophilus influenzae by sialyl gangliosides. Infect Immun. 1991:4473–4477. doi: 10.1128/iai.59.12.4473-4477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barghouthi S, Guerdoud LM, Speert DP. Inhibition by dextran of Pseudomonas aeruginosa adherence to epithelial cells. Am J Respir Crit Care Med. 1996;154:1788–1793. doi: 10.1164/ajrccm.154.6.8970372. [DOI] [PubMed] [Google Scholar]
- 50.Fakih MG, Murphy TF, Pattoli MA, Berenson CS. Specific binding of Haemophilus influenzae to minor gangliosides of human respiratory epithelial cells. Infect Immun. 1997;65:1695–1700. doi: 10.1128/iai.65.5.1695-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Essery SD, Weir DM, James VS, Blackwell CC, Saadi AT, Busuttil A, et al. Detection of microbial surface antigens that bind Lewisa antigen. FEMS Immunol Med Microbiol. 2006;9:15–22. doi: 10.1111/j.1574-695X.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Portner A, Scroggs RA, Uchikawa M, Koyama N, Matsuo K, et al. Receptor specificities of human respiroviruses. J Virol. 2001;75:4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki Y, Nagao Y, Kato H, Suzuki T, Matsumoto M, Murayama J. The hemagglutinins of the human influenza viruses A and B recognize different receptor microdomains. Biochim Biophys Acta. 1987;903:417–424. doi: 10.1016/0005-2736(87)90048-4. [DOI] [PubMed] [Google Scholar]
- 54.Hosoya M, Balzarini J, Shigeta S, De Clerq E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob Agents Chemother. 1991;35:2515–2520. doi: 10.1128/aac.35.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrler G, Klenk HD. The surface receptor is a major determinant of the cell tropism of influenza C virus. Virol. 1987;159:102–108. doi: 10.1016/0042-6822(87)90352-7. [DOI] [PubMed] [Google Scholar]
- 56.Ozcelik P, Bezerci FB, Suzuki Y, Uzawa H, Nishida Y, Kobayashi K, et al. Sulfatide and its synthetic analogues recognition by Moraxella catarrhalis. Microbiol Immunol. 2006;50:967–970. doi: 10.1111/j.1348-0421.2006.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed K, Suzuki Y, Miyamoto D, Nagatake T. Asialo-GM1 and asialo-GM2 are putative adhesion molecules for Moraxella catarrhalis. Med Microbiol Immunol. 2002;191:5–10. doi: 10.1007/s00430-002-0109-2. [DOI] [PubMed] [Google Scholar]
- 58.Roberts DD, Olson LD, Barile MF, Ginsburg V, Krivan HC. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989;264:9289–9293. [PubMed] [Google Scholar]
- 59.Krivan HC, Olson LD, Barile MF, Ginsburg V, Roberts DD. Adhesion of Mycoplasma pneumoniae to sulphated glycolipids and inhibition by dextran sulphate. J Biol Chem. 1989;264:9283–9288. [PubMed] [Google Scholar]
- 60.Hugosson S, Angström J, Olsson BM, Bergström J, Fredlund H, Olcen P, et al. Glycosphingolipid binding specificities of Neisseria meningitidis and Haemophilus influenzae: detection, isolation and characterization of a binding-active glycosphingolipid from human oropharyngeal epithelium. J Biochem. 1998;124:1138–1152. doi: 10.1093/oxfordjournals.jbchem.a022232. [DOI] [PubMed] [Google Scholar]
- 61.Lingwood CA, Cheng M, Krivan HC, Woods D. Glycolipid receptor binding specificity of exoenzyme S from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1991;175:1076–1081. doi: 10.1016/0006-291x(91)91675-3. [DOI] [PubMed] [Google Scholar]
- 62.Duran JA, Malvar A, Rodriguez-Ares MT, Garcia-Riestra C. Heparin inhibits Pseudomonas aeruginosa adherence to soft contact lens. Eye. 1993;7:152–154. doi: 10.1038/eye.1993.32. [DOI] [PubMed] [Google Scholar]
- 63.Neyts J, Reyman D, Letourneur D, Jozefonvicz J, Schols D, Este J, et al. Differential antiviral activity of derivatized dextrans. Biochem Pharmacol. 1995;50:743–751. doi: 10.1016/0006-2952(95)00193-4. [DOI] [PubMed] [Google Scholar]
- 64.Barthelson R, Mobasseri A, Zopf D, Simon P. Adherence of Streptococcus pneumoniae to respiratory epithelial cells is inhibited by sialylated oligosaccharides. Infect Immun. 1998;66:1439–1444. doi: 10.1128/iai.66.4.1439-1444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Payne D, Tatham D, Williamson ED, Titball RW. The pH 6 antigen of Yersinia pestis binds to β1-linked galactosyl residues in glycosphingolipids. Infect Immun. 1998;66:4545–4548. doi: 10.1128/iai.66.9.4545-4548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kienle Z, Emody L, Svanborg C, O'Toole PW. Adhesive properties conferred by the plasminogen activator of Yersinia pestis. J Gen Microbiol. 1992;138:1679–1687. doi: 10.1099/00221287-138-8-1679. [DOI] [PubMed] [Google Scholar]
- 67.Lähteenmäki K, Virkola R, Sarén A, Emödy L, Korhonen TK. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect Immun. 1998;66:5755–5762. doi: 10.1128/iai.66.12.5755-5762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lähteenmäki K, Kukkonen M, Korhonen TK. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 2001;504:69–72. doi: 10.1016/s0014-5793(01)02775-2. [DOI] [PubMed] [Google Scholar]
- 69.Sahly H, Keisari L, Crouch E, Sharon N, Ofek I. Recognition of bacterial surface polysaccharides by lectins of the innate immune system and its contribution to defense against infection: the case of pulmonary pathogens. Infect Immun. 2008;76:1322–1332. doi: 10.1128/IAI.00910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parente F, Cucino C, Anderloni A, Grandinetti G, Bianchi Porro G. Treatment of Helicobacter pylori infection using novel antiadhesion compound (3' sialyllactose sodium salt). A double blind, placebo-controlled clinical study. Helicobacter. 2003;8:252–256. doi: 10.1046/j.1523-5378.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 71.Ukkonen P, Varis K, Jernfors M, Herva E, Jokinen J, Ruokokoski D, et al. Treatment of acute otitis media with an antiadhesive oligosaccharide: a randomised, double-blind, placebo-controlled trial. Lancet. 2000;356:1398–1402. doi: 10.1016/S0140-6736(00)02843-9. [DOI] [PubMed] [Google Scholar]
- 72.Idänpään-Heikkilä I, imon PM, Zopf D, Vullo T, Cahill P, Sokol H, et al. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis. 1997;176:704–712. doi: 10.1086/514094. [DOI] [PubMed] [Google Scholar]
- 73.Reuter JD, Myc A, Hayes MH, Gan Z, Roy R, Qin D, et al. Inhibition of viral adhesion and infection by sialic-acid-conjugated dendritic polymers. Bioconj Chem. 1999;10:271–278. doi: 10.1021/bc980099n. [DOI] [PubMed] [Google Scholar]
- 74.Fouet A, Mesnage S, Tosi-Coutre E, Gounon P, Mock M. Bacillus anthracis surface: capsule and S-layer. J Appl Microbiol. 1999;87:251–255. doi: 10.1046/j.1365-2672.1999.00882.x. [DOI] [PubMed] [Google Scholar]
- 75.Sutherland MD, Kozel TR. Macrophage uptake, intracellular localization, and degradation of poly-γ-D-glutamic acid, the capsular antigen of Bacillus anthracis. Infect Immun. 2009;77:532–538. doi: 10.1128/IAI.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kern JW, Schneewind O. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol Microbiol. 2008;68:504–515. doi: 10.1111/j.1365-2958.2008.06169.x. [DOI] [PubMed] [Google Scholar]
- 77.Ariel N, Zvi A, Makarova KS, Chitlaru T, Elhanany E, Velan B, et al. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect Immun. 2003;71:4563–4579. doi: 10.1128/IAI.71.8.4563-4579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Liang X, Chen Y, Koehler TM, Höök M. Identification and biochemical characterization of two novel collagen binding MSCRAMMs of Bacillus anthracis. J Biol Chem. 2004;279:51760–51768. doi: 10.1074/jbc.M406417200. [DOI] [PubMed] [Google Scholar]
- 79.Oliva CR, Swiecki MK, Griguer CE, Lisanby MW, Bullard DC, Turnbough CL, et al. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. PNAS. 2008;105:1261–1266. doi: 10.1073/pnas.0709321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakraborty S, Monfett M, Maier TM, Benach JL, Frank DW, Thanassi DG. Type IV pili in Francisella tularensis: roles of pilF and pilT in fiber assembly, host cell adherence and virulence. Infect Immun. 2008;76:2852–2861. doi: 10.1128/IAI.01726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salomonsson E, Forsberg A, Roos N, Holz C, Maier B, Koomey M, et al. Functional analysis of pilin-like proteins from Francisella tularensis: complementation of type IV pilus phenotypes in Neisseria gonorrhoeae. Microbiol. 2009;155:2546–2559. doi: 10.1099/mic.0.028183-0. [DOI] [PubMed] [Google Scholar]
- 82.Melillo A, Sledjeski DD, Lipski S, Wooton RM, Basrur V, Lafontaine ER. Identification of a Francisella tularensis LVS outer membrane protein that confers adherence to A549 human lung cells. FEMS Microbiol Lett. 2006;263:102–108. doi: 10.1111/j.1574-6968.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 83.Balagopal A, MacFarlane A, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulert GS, Allen LH. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leuk Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben Nasr A, Haithcoat J, Masterson JE, Gunn JS, Eaves-Pyles T, Klimpel JR. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leuk Biol. 2006;80:774–786. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 86.Casteñeda-Roldán EI, Ouahrani-Bettache S, Saldaña Z, Avelino F, Rendón MA, Dornand J, et al. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell Microbiol. 2006;8:1877–1887. doi: 10.1111/j.1462-5822.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 87.Rocha Gracia R, Casteñeda-Roldán EI, Giono-Cerezo S, Girón JA. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol Lett. 2002;231:219–224. doi: 10.1111/j.1574-6968.2002.tb11309.x. [DOI] [PubMed] [Google Scholar]
- 88.Ludwig GV, Kondig JP, Smith JF. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J Virol. 1996;70:5592–5599. doi: 10.1128/jvi.70.8.5592-5599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang E, Brault AC, Powers AM, Kang W, Weaver SC. Glycosaminoglycan binding properties of natural Venezuelan Equine Encephalitis virus isolates. J Virol. 2003;77:1204–1210. doi: 10.1128/JVI.77.2.1204-1210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liszewski MK, Bertram P, Leung MK, Hauhart R, Zhang L, Atkinson JP. Smallpox inhibitor of complement enzymes (SPICE): regulation of complement activation on cells and mechanism of its cellular attachment. J Immunol. 2008;181:4199–4207. doi: 10.4049/jimmunol.181.6.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan SY, Empig CJ, Welte FJ, Speck RF, Schmaljohn A, Kreisberg JF, et al. Folate receptor-α is a cofactor for cellular entry by Marburg and Ebola viruses. Cell. 2001;106:117–126. doi: 10.1016/s0092-8674(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 92.Marzi A, Gramberg T, Simmons G, Möller P, Rennekamp AJ, Krumbiegel M, et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of Severe Acute Respiratory Syndrome coronavirus. J Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du Y, Rosqvist R, Forsberg Å. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect Immun. 2002;70:1453–1460. doi: 10.1128/IAI.70.3.1453-1460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu F, Chen H, Galván EM, Lasaro MA, Schifferli DM. The effects of PsaA and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect Immun. 2006;74:5636–5644. doi: 10.1128/IAI.00612-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lobo LA. Adhesive properties of the purified plasminogen activator Pla of Yersinia pestis. FEMS Microbiol Lett. 2006;262:158–162. doi: 10.1111/j.1574-6968.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 96.Galván EM, Chen H, Schifferli DM. The Psa fimbriae of Yersinia pestis interact with phosphatidylcholine on alveolar epithelial cells and pulmonary surfactant. Infect Immun. 2007;75:1272–1279. doi: 10.1128/IAI.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zav'yalov VP, Abramov VM, Cherepanov PG, Spirina GV, Chernovskaya TV, Vasiliev AM, et al. pH 6 antigen (PsaA protein) of Yersinia pestis, a novel bacterial Fc-receptor. FEMS Immunol Med Microbiol. 1996;14:53–57. doi: 10.1111/j.1574-695X.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 98.Makoveichuk E, Cherepanov P, Lundberg S, Forsberg A, Olivecrona G. pH 6 antigen of Yersinia pestis interacts with plasma lipoproteins and cell membranes. J Lipid Res. 2003;44:320–330. doi: 10.1194/jlr.M200182-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Kolodziejek AM, Sinclair DJ, Seo KS, Schnider DR, Deobald CF, Rohde HN, et al. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiol. 2007;153:2941–2951. doi: 10.1099/mic.0.2006/005694-0. [DOI] [PubMed] [Google Scholar]
- 100.Felek S, Krukonis ES. The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence. Infect Immun. 2009;77:825–836. doi: 10.1128/IAI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang P, Skurnik M, Zhang S-S, Schwartz O, Kalyanasundaram R, Bulgheresi S, et al. Human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infect Immun. 2008;76:2070–2079. doi: 10.1128/IAI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felek S, Lawrenz MB, Krukonis ES. The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiol. 2008;158:1802–1812. doi: 10.1099/mic.0.2007/010918-0. [DOI] [PubMed] [Google Scholar]
- 103.Lawrenz MB, Lenz JD, Miller VL. A novel Autotransporter adhesin is required for efficient colonization during bubonic plague. Infect Immun. 2009;77:317–326. doi: 10.1128/IAI.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kachlany SC, Planet PJ, Bhattacharjee MK, Kollia E, DeSalle R, Fine DH, et al. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in Bacteria and Archaea. J Bacteriol. 2000;182:6169–6176. doi: 10.1128/jb.182.21.6169-6176.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rojas CM, Ham JH, Deng W-L, Doyle JJ, Colimer A. HecA, a member of a class of adhesins produced by diverse pathogenic bacteria, contributes to the attachment, aggregation, epidermal cell killing, and virulence phenotypes of Erwinia chrysanthemi EC16 on Nicotiana clevelandii seedlings. PNAS. 2002;99:13142–13147. doi: 10.1073/pnas.202358699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kozaki S, Kamata Y, Watarai S, Nishiki T, Mochida S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog. 1998;25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- 107.Kitamura M, Iwamori M, Nagai Y. Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim Biophys Acta. 1980;628:328–335. doi: 10.1016/0304-4165(80)90382-7. [DOI] [PubMed] [Google Scholar]
- 108.Takamizawa K, Iwamori M, Kozaki S, Sakaguchi G, Tanaka R, Takayama H, et al. TLC immunostaining characterization of Clostridium botulinum type A neurotoxin binding to gangliosides and free fatty acids. FEBS Lett. 1986;201:229–332. doi: 10.1016/0014-5793(86)80614-7. [DOI] [PubMed] [Google Scholar]
- 109.Yowler BC, Kensinger RD, Schengrund CL. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem. 2002;277:32815–32819. doi: 10.1074/jbc.M205258200. [DOI] [PubMed] [Google Scholar]
- 110.Ochanda JO, Syuto B, Ohishi J, Naiki M, Kubo S. Binding of Clostridium botulinum neurotoxin to gangliosides. J Biochem. 1986;100:27–33. doi: 10.1093/oxfordjournals.jbchem.a121702. [DOI] [PubMed] [Google Scholar]
- 111.Tsukamoto K, Kohda T, Mukamoto M, Takeuchi K, Ihara H, Saito M, et al. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J Biol Chem. 2005;280:35164–35171. doi: 10.1074/jbc.M507596200. [DOI] [PubMed] [Google Scholar]
- 112.Kamata Y, Kozaki S, Sakaguchi G, Iwamori M, Nagai Y. Evidence for direct binding of Clostridium botulinum type E derivative toxin and its fragments to gangliosides and free fatty acids. Biochem Biophys Res Commun. 1986;140:1015–1019. doi: 10.1016/0006-291x(86)90736-9. [DOI] [PubMed] [Google Scholar]
- 113.Rummel A, Karnath T, Henke T, Bigalke H, Binz T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J Biol Chem. 2004;279:30865–30870. doi: 10.1074/jbc.M403945200. [DOI] [PubMed] [Google Scholar]
- 114.Chatterjee S, Khullar M, Shi WY. Digalactosylceramide is the receptor for staphylococcal enterotoxin-B in human kidney proximal tubular cells. Glycobiology. 1995;5:327–333. doi: 10.1093/glycob/5.3.327. [DOI] [PubMed] [Google Scholar]
- 115.Essery SD, Saadi AT, Twite SJ, Weir DM, Blackwell CC, Busuttil A. Lewis antigen expression on human monocytes and binding of pyrogenic toxins. Agents Actions. 1994;41:108–110. doi: 10.1007/BF01986408. [DOI] [PubMed] [Google Scholar]
- 116.Itakura Y, Nakamura-Tsuruta S, Kominami J, Sharon N, Kasai K, Hirabayashi J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: a search by frontal affinity chromatography. J Biochem. 2007;142:459. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- 117.Blome MC, Schengrund CL. Multivalent binding of ricin to bovine serum albumin-based neoglycoconjugates. Toxicon. 2008;51:1214–1224. doi: 10.1016/j.toxicon.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 118.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 119.Sylvestre P, Coutre-Tosi E, Mock M. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol Microbiol. 2002;45:169–178. doi: 10.1046/j.1365-2958.2000.03000.x. [DOI] [PubMed] [Google Scholar]
- 120.Fox A, Stewart GC, Waller LN, Fox KF, Harley WM, Price RL. Carbohydrates and glycoproteins of Bacillus anthracis and related bacilli: targets for biodetection. J Microbiol Methods. 2003;54:143–152. doi: 10.1016/s0167-7012(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 121.Waller LN, Fox N, Fox KF, Fox A, Price RL. Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J Microbiol Methods. 2004;58:23–30. doi: 10.1016/j.mimet.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 122.Ji X, Olinger GG, Aris S, Chen Y, Gewurz H, Spear GT. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J Gen Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- 123.Lasala F, Arce E, Otero JR, Rojo J, Delgado R. Mannosyl glycodendritic structure inhibits DC-SIGN-mediated Ebola virus infection in cis and in trans. Antimicrob Ag Chemother. 2003;47:3970–3972. doi: 10.1128/AAC.47.12.3970-3972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krishna K, Chitkara RK. Pneumonic plague. Sem Resp Infect. 2003;18:159–167. [PubMed] [Google Scholar]
- 125.Thomas RJ, Hacking A, Brooks TJG. Structural modification of a base disaccharide alters antiadhesion properties towards Yersinia pestis. FEMS Immunol Med Microbiol. 2007;49:410–414. doi: 10.1111/j.1574-695X.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 126.Hambrook J, Titball R, Lindsay C. The interaction of Pseudomonas aeruginosa PAK with human and animal respiratory cell lines. FEMS Microbiol Lett. 2004;238:49–55. doi: 10.1016/j.femsle.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 127.Huang X-Z, Lindler LE. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect Immun. 2004;72:7212–7219. doi: 10.1128/IAI.72.12.7212-7219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown NF, Boddey JA, Flegg CA, Beacham IR. Adherence of Burkholderia pseudomallei cells to cultured human epithelial cell lines is regulated by growth temperature. Infect Immun. 2002;70:974–980. doi: 10.1128/iai.70.2.974-980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmed K, Encisco HDR, Masaki H, Tao M, Omori A, Tharavichikul P, et al. Attachment of Burkholderia pseudomallei to pharyngeal epithelial cells: a highly pathogenic bacteria with low attachment ability. Am J Trop Med Hyg. 1999;60:90–93. doi: 10.4269/ajtmh.1999.60.90. [DOI] [PubMed] [Google Scholar]
- 130.Essex-Lopresti AE, Boddey JA, Thomas R, Smith MP, Hartley MG, Atkins T, et al. A type IV pilin, PilA, contributes to adherence of Burkholderia pseudomallei and virulence in vivo. Infect Immun. 2005;73:1260–1264. doi: 10.1128/IAI.73.2.1260-1264.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boddey JA, Flegg CA, Day CJ, Beacham IR, Peak IR. Temperature-regulated microcolony formation by Burkholderia pseudomallei requires pilA and enhances association with cultured human cells. Infect Immun. 2006;74:5374–5381. doi: 10.1128/IAI.00569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kozaki S, Sakaguchi G, Nishimura M, Iwamori M, Nagai Y. Inhibitory effect of Ganglioside GT1b on the activities of Clostridium botulinum toxins. FEMS Microbiol Lett. 1984;212:219–223. [Google Scholar]
- 133.Lambert JM, Goldmacher VS, Collinson AR, Nadler LM, Blattler WA. An immunotoxin prepared with blocked ricin: a natural plant toxin adapted for therapeutic use. Cancer Res. 1991;51:6236–6242. [PubMed] [Google Scholar]
- 134.Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, Ackerman EJ, et al. Cell surface and intracellular functions for ricin galactose binding. J Biol Chem. 1992;267:11917–11922. [PubMed] [Google Scholar]
- 135.Steeves RM, Denton ME, Barnard FC, Henry A, Lambert JM. Identification of three oligosaccharide binding sites in ricin. Biochem. 1999;38:11677–11685. doi: 10.1021/bi990493o. [DOI] [PubMed] [Google Scholar]
- 136.Ganguly D, Mukhopadhyay C. Binding diversity of the two binding sites of ricin B lectin. Biopolymers. 2006;83:83–94. doi: 10.1002/bip.20530. [DOI] [PubMed] [Google Scholar]
- 137.Ganguly D, Mukhopadhyay C. Extended binding site of ricin B lectin for oligosaccharide recognition. Biopolymers. 2007;86:311–320. doi: 10.1002/bip.20746. [DOI] [PubMed] [Google Scholar]
- 138.Dawson RM, Alderton MR, Wells D, Hartley PG. Monovalent and polyvalent carbohydrate inhibitors of ricin binding to a model of the cell surface receptor. J Appl Toxicol. 2006;26:247–252. doi: 10.1002/jat.1136. [DOI] [PubMed] [Google Scholar]
- 139.Chatterjee S, Jett M. Glycosphingolipids: the putative receptor for Staphylococcus aureus enterotoxin-B in human kidney proximal tubular cells. Mol Cell Biochem. 1992;113:25–31. doi: 10.1007/BF00230882. [DOI] [PubMed] [Google Scholar]
- 140.Kudo S, Yazawa S. Binding of bacterial toxins to glycoproteins in the envelopes of rainbow trout eggs. Histochem J. 1995;27:300–308. doi: 10.1007/BF00398972. [DOI] [PubMed] [Google Scholar]
- 141.Lindhorst TK, Kieburg C, Krallmann-Wenzel U. Inhibition of the type 1 fimbriae-mediated adhesion of Escherichia coli to erythrocytes by multiantennary α-mannosyl clusters: the effect of multivalency. Glycoconj J. 1998;15:605–613. doi: 10.1023/a:1006920027641. [DOI] [PubMed] [Google Scholar]
- 142.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, et al. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 143.Autar R, Salam Khan A, Schad M, Hacker J, Liskamp RMJ, Pieters RJ. Adhesion inhibition of F1C-fimbriated Escherichia coli and Pseudomonas aeruginosa PAK and PAO by multivalent carbohydrate ligands. Chem Bio Chem. 2003;4:1317–1325. doi: 10.1002/cbic.200300719. [DOI] [PubMed] [Google Scholar]
- 144.Lindberg AA, Brown JE, Strömberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae Type 1. J Biol Chem. 1987;262:1779–1785. [PubMed] [Google Scholar]
- 145.Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-from E. coli. Nature. 1992;355:748–750. doi: 10.1038/355748a0. [DOI] [PubMed] [Google Scholar]
- 146.Zaretsky FR, Pearce-Pratt R, Phillips DM. Sulfated polyanions block Chlamydia trachomatis infection of cervix-derived human epithelia. Infect Immun. 1995;63:3520–3526. doi: 10.1128/iai.63.9.3520-3526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doyle RJ. Contribution of the hydrophobic effect to microbial infection. Microb Infect. 2002;2:391–400. doi: 10.1016/s1286-4579(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 148.Falkowski W, Edwards M, Schaeffer AJ. Inhibitory effect of substituted aromatic hydrocarbons on adherence of Escherichia coli to human epithelial cells. Infect Immun. 1986;52:863–866. doi: 10.1128/iai.52.3.863-866.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wittschier N, Lengsfeld C, Vorthems S, Stratmann U, Ernst JF, Verspohl, et al. Large molecules as anti-adhesive compounds against pathogens. J Pharm Pharmacol. 2007;59:777–786. doi: 10.1211/jpp.59.6.0004. [DOI] [PubMed] [Google Scholar]
- 150.Howell AB. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51:732–737. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- 151.Weiss EI, Houri-Haddad Y, Greenbaum E, Hochman N, Ofek I, Zakay-Rones Z. Crnaberry juice constituents affect influenze virus adhesion and infectivity. Antiviral Res. 2005;66:9–12. doi: 10.1016/j.antiviral.2004.12.011. [DOI] [PubMed] [Google Scholar]