Abstract
Negative emotional stimuli activate a broad network, including the medial prefrontal (mPFC) and anterior cingulate (ACC) cortices. An early influential view dichotomized these regions into dorsal-caudal “cognitive” and ventral-rostral “affective” subdivisions. In this review, we examine a wealth of recent research on negative emotions in animals and humans, using the example of fear/anxiety, and conclude that, contrary to the traditional dichotomy, both subdivisions make key contributions to emotional processing. Specifically, dorsal-caudal regions of the ACC/mPFC are involved in appraisal and expression of negative emotion, while ventral-rostral portions of the ACC/mPFC have a regulatory role with respect to limbic regions involved in generating emotional responses. Moreover, this new framework is broadly consistent with emerging data on other negative and positive emotions.
Controversies about anterior cingulate/medial prefrontal functions
Although the medial walls of the frontal lobes, comprised of the anterior cingulate cortex (ACC) and the medial prefrontal cortex (mPFC), have long been thought to play a critical role in emotional processing 1, it has remained uncertain what exactly their functional contributions might be. Some investigators have described evaluative (appraisal) functions of the ACC and mPFC, such as the representation of the value of stimuli or actions 2-4, or the monitoring of somatic states 5. Others hold the ACC to be primarily a generator of physiological or behavioral responses 6, 7. Still others have described a regulatory role for these regions, such as in the top-down modulation of limbic and endocrine systems for the purpose of emotion-regulation 3, 8-11. An additional source of uncertainty lies with the way in which any one of these proposed functions might map onto distinct sub-regions of the ACC or mPFC (see Box 1).
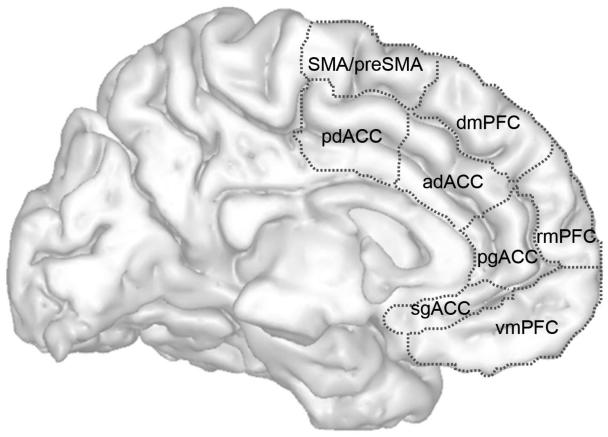
Box 1. Anatomy of the ACC and mPFC.
Within the ACC, a subdivision can be made between a more ventral portion, comprised of areas 24a, 24b, 24c, 25, 32 an 33 (pregenual (pgACC) and subgenual ACC (sgACC) in figure 1) and a more dorsal portion, comprised of areas 24a′, 24b′, 24c′, 24d, 32′ and 33 (dorsal ACC (dACC) in figure 1). This distinction is consistent with that of Vogt and colleagues 63 between an anterior and a midcingulate cortex. In the dACC a further distinction exists between anterior and posterior portions of the dACC (adACC and pdACC), similar to Vogt et al.'s partitioning of the midcingulate into anterior and posterior portions 64 and consistent with partitioning between rostral and caudal cingulate zones 65.
These subdivisions are also reflected in patterns of connectivity. Connectivity with core emotion-processing regions such as the amygdala, periaqueductal gray (PAG) and hypothalamus is strong throughout the sgACC, pgACC and adACC, but very limited in the pdACC 46, 66-70. In general, cingulo-amygdalar connectivity is focused on the basolateral complex of the amygdala.
ACC subregions may also be distinguished based on connectivity with premotor and lateral prefrontal cortices, which are heaviest in the pdACC and adACC 67, 71. In summary, the pattern of anatomical connectivity supports an important role for the sgACC, pgACC and adACC in interacting with the limbic system, including its effector regions, and for the adACC and pdACC in communicating with other dorsal and lateral frontal areas which are important for top-down forms of regulation 72.
Like the ACC, the mPFC can be divided into several functionally distinct subregions, though borders between these subregions are in general less clear, and differential anatomical connectivity is less well described. Amygdalar, hypothalamic and PAG connectivity with mPFC subregions is considerably lighter than the connectivity of adjacent ACC subregions, with the strongest connections seen for the ventromedial (vmPFC) and and dorsomedial PFC (dmPFC) 46, 68-70.
Much like the nearby ACC subregions, the Supplementary Motor Area (SMA) is heavily interconnected with primary motor cortex, and originates direct corticospinal projections 65, 73. The pre-SMA, by contrast, is connected with lateral prefrontal cortices, but not with primary motor cortex 65, 73. Premotor and lateral prefrontal connections are also present, though to a lesser degree, in the dmPFC 71. Thus, the patterns of connectivity are similar between abutting ACC and mPFC subregions, with the difference being primarily in the density of limbic connectivity, which is substantially greater in the ACC.
Undoubtedly the most influential functional parcellation of this type has been the proposal that there exists a principal dichotomy between caudal-dorsal midline regions that serve a variety of cognitive functions, and rostral-ventral midline regions that are involved in some form of emotional processing 12. However, even this broadly- and long-held view of basic functional specialization in these regions has been shaken by considerable evidence accumulating over the past decade to indicate that many types of emotional processes reliably recruit caudal-dorsal ACC and mPFC regions 13, 14.
Here, we review recent human neuroimaging, animal electrophysiology, and human and animal lesion studies that have produced a wealth of data about the role of the ACC and mPFC in the processing of anxiety and fear. We chose to focus primarily on the negative emotions of anxiety and fear because they are by far the most experimentally tractable and most heavily studied, and they afford the closest link between animal and human data. We subsequently briefly examine whether a conceptual framework derived from fear/anxiety generalizes to other emotions.
Given the complexity 15 and multi-dimensional nature 16 of emotional responses, we will speak of the specific functions or processes that constitute an emotional reaction, regardless of whether they are classically seen as ‘emotional’ (e.g., a withdrawal response or a feeling) or ‘cognitive’ (e.g., attentional focussing on a relevant stimulus). We also distinguish between processes involved in emotional stimulus appraisal and consequential response expression 17 and those involved in emotion regulation. Regulation occurs when stimuli induce conflicting appraisals and hence incompatible response tendencies or when goal-directed activity requires suppression of interference from a single, emotionally salient, task-irrelevant stimulus source. We found that an appraisal/expression versus regulation contrast provides a robust framework for understanding ACC/mPFC function in negative emotion.
Fear conditioning and extinction in humans
The paradigms used in the acquisition and extinction of learned fear are particularly valuable for isolating the neural substrates of fear processing because the anticipatory fear or anxiety triggered by the previously neutral conditioned stimulus (CS) can be dissociated from the reaction to the aversive unconditioned stimulus (US) per se. This is not possible in studies that, for example, use aversive images to evoke emotional responses. Furthermore, comparison between fear conditioning and fear extinction allows us to make an initial coarse distinction between regions associated with either the appraisal of fear-relevant stimuli and generation of fear responses (fear conditioning), or the inhibitory regulation of these processes (extinction).
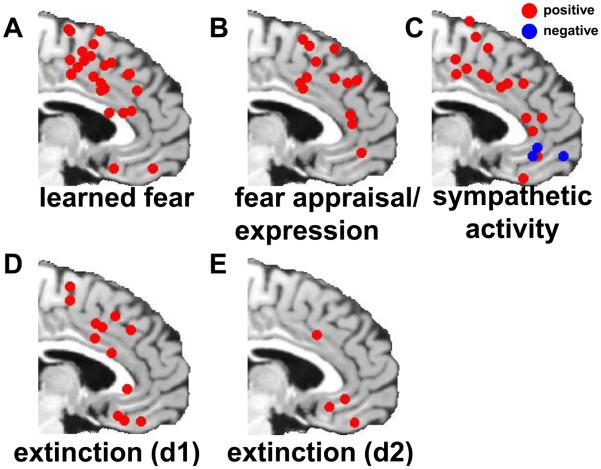
Several recent quantitative meta-analytic reviews of human neuroimaging studies examined activations associated with fear CS presentation, compared to a control CS never paired with the US 13, 14, 18. In Figure 1A we present plots of the location of each activation peak reported in the ACC or mPFC in the relevant fear conditioning studies, collapsing across left and right hemispheres. It is readily apparent that activations in fear conditioning studies are not evenly distributed throughout the ACC and mPFC, but rather are clustered heavily within the dorsal ACC (dACC), dorsomedial PFC (dmPFC), supplementary motor area (SMA) and pre-SMA. These activations, however, may reflect a variety of different processes that occur simultaneously or in rapid temporal succession, for example CS appraisal and expression of conditioned responses (CRs). These processes are intermixed with, and supported by, learning processes, that is, acquisition, consolidation, and storage of a fear memory (CS-US association), and retrieval of the fear memory upon subsequent CS presentations.
Figure 1.
Activation foci associated with fear and its regulation. Predominantly dorsal ACC/mPFC activations are seen during classical (Pavlovian) fear conditioning (A), as well as during instructed fear paradigms, which circumvent fear learning (B). Likewise, sympathetic nervous system activity correlates positively primarily with dorsal ACC/mPFC regions and negatively primarily with ventral ACC/mPFC regions, supporting a role for the dorsal ACC/mPFC in fear expression (C). During within-session extinction, activation is seen in both the dorsal and ventral ACC/mPFC (D), while during subsequent delayed recall and expression of the extinction memory, when the imaging data is less confounded by residual expression of fear responses, activation is primarily in the ventral ACC/mPFC (E). Information of the studies selected for this and all following peak voxel plots can be found in the online supplemental materials.
The acquisition component of fear conditioning can, to some extent, be circumvented by instructing subjects about CS-US contingencies at the beginning of the experiment. Such “instructed fear” experiments nevertheless also consistently activate the dorsal ACC/mPFC 14, 19(see Figure 1B). Similarly, recalling and generating fear in the absence of reinforcement several days after conditioning activates dorsal midline areas, and is not confounded by fear learning 20. Rostral parts of the dorsal ACC/mPFC are specifically involved in the (conscious) appraisal, but not direct expression, of fear responses, as shown by reduction of rostral dACC/dmPFC activity to threat by high working memory load, in the context of unchanged physiological reactivity 2, 14 and correlations of rostral dACC/dmPFC activity with explicit threat evaluations but not physiological threat reactions 21.
Response expression, on the other hand, appears to involve more caudal dorsal areas, in SMA/pre-SMA, pdACC and caudal parts of dmPFC and adACC, though some of the evidence for this contention is indirect and based on studies of the arousal component inherent to most fear/anxiety responses. For example, figure 1C shows clusters which correlate with sympathetic nervous system activity, irrespective of whether the context was fear-related or not. Positive correlations are found throughout the mPFC, but again primarily clustered in mid-to-caudal dorsal mPFC areas. Lesion (e.g. Critchley et al.22) and electrical stimulation studies 23 confirm this anatomical distribution.
Considering these data in conjunction with observations that dACC activity correlates with fear-conditioned skin conductance responses (e.g. Milad et al.24 and with increases in heart rate induced by a socially threatening situation 25, as well as findings that direct electrical stimulation of the dACC can elicit subjective states of fear 26, strongly suggests that the dorsal ACC/mPFC is involved in generating fear responses. Neuroimaging studies of autonomic nervous system activity also indirectly suggest that the same areas do not exclusively function in response expression, but may also support appraisal processes. For example, the dorsal ACC/mPFC is associated with interoceptive awareness of heart beats (e.g. Critchley et al.27) and, importantly, recruitment of the dorsal ACC/mPFC during interoceptive perception is positively correlated with subjects’ trait anxiety levels 27. Thus, the dorsal ACC/mPFC appears to function generally in the appraisal and expression of fear or anxiety. These studies leave uncertain the role that the dorsal ACC/mPFC may play in the acquisition of conditioned fear, although converging evidence from studies in rodents (see Box 2) suggests only a minor role in acquisition.
Box 2. Studies of fear conditioning and extinction in rodents.
A rich literature has developed examining the role of the rodent medial frontal cortex in the acquisition and extinction of conditioned fear, as well as the expression of conditioned and unconditioned fear 74. These studies allow a greater degree of causal inference than imaging studies. Much like the human dorsal ACC/mPFC, the rodent mPFC is strongly activated during fear conditioning 75, 76. Lesion or acute inactivation studies have found a role for the ventrally-located infralimbic (IL) and dorsally-located prelimbic (PL) subregions in conditioned fear expression, when recall tests are performed within a few days after initial conditioning 77-81. Interestingly, the mPFC does not appear to be required during fear acquisition itself, as evidenced by intact initial fear learning after disruption of IL or PL prior to conditioning 82-85. As with expression of fear memories, activity in the rodent mPFC is also required for expression of unconditioned fear 86, 87.
In terms of extinction, the recall and expression of an extinction memory >24 hours after learning requires activity in IL 80, 82, 84, 88 and to some degree PL 85, 89. By contrast, within-session extinction of CRs during the repeated non-reinforced presentations of the CS does not require activity in IL or PL 80, 82, 84, 88. Thus, the role of the mPFC during extinction closely follows its role during fear conditioning – that is, being required for recall or expression, but not for initial acquisition.
Electrical microstimulation of the rodent mPFC generally does not directly elicit fear behavior or produce overt anxiolysis, but rather exerts a modulatory function, gating behavioral output elicited by external fear-eliciting stimuli or by direct subcortical stimulation 90-93. Curiously given the role of the mPFC in fear expression, these effects have generally, but not exclusively, been found to be fear-inhibitory, and occur with stimulation in all mPFC subregions 90-93. Of note, however, one recent study found a fear-enhancing effect of PL stimulation, but a fear-inhibiting effect of IL stimulation 92. Together, these findings suggest that a model of mPFC function in fear or extinction must account for interactions of the mPFC with other elements of the fear circuit, since the mPFC itself functions primarily by modifying activity in other brain areas.
With respect to one important interacting partner, the amygdala, stimulation in the IL or PL has been reported to inhibit the activity of output neurons in the central amygdalar nucleus (CEA)94, as well as the basolateral amygdalar complex (BLA) 95. IL and PL stimulation can also directly activate BLA neurons 96. Thus, the mPFC can promote fear expression through BLA activation, and can inhibit amygdala output through CEA inhibition. CEA inhibition, however, is achieved through the action of excitatory glutamatergic mPFC projections onto inhibitory interneurons in the amygdala, likely through the intercalated cell masses 97, 98. Innervation of the intercalated cell masses originates more prominently from IL than PL 99, 100, supporting a preferential role for IL in inhibitory regulation of the amygdala.
To elucidate how fear may be regulated, we next discuss activations associated with extinction of learned fear. In extinction, the CS is repeatedly presented in the absence of reinforcement, leading to the formation of a CS-no US association (or extinction memory) that competes with the original fear memory for control over behavior 28-30. Hence, extinction induces conflicting appraisals of, and response tendencies to, the CS as it now signals both threat and safety, a situation that requires regulation, as outlined in the introduction. We further distinguish between within-session extinction (Figure 1D; “day 1”) and extinction recall, as tested by CS presentation on a subsequent day (Figure 1E; “day 2”). Within-session extinction is associated with activation in both the dorsal ACC/mPFC (dACC, dmPFC, SMA/pre-SMA), as well as the ventral ACC/mPFC (pgACC and vmPFC; see Figure 1D). Given the close association of dorsal ACC/mPFC with fear conditioning responses, it should be noted that the activations observed within these regions during fear extinction may in fact reflect remnants of fear conditioning, because in early extinction trials the CS will continue to elicit a residual CR. Activation within the ventral ACC/mPFC is thus a candidate neural correlate of the fear inhibition that occurs during extinction (for converging rodent data, see Box 2). Accordingly, acute reversal of a fear conditioning contingency, during which a neutral, non-reinforced, CS is paired with an aversive stimulus while the previously reinforced CS is not and now inhibits fear, is associated with activation in the pgACC 31. Likewise, exposure to distant threat is associated with ventral ACC/mPFC activation, presumably acting in a regulatory capacity to facilitate planning of adaptive responses, while more imminent threat is associated with dorsal ACC/mPFC activation, which is consistent with greater expression of fear responses 32. Along with ventral ACC/mPFC activation during extinction, decreases in amygdalar responses have also been reported 33, 34, consistent with the idea that amygdalar inhibition is an important component of extinction.
In support of this conclusion, recall of extinction >24 hours after conditioning, a process that is less confounded by residual CRs, yields primarily ventral ACC/mPFC activations (pgACC, sgACC, vmPFC; see figure 1E). It should be stressed, however, that extinction, like conditioning, involves multiple component processes, including acquisition, consolidation, storage, and retrieval of the extinction memory, and the related appraisal of the CS as safe, of which CR inhibition is only the endpoint. The limited number of human neuroimaging studies of extinction do not allow a reliable parcellation of these processes, although a rich literature in rodents suggests that, like for fear conditioning, the role of the mPFC is primarily in expression rather than acquisition of inhibitory fear memories (see Box 2). Moreover, our conclusions are also supported by findings of negative correlations primarily between ventral areas (pgACC and vmPFC) and sympathetic activity (Figure 1C), and with activation in an area consistent with the periaqueductal gray (PAG), which mediates heart rate increases under social threat 25, 35.
In summary, neuroimaging studies of the learning and extinction of fear in humans reveal evidence for an important differentiation between dorsal ACC/mPFC subregions, which are implicated in threat appraisal and the expression of fear, and ventral ACC/mPFC subregions, which are involved in the inhibition of conditioned fear through extinction.
Emotional conflict regulation
Convergent evidence for the functional differentiation between the dorsal and ventral ACC/mPFC comes from work on “emotional conflict”. Two recent studies employed a task that required subjects to categorize face stimuli according to their emotional expression (fearful vs. happy), whilst attempting to ignore emotionally congruent or incongruent word labels (HAPPY, FEAR) superimposed over the faces. Emotional conflict, created by a word label that is incongruent with the facial expression, was found to substantially slow reaction times 8, 36. Moreover, when incongruent trials were preceded by an incongruent trial, reaction times were faster than if incongruent trials were preceded by a congruent trial 8, 36, an effect that has previously been observed in traditional, non-emotional conflict tasks, such as the Stroop or flanker protocols 37. According to the “conflict-monitoring model” 38, this data pattern stems from a conflict-driven regulatory mechanism, where conflict from an incongruent trial triggers an up-regulation of top-down control, reflected in reduced conflict on the subsequent trial. This model allows one to distinguish between brain regions involved in conflict evaluation and those involved in conflict regulation 38, 39. In the studies of emotional conflict, regions which activated more to post-congruent incongruent trials than post-incongruent incongruent trials, interpreted as being involved in conflict evaluation, included the amygdala, dACC/dmPFC and dorsolateral PFC 8, 36. The role of dorsal ACC/mPFC areas in detecting emotional conflict is further echoed by other studies of various forms of emotional conflict or interference, the findings of which we plot in Figure 2A.
Figure 2.
(A) Emotional conflict across a variety of experimental paradigms is associated with activation in the dorsal ACC/mPFC. (B) Decreasing negative emotion through reappraisal is associated with preferential activation of the dorsal ACC/mPFC. Targets of amygdalar connectivity during tasks involving appraisal/expression (C) or regulation (D) of negative emotion. Positive connectivity is seen primarily during appraisal/expression tasks, and most heavily in the dorsal ACC/mPFC. By contrast, negative connectivity is seen most heavily in the ventral ACC/mPFC across both appraisal/expression and regulation tasks. These connectivity findings are therefore consistent with the dorsoventral functional-anatomical parcellation of the ACC/mPFC derived from activation analyses.
By contrast, regions more active in post-incongruent incongruent trials are interpreted as being involved in conflict regulation, and prominently include the pgACC 8, 36. Regulation-related activation in the pgACC was also accompanied by a simultaneous and correlated reduction of conflict-related amygdalar activity and does not seem to involve biasing of early sensory processing streams 39, but rather the regulation of affective processing itself 36. These data echo the dorsal-ventral dissociation discussed above with respect to fear expression and extinction in the ACC/mPFC.
The circuitry we find to be specific for regulation of emotional conflict (ventral ACC/mPFC and amygdala) is very similar to that involved in extinction. While these two processes are unlikely to be isomorphic, and each can be understood without reference to the other, we consider the striking similarity between extinction and emotional conflict regulation to be potentially important. Much like the relationship between improved emotional conflict regulation and decreased conflict evaluation-related activation in the dorsal ACC/mPFC, more successful extinction is associated with decreased CS-driven activation in the dorsal ACC/mPFC of humans and rodents 40, 41. As such, the most parsimonious explanation for these data is that emotional conflict evaluation-related functions involve overlapping neural mechanisms with appraisal and expression of fear, and that regulation of emotional conflict also involves circuitry that overlaps with fear extinction. These conceptual and functional-anatomical similarities between evaluation and regulation of emotional conflict and fear also support the generalizeability of our account of ACC/mPFC functional subdivisions beyond simply fear-related processing, but more generally to negative emotional processing. Of note, while the intensity of the negative emotion elicited during fear conditioning and evoked by emotional conflict differ significantly, they nonetheless engage a similar neural circuitry, likely since both fear and emotional conflict reflect biologically salient events.
Top-down control of emotion
During emotional conflict regulation, emotional processing is spontaneously modulated in the absence of an explicit instruction to regulate emotion. Emotional processing can also be modulated through deliberate and conscious application of top-down executive control over processing of an emotional stimulus. The best-studied strategy for the latter type of regulation is reappraisal, a cognitive technique whereby the appraisal of a stimulus is modified in order to change its ability to elicit an emotional reaction 42. Reappraisal involves both the initial emotional appraisal process, as well as the reappraisal process proper, where an additional positive appraisal is created that competes with the initial negative emotional appraisal. Thus, we would expect reappraisal to involve the dorsal ACC/mPFC regions that we observed to be important for emotional conflict detection (see Figure 2A). Consistent with this prediction, a meta-analytic study found reappraisal to be reliably associated with activation in the dorsal ACC/mPFC 43 (see Figure 2B).
This reappraisal meta-analysis, interestingly, did not implicate a consistent role for the ventral ACC/mPFC 43, suggesting that reappraisal does not primarily work by suppressing the processing of the undesired emotional stimulus. Nevertheless, activity in the ventral ACC/mPFC has in some instances been found to be negatively correlated with activity in the amygdala in paradigms in which reappraisal resulted in the downregulation of amygdalar activity in response to negative pictures 44, 45. Thus, the ventral ACC/mPFC may be a mediator between activation in dorsal medial and lateral prefrontal areas, involved in reappraisal 43, and the amygdala, with which lateral prefrontal structures in particular have little or no direct connectivity 46. Consistent with this idea the ventral ACC/mPFC is also engaged when subjects perform affect labeling of emotional faces 47 or when they self-distract from a fear-conditioned stimulus 48, two other emotion regulation strategies that result in downregulation of amygdalar activity.
These data suggest that controlled top-down regulation, like emotional conflict regulation, employs ventral ACC/mPFC areas to inhibit negative emotional processing in the amygdala, thus dampening task interference. The ventral ACC/mPFC may thus perform a generic negative emotion inhibitory function that can be recruited by other regions (e.g. dorsal ACC/mPFC and lateral PFC) whenever there is a need for suppressing limbic reactivity 10. This would be a prime example of a parsimonious use of basic emotional circuitry, conserved between rodents and humans (see Box 2), for the purpose of higher-level cognitive functions possible only in humans.
Amygdala-ACC/mPFC functional connectivity
Our analysis of the neuroimaging data has emphasized task-based activation studies. Complementary evidence can be found in analyses of functional connectivity, as ACC/mPFC subregions can be distinguished through their differential anatomical connectivity (Box 1). In some ways, psychological context-specific temporal covariation (i.e. task-dependent connectivity) between regions may provide an even stronger test of the nature of inter-regional relationships than consistency with which regions simply coactivate in a task. Figure 2C and 2D show the ACC/mPFC connectivity peaks for all such connectivity studies, irrespective of the specific paradigm or instructions used (primarily general negative stimuli), as long as the task allowed a discrimination between appraisal/expression (Figure 2C) or regulation (Figure 2D). The spatial distribution of positive and negative connectivity peaks during reactivity tasks shows a relative preponderance of positive connectivity peaks in the dorsal ACC/mPFC and of negative connectivity peaks in the ventral ACC/mPFC. In addition, during regulation tasks, connectivity was restricted to the ventral ACC/mPFC, and was primarily negative (Figure 2D). These data thus lend further support to our proposal of a dorso-ventral separation in terms of negative emotion generation (appraisal/expression) and inhibition (regulation).
Integration with other perspectives on ACC/mPFC function and other emotions
Though less developed than the literature on fear/anxiety, studies on other emotions are broadly consistent with our formulation of ACC/mPFC function. On the negative emotion appraisal/expression side, direct experience of pain, or empathy for others experiencing pain, activate the dorsal ACC/mPFC 49, and lesions of the dACC also serve as treatments for chronic pain 50. Similarly, increased sensitivity to a range of negative emotions is associated with greater engagement of the dorsal ACC/mPFC, including in disgust 51 and rejection 52, and TMS-induced disruption of the dmPFC interferes with anger processing 53. Uncertainty or ambiguity, which can induce anxiety and relates to emotional conflict, leads to activation in the dACC/dmPFC 54. On the regulation side, endogenously driven analgesia, by means of the ‘placebo effect’, has been closely tied to the pgACC, which is thought to engage in top-down modulation of regions that generate opioid-mediated anti-nociceptive responses, such as the amygdala and periaqueductual gray 55, 56. Still unclear is how sadness is evaluated and regulated, and what role the sgACC plays in these processes, as it is a common activation site in response to sad stimuli 57.
Positive emotion, which can serve to regulate and diminish negative emotion, has been associated in a meta-analysis with activation in the sgACC, vmPFC and pgACC 58. Extinction of appetitive learning activates the vmPFC 59, much as extinction of learned fear does. The evaluation of positive stimuli and reward is more complicated. For instance, Rushworth and co-workers proposed that the processes carried out by the adACC are mirrored by similar contributions to reinforcement-guided decision-making from the orbitofrontal cortex, with the distinction that the adACC is concerned with computing reinforcement value of actions whereas the orbitofrontal cortex is concerned with gauging the reinforcement values of stimuli 60.
Taken together, these data broadly support our dorsal/ventral distinction along appraisal/expression versus regulation lines, with respect specifically to negative emotion. On the other hand, it is not obvious how to accommodate our analysis with the suggestion that the vmPFC specifically assesses stimulus values 10, but not action values, with the opposite being the case for the dACC 60. As such, this should be seen as an early attempt to integrate these (and other non-discussed) models of ACC/mPFC function, and serve to stimulate further research in this area.
It is also worth examining why the conceptualization proposed in this review differs significantly from the earlier view of a cognitive/affective division 12. Though the meta-analysis reported in the earlier paper did not indicate which specific studies were included, it appears that much of the support for this scheme comes from studies of patients with affective disorders, wherein ventral ACC/mPFC dysfunction can be more readily seen in the context of deficits in regulation 40, 61. Moreover, the dorsal/ventral dissociation between dACC activation in a “counting Stroop” and pgACC in an “emotional counting Stroop” 12 has not held up to subsequent evidence (see Figure 2A) or direct contrasts between emotional and non-emotional conflict processing 36, nor does the emotional counting Stroop involve a true Stroop conflict effect, in the way that the counting Stroop does 62.
Concluding remarks
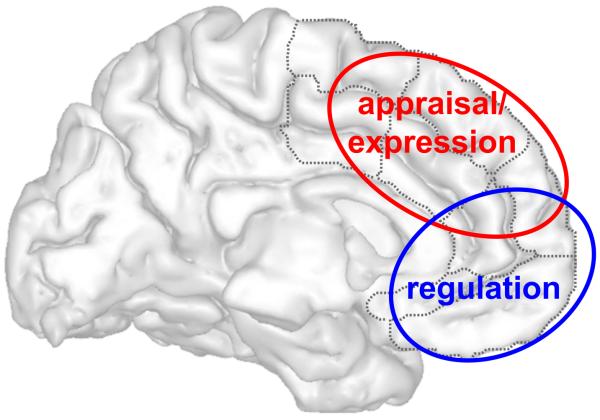
This review has highlighted several important themes. First, the empirical data do not support the long-held popular view that dorsal ACC/mPFC regions are involved in “cognitive” but not “emotional” functions, while ventral regions do the reverse 12. Rather, the key functional distinction between these regions relates to evaluative function, on the one hand, and regulatory function, on the other hand, for the dorsal and ventral ACC/mPFC, respectively (see Figure 3). This new framework also broadly generalizes to other negative and positive emotions, and points to multiple exciting lines of future research (see Box 3).
Figure 3.
A graphical depiction of the ACC/mPFC functional model aligned across an appraisal/expression versus regulation dimension for negative emotion. The imperfect separation of these functions across the dorsal and ventral ACC/mPFC noted in the reviewed studies is represented schematically as an intermixing of red (appraisal/expression) and blue (regulation) circles.
Box 3. Future directions.
Further work is needed in particular in exploring the neurophysiological basis for appraisal/expression versus regulation-related signaling in the ACC/mPFC of experimental animals. Specifically, how does this coding take place at the single-cell level and how do these effects result in the dorsal/ventral division in ACC/mPFC function seen in human imaging studies?
We have left out discussion of other regions, such as the insula and brainstem, which are likely important partners of the ACC/mPFC, though far less is known about these interactions. Additional work is required to bring to these interaction the depth of understanding currently available for interactions with the amygdala. Moreover, a better systems-level understanding of how ACC/mPFC activity is shaped by its input regions, such as the amygdala, hippocampus and thalamus, is necessary.
Though we hint at levels of similarity between our model of ACC/mPFC in negative emotion and other models of the roles of this region in other functions, additional work is required to directly contrast and harmonize other conceptualizations of ACC/mPFC functions, in order to create a more comprehensive framework capable of making predictions about a wide range of task contexts.
With a few notable exceptions 40, 61 the sophisticated cognitive neuroscience models described above have not been extended to populations with anxiety-related disorders. A great deal of work will be needed to translate our increasingly nuanced descriptions of ACC/mPFC functions into a better understanding of psychopathology.
Supplementary Material
Figure I.
Parcellation of anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) subregions. Abbreviations: sg=subgenual, pg=pregenual, vm=ventromedial, rm=rostromedial, dm=dorsomedial, ad=anterior dorsal, pd=posterior dorsal, SMA=supplementary motor area.
Acknowledgements
We would like to thank Gregory Quirk, Kevin LaBar, James Gross and Carsten Wotjak for their helpful comments and criticisms of this manuscript.
This work was supported by NIH grants P30MH089888 and R01MH091860, and the Sierra-Pacific Mental Illness Research Education and Clinical Center at the Palo Alto VA Health Care System
Glossary
- Fear conditioning
Refers to a learning paradigm in which a previously neutral stimulus, termed the “conditioned stimulus” (CS) is temporally paired with a non-learned aversive stimulus, termed the “unconditioned stimulus” (US). After pairing, the CS predicts the US, and hence elicits a “conditioned response” (CR). For example, pairing a tone with a foot shock results in elicitation of fear behavior during subsequent response to a non-paired tone.
- Extinction
Refers to a learning process created by repeatedly presenting the CS without pairing with a US (i.e. teaching the animal that the CS no longer predict the US), after fear conditioning had been established. This results in formation of an extinction memory, which inhibits expression of, but does not erase, the original fear memory.
- Appraisal
The evaluation of the meaning of an internal or external stimulus to the organism. Only stimuli that are appraised as motivationally significant will induce an emotional reaction, and the magnitude, duration, and quality of the emotional reaction are a direct result of the appraisal process. Moreover, appraisal may be automatic and focus on basic affective stimulus dimensions such as novelty, valence/value, or expectation discrepancy or may be slower and sometimes even require controlled conscious processing, which permits a more sophisticated context-dependent analysis.
- Regulation
A general process by which conflicting appraisals, and response tendencies, are arbitrated between in order to allow selection of a course of action. Typically, regulation is thought to have an element of inhibition and/or enhancement for managing the competing appraisals and response tendencies.
- Reappraisal
A specific method for explicit emotion regulation, wherein conscious, deliberate effort is engaged to alter the meaning (appraisal) of an emotional stimulus. For example, a picture of a woman crying can be reappraised from a negative meaning to a positive one by favoring an interpretation that she is crying tears of joy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Amit Etkin receives consulting fees from NeoStim. The other authors report no financial conflicts.
References
- 1.Papez JW. A proposed mechanism of emotion. Archives of Neurological Psychiatry. 1937;38:725–743. [Google Scholar]
- 2.Kalisch R, et al. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Rushworth MF, et al. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A, et al. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 7.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 8.Etkin A, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt BA, et al. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 12.Bush G, et al. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 13.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mechias ML, et al. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Levenson RW. Blood, sweat, and fears: the autonomic architecture of emotion. Ann N Y Acad Sci. 2003;1000:348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- 16.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 17.Roseman IJ, Smith CA. Appraisal theory: Overview, assumptions, varieties, controversies. In: Scherer KR, et al., editors. Appraisal processes in emotion: theory, methods, research. OUP; 2001. pp. 3–19. [Google Scholar]
- 18.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 19.Klucken T, et al. Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture-picture conditioning paradigm. Neuroscience. 2009;158:721–731. doi: 10.1016/j.neuroscience.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Kalisch R, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex. 2009;19:187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raczka KA, et al. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing. Mol Psychiatry. 2010;15:1045, 1067–1074. doi: 10.1038/mp.2010.79. [DOI] [PubMed] [Google Scholar]
- 22.Critchley HD, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 23.Gentil AF, et al. Physiological responses to brain stimulation during limbic surgery: further evidence of anterior cingulate modulation of autonomic arousal. Biol Psychiatry. 2009;66:695–701. doi: 10.1016/j.biopsych.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Milad MR, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Wager TD, et al. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer G, et al. Stereotactic cingulotomy with results of acute stimulation and serial psychological testing. In: Laitinen LV, Livingston KE, editors. Surgical approaches in psychiatry. University Park Press; 1973. [Google Scholar]
- 27.Critchley HD, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 28.Bouton ME. Context and behavioral processes in extinction. Learn.Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 29.Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q.J.Exp.Psychol.B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- 30.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 31.Schiller D, et al. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobbs D, et al. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milad MR, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelps EA, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 35.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egner T, et al. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 37.Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- 38.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 39.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 40.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgos-Robles A, et al. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 43.Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Johnstone T, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amaral DG, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- 47.Lieberman MD, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 48.Delgado MR, et al. Diminishing Fear: Shared Neural Circuitry Underlies Emotion Regulation and Extinction. Neuron. 2008 in press. [Google Scholar]
- 49.Lamm C, et al. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. doi: 10.1016/j.neuroimage.2010.10.014. in press. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson HA, et al. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999;45:1129–1134. doi: 10.1097/00006123-199911000-00023. discussion 1134-1126. [DOI] [PubMed] [Google Scholar]
- 51.Mataix-Cols D, et al. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. Eur J Neurosci. 2008;27:3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- 52.Eisenberger NI, et al. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 53.Harmer CJ, et al. Transcranial magnetic stimulation of medial-frontal cortex impairs the processing of angry facial expressions. Nat Neurosci. 2001;4:17–18. doi: 10.1038/82854. [DOI] [PubMed] [Google Scholar]
- 54.Nomura M, et al. Frontal lobe networks for effective processing of ambiguously expressed emotions in humans. Neurosci Lett. 2003;348:113–116. doi: 10.1016/s0304-3940(03)00768-7. [DOI] [PubMed] [Google Scholar]
- 55.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Petrovic P, et al. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 57.Phan KL, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 58.Wager TD, et al. The Neuroimaging of Emotion. In: Lewis M, editor. Handbook of Emotion. Third edn The Guilford Press; 2008. [Google Scholar]
- 59.Finger EC, et al. Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage. 2008;43:748–755. doi: 10.1016/j.neuroimage.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rushworth MF, et al. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Etkin A, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Algom D, et al. A rational look at the emotional stroop phenomenon: a generic slowdown, not a stroop effect. J Exp Psychol Gen. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- 63.Vogt BA. Cingulate Gyrus. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2nd edn Elsevier; 2004. pp. 915–949. [Google Scholar]
- 64.Vogt BA, et al. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 66.Ghashghaei HT, et al. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beckmann M, et al. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiba T, et al. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- 69.Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 70.An X, et al. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 71.Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- 72.Mansouri FA, et al. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 73.Nachev P, et al. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 74.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgos-Robles A, et al. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herry C, et al. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. Journal of Neurophysiology. 1999;82:2827–2832. doi: 10.1152/jn.1999.82.5.2827. [DOI] [PubMed] [Google Scholar]
- 77.Resstel LB, et al. The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex. 2008;18:2027–2035. doi: 10.1093/cercor/bhm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blum S, et al. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- 79.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- 81.Laviolette SR, et al. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J.Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quirk GJ, et al. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Runyan JD, et al. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sierra-Mercado D, Jr., et al. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 85.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 86.Maaswinkel H, et al. Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav.Brain Res. 1996;79:51–59. doi: 10.1016/0166-4328(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 87.Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- 88.Lebron K, et al. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn.Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 89.Hugues S, et al. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- 90.al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J of the Auton Nerv Sys. 1989;28:117. doi: 10.1016/0165-1838(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 91.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 92.Vidal-Gonzalez I, et al. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zbrozyna AW, Westwood DM. Stimulation in prefrontal cortex inhibits conditioned increase in blood pressure and avoidance bar pressing in rats. Physiol Behav. 1991;49:705–708. doi: 10.1016/0031-9384(91)90306-9. [DOI] [PubMed] [Google Scholar]
- 94.Quirk GJ, et al. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J.Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenkranz JA, et al. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J.Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Likhtik E, et al. Prefrontal control of the amygdala. J.Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amano T, et al. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 99.McDonald AJ, et al. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 100.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.