Abstract
Objectives
The Cox-Maze III procedure(CMP) achieved high cure rates and became the surgical gold standard for the treatment of atrial fibrillation(AF). Due to its invasiveness, a more simplified ablation-assisted procedure(CMP-IV) has been performed at our institution since January, 2002. The study examined multiple preoperative and perioperative variables to determine predictors of late recurrence.
Methods
Data were collected prospectively on 282 patients who underwent the CMP-IV from January 2002 through December 2009. Forty-two percent of patients had paroxysmal and 58% had either persistent or long-standing persistent AF. All patients were available for follow-up. Follow-up included ECGs in all patients. Since 2006, 24 hour holter monitoring was obtained in 94% of patients at 3, 6 and 12 months. Data were analyzed by logistic regression analysis at 12 months with 13 preoperative and perioperative variables used as co-variants.
Results
Sixty-six percent of patients had a concomitant procedure. Following an ablation-assisted CMP, the freedom from AF was 89%, 93%, and 89% at 3, 6, and 12 months, respectively. The freedom from both AF and antiarrhythmic drugs was 63%, 79%, and 78% at 3, 6, and 12 months. The risk factors for AF recurrence at one year were enlarged left atrial(LA) diameter(p=0.027), failure to isolate the entire posterior left atrium(p=0.022), and early atrial tachyarrhythmias (ATAs)(p=0.010).
Conclusions
The CMP-IV has a high success rate at one year, even with improved follow-up and stricter definitions of failure. In patients with large LA, there may be a need for more extensive size reduction or expanded lesion sets.
INTRODUCTION
The Cox-Maze procedure was introduced in 1987 at our institution for the treatment of atrial fibrillation (AF) by Dr. James L. Cox.1 The first two iterations of this procedure were abandoned because of a high incidence of pacemaker implantation and technical difficulty. The final version introduced was the Cox-Maze III procedure, and it remained the gold standard for the treatment of AF for more than a decade. In a follow-up study of 198 consecutive patients from our institution, the overall freedom from symptomatic atrial fibrillation was 97% with a mean follow-up of 5.4 ± 2.9 years. 2 While this was a high success rate, the patients did not undergo rigorous follow-up by present standards. Most of the rhythms were documented only by means of a mailed questionnaire or telephone interview. Very few of the patients had any monitoring more than an electrocardiogram to document their rhythm. Moreover, the most recent consensus statement on ablation for AF has proposed that success be defined as freedom from atrial fibrillation, atrial tachycardia, or atrial flutter (ATAs) off of antiarrhythmic drugs.3 In our previous report, success was considered simply freedom from symptomatic AF. Our results with the original cohort of the cut-and-sew Cox-Maze III procedure showed that 27% of patients were on antiarrhythmic drugs at last follow-up.2
In 2002, a new iteration of the Cox-Maze procedure was introduced, termed the Cox-Maze IV procedure, which replaced most of the incisions with linear lines of bipolar radiofrequency ablation (Figure 1).4 Initially, the procedure had only a single inferior connecting ablation line between the pulmonary veins in an attempt to better preserve left atrial function. However, a second connecting lesion superiorly was added several years later to anatomically isolate the entire posterior left atrium (termed box lesion set) in an attempt to improve early and late results. At this time, we also adopted a more rigorous follow-up of the patients, with all patients having either electrocardiographic or 24 hour holter monitoring at 3, 6, and 12 months and annually thereafter. An examination of predictors of failure following the Cox-Maze procedure was published by our group in 2005.5 However, the great majority of patients in this series underwent a Cox-Maze III procedure. As stated above, the definition of failure was not rigorous, and patients rarely had electrocardiographic monitoring. The only risk factors for late recurrence of atrial fibrillation were duration of preoperative atrial fibrillation and earlier version of the Cox-Maze procedure, with both the Cox-Maze I and II having higher failure rates than the Cox-Maze III.
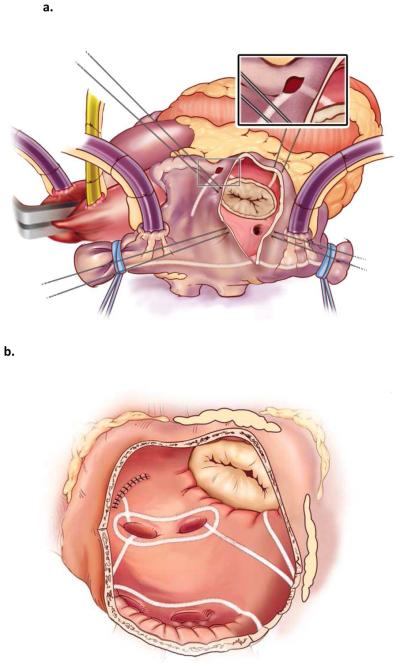
Figure 1.
a. Right atrial lesion set of the Cox-Maze IV procedure
b. Left atrial lesion set of the Cox-Maze IV procedure
In order to better define predictors of failure in the modern era, this study was designed to look at our experience with the Cox-Maze IV procedure alone, in which follow-up has been more rigorous and intensive, with 24 hour holter monitoring obtained in the majority of patients at 3, 6 and 12 months. Moreover, patients were considered to be a success only if they were both free of ATAs and off antiarrhythmic drugs. A previous report from our institution has shown that this is important in order to accurately define criteria for successful outcome.6 In this study, our prospective database was reviewed to evaluate the predictors of late ATA recurrence in 282 consecutive patients who underwent a Cox-Maze IV procedure.
METHODS
From January 2002 through December 2009, 282 patients underwent a Cox-Maze IV procedure for AF. All procedures were performed on cardiopulmonary bypass. The right atrial lesion set was done on the beating heart, while the left atrial (LA) lesions were performed under cardioplegic arrest. As described previously, two small atriotomies were performed. The rest of the incisions of the Cox Maze III procedure were replaced with linear ablation lines using either the AtriCure or Medtronic bipolar radiofrequency clamp. At the tricuspid and mitral valve annuli, cryoablation was used in the vast majority of cases. In a small number of cases, a unipolar ablation pen, either the AtriCure Max1 or the Medtronic Cardioblate XL pen was used. The coronary sinus was ablated both with the bipolar clamp and also with epicardial cryoablation.
In 245 patients (87%), electrical pulmonary vein isolation was confirmed by pacing demonstrating exit block from each of the pulmonary veins. Of the 37 patients who did not have pacing thresholds or exit blocked checked, 12 patients had a suspicious LA appendage clot seen and cardioversion could not be performed. Thirteen patients remained in AF despite cardioversion or were in heart block. Initially, we only performed a single inferior connecting lesion between the pulmonary veins leaving most of the posterior left atrium in electrical continuity with the remainder of the atrial myocardium. In 2005, a second superior connecting lesion was added (box lesion set) to anatomically isolate the entire posterior LA (Figure 1). Pacing was not performed to confirm the electrical isolation of the posterior left atrium. While the majority of patients underwent their procedures through a median sternotomy, 29 patients had a less-invasive right minithoracotomy approach. These patients had an identical lesion set, that has been previously described.7 However, the left atrial appendage was oversewn from the endocardial surface instead of being amputated. Of the entire group, 95 patients (34%) underwent a stand-alone Maze. The majority underwent concomitant surgical procedures, with the most common being either a mitral valve procedure or coronary artery bypass grafting (Table 1). Atrial fibrillation was defined as paroxysmal, persistent, or long-standing persistent per recent guidelines.3 The patients' clinical profiles and postoperative outcomes were recorded prospectively in a longitudinal database containing 386 variables. All patients were available for follow-up and every patient had a minimum of three month follow-up.
Table 1.
Concomitant cardiac procedures
| n = 187 | # | % |
|---|---|---|
| CM + mitral valve procedure | 79 | 42 |
| CM + mitral valve + tricuspid procedure | 14 | 7 |
| CM + CABG | 26 | 14 |
| CM + CABG + mitral valve procedure | 17 | 9 |
| CM + aortic valve procedure | 16 | 9 |
| CM + aortic valve + CABG | 13 | 7 |
| CM + mitral valve + aortic valve procedure | 5 | 3 |
| CM + septal myectomy +/− additional procedures | 7 | 4 |
| CM + other procedures | 10 | 5 |
CABG = coronary artery bypass grafting; CM = Cox-Maze procedure.
In this series, patients were begun on class I or III antiarrhythmic drugs and warfarin prior to hospital discharge. If they were in sinus rhythm at two months, the antiarrhythmic drugs were discontinued. At three months, a 24 hour holter monitor and echocardiogram were obtained. If patients had no evidence of atrial tachyarrhythmias or atrial stasis by echocardiogram, their anticoagulation was stopped.
Follow-up was conducted by obtaining electrocardiograms in all patients at 3, 6 and 12 months. Since 2006 when the new guidelines were first circulated, 24 hour holter monitoring or pacemaker interrogation was obtained in 94% of patients. Late recurrence was defined as any episode of atrial fibrillation, atrial flutter, or atrial tachycardia that lasted more than 30 seconds. Any patient requiring an interventional procedure after three months was deemed a permanent failure. Patients were only considered to be a success if they were both off antiarrhythmic drugs and free of atrial tachyarrhythmias. For the purpose of this analysis, we defined antiarrhythmic drugs as either Vaughan-Williams type I or III. Beta blockers or calcium channel blockers were not considered to be antiarrhythmic drugs. Only patients who were alive at 12 months were included in the analysis. This study was approved by the Washington University School of Medicine institutional review board. Informed consent and permission for release of information were obtained from each participant.
Statistical Analysis
Continuous variables are expressed as mean ± SD unless otherwise specified and categoric data are expressed as counts and proportions. Comparisons were done with paired, two-tailed t test for means of normally distributed continuous variables. Either the χ2 or the Fisher exact test was used to compare categoric data. Thirteen preoperative and perioperative variables were evaluated in a univariate analysis to identify potential predictors of late ATA recurrence. These included age, gender, type and duration of AF, NYHA class, type of bipolar radiofrequency device, left ventricular ejection fraction, failed catheter ablation, left atrial diameter, pulmonary vein box vs. non-box lesion set, concomitant vs. stand-alone Cox-Maze procedure, early ATAs (within the first postoperative month), and early postoperative pacemaker implantation within the first 90 days. Significant covariates on univariate analysis (p ≤ 0.10) or covariates deemed clinically relevant based on our experience were entered into a multivariate binary logistic regression analysis. All data analyses were performed using SPSS (SPSS 11.0 for Windows; SPSS, Chicago, IL).
RESULTS
Demographics
The mean age of patients undergoing a Cox-Maze IV procedure was 63 ± 12 years (range, 23 to 83 years: Table 2). There were 177 men and 105 women. Forty-two percent of patients were in paroxysmal AF. Ten percent were in persistent and 48% in long-standing persistent AF. The median duration of AF was 3.7 years. Most patients (66%) underwent concomitant cardiac operations. In patients undergoing a stand-alone Cox-Maze procedure, 46% (n=44) were referred for failure of medical therapy, 40% (n = 38) had failed a catheter ablation, and 14% of patients (n=13) were referred for either a stroke or TIA. In the entire series, 52 (18%) patients had failed previous catheter ablation.
Table 2.
Patient characteristics
| Variables | Stand alone CM (n = 95) | Concomitant CM (n = 187) | ||
|---|---|---|---|---|
| # | % or (range) | # | % or (range) | |
| Preoperative | ||||
| Age (years) | 56 ± 10 | (28 - 77) | 66 ± 12 | (23 - 83) |
| Male gender | 73 | 77 | 104 | 56 |
| AF duration (years) | ||||
| Mean | 7.5 ± 6.7 | (0.1 - 28.0) | 5.8 ± 7.9 | (0.1 – 46.0) |
| Median | 6.0 | 3.0 | ||
| Paroxysmal AF | 29 | 31 | 89 | 48 |
| NYHA Class 3 or 4 | 19 | 20 | 141 | 75 |
| Sleep apnea | 18 | 19 | 30 | 16 |
| History of failed catheter ablation |
38 | 40 | 14 | 7 |
| Operative | ||||
| Mean CCT (minutes) | 43 ± 15 | 96 ± 27 | ||
| Box lesion around PVs | 73 | 77 | 151 | 81 |
| LA diameter (cm) | 4.8 ± 1.1 | (2.5 – 8.0) | 5.5 ± 1.2 | (2.9 – 10.0) |
| Postoperative | ||||
| Operative mortality (≤ 30 days) |
1 | 1 | 6 | 3 |
| Early ATA | 42 | 44 | 108 | 58 |
| Median hospital LOS (days) | 7 | (4 - 53) | 10 | (4 – 73) |
AF = atrial fibrillation; ATA = atrial tachyarrhythmias; CCT = aortic cross clamp time; CM = Cox-Maze procedure; LA = left atrium; LOS = length of stay; NYHA=New York Heart Association; PVs = pulmonary veins. Early ATA included atrial fibrillation, atrial tachycardia, and atrial flutter.
In the concomitant group, half of the patients underwent mitral or mitral plus tricuspid valve surgery (93 patients, 50%). Forty-three patients (23%) underwent coronary bypass grafting with or without mitral valve repair. In twenty-nine patients (16%), the primary indication was for aortic valve replacement. The rest of the patients underwent more complicated concomitant procedures (Table 1).
Peri-operative results
The overall operative mortality was 2% in the entire series and 1% in the standalone Cox-Maze cohort. The mean aortic cross-clamp time for patients undergoing a Cox-Maze with concomitant surgery was 96 ± 27 minutes. This was shorter in patients undergoing a lone Cox-Maze procedure (43 ± 15 minutes). Previous published work from our group has shown that these times were significantly shorter when compared to our experience with the traditional cut-and-sew Cox-Maze III procedure.8 The median length of stay was 9 days (range 4 – 73). Eleven percent of patients had major perioperative complications (n=31). These complications include reoperation for bleeding (n=14), intra-aortic balloon pump placement (n=9), stroke (n=5), renal failure (n=12), and mediastinitis (n=1). Seven patients experienced more than one major perioperative complication. Early postoperative atrial arrhythmias were documented in 53% of patients (n=150). These dysrhythmias were usually transient, and most resolved over the first month. Early permanent pacemaker placement within 30 days of operation occurred in 9% (n=24) of patients. A large majority of these patients had documented evidence of sick sinus syndrome preoperatively.
Late follow-up
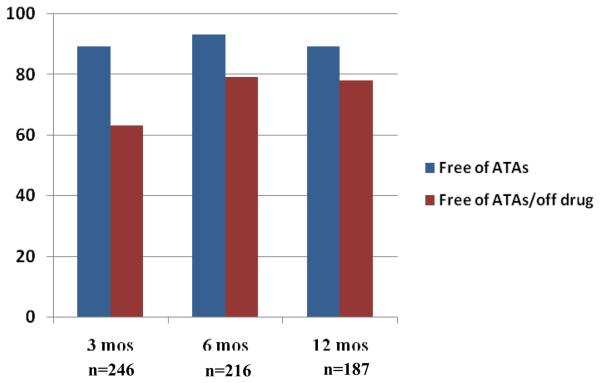
Follow-up was available in all patients. Following a Cox-Maze IV procedure, the freedom from ATAs were 89, 93 and 89% at 3, 6 and 12 months, respectively (Figure 2). The freedom from both antiarrhythmic drugs and arrhythmias was 63, 79 and 78% as 3, 6 and 12 months, respectively. Of the 70% (n=178) with prolonged monitoring, 92% (n=164) were free of ATAs and 79% (n=141) were free of ATAs and off antiarrhythmic medications. There was no difference in late success rate for patients with paroxysmal versus persistent or long-standing atrial fibrillation (p = 0.378). There also was no difference in success rates for patients undergoing stand-alone versus concomitant procedures (p=0.361). Of the 16 patients with ATAs at 6 months, 9 patients had recurrent AF, 5 patients had atrial flutter, and one patient had atrial tachycardia. At 12 months, 15 patients had recurrent AF and 5 patients had atrial flutter.
Figure 2.
Freedom from atrial tachyarrhythmias with and without antiarrhythmic drugs at 3, 6, and 12 months with number of patients at risk.
Recurrence of atrial tachyarrhythmias
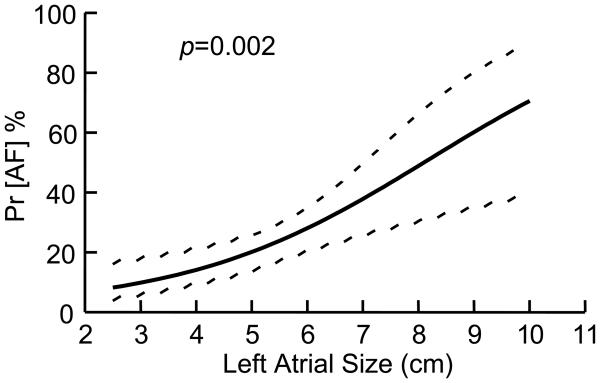
A univariate analysis of preoperative and perioperative variables was performed to determine potential predictors of late ATA recurrence. The only significant predictors of recurrence on univariate analysis were the absence of a box lesion set, increasing left atrial diameter, and early postoperative ATAs. The following variables were entered into a multivariate logistic regression to determine risk factors for failure following a Cox-Maze procedure: age, AF duration, box versus non-box lesion set, left atrial diameter, early postoperative ATAs, and early pacemaker implantation. Left atrial size was a significant predictor of failure, with an odds ratio of 1.42 (Table 3). The probability of AF recurrence increased with increasing LA diameter (Figure 3). The failure to anatomically isolate the entire posterior left atrium (non-box lesion set) was also predictive of failure with a p-valve of 0.022. Finally, patients with early postoperative ATAs were significantly more likely to fail with an odds ratio of 3.05. As opposed to our prior publication, duration of preoperative atrial fibrillation was not a predictor of failure. Other variables which were not predictors of failure at 12 months by multivariate analysis included age and early pacemaker implantation.
Table 3.
Multivariate logistic regression analysis of risk factors for failure following Cox-Maze procedure IV (38/187, 20.3%).
| Variable | N | Odds Ratio | 95% CI | p-value |
|---|---|---|---|---|
| Left atrial diameter | 187 | 1.420 | 1.04 – 1.94 | 0.027 |
| Box lesion set around pulmonary veins | 137 | 0.382 | 0.167 – 0.871 | 0.022 |
| Early atrial tachyarrhythmias | 96 | 3.020 | 1.079 – 8.455 | 0.010 |
Figure 3.
Relationship between left atrial size and probability of atrial fibrillation recurrence
DISCUSSION
The Cox-Maze procedure has been the most successful surgical treatment for atrial fibrillation. 2, 9-11 Our institution has had the longest experience with this operation, and has maintained a prospective database over the last two decades following all of our patients. While we have published extensively on our series, our prior reports have generally not followed recent guidelines in terms of recommended follow-up or definition of failure.3 In this report of over 280 patients, follow-up was more rigorously collected with a strict definition of AF recurrence consistent with the recent consensus statement. Moreover, this report only included patients undergoing an ablation-assisted Cox-Maze IV procedure. This is more relevant to modern practice, since we have not performed a cut-and-sew Maze procedure in eight years. Furthermore, with the more rigorous follow-up and definition of failure, the present study provides a much more accurate representation of late recurrence, allowing for a better elucidation of preoperative and postoperative risk factors leading to failure of the Cox-Maze procedure.
In this case series, the Cox-Maze procedure had excellent results at one year, with 89% of patients free from atrial dysrhythmias and 78% free of arrhythmias and off drugs. In patients undergoing a stand-alone procedure, the success rates were 91% and 74%, respectively. This compares favorably with our historical data from the Cox-Maze III procedure, in which only symptomatic follow-up was obtained.2 In our previous report, 80% of patients undergoing a stand-alone procedure were both free of atrial fibrillation and antiarrhythmic drugs at last follow-up. In the concomitant group, 73% of patients were in sinus rhythm and off antiarrhythmic drugs at last follow-up. While follow-up in our previous report was longer, virtually none of these patients had electrocardiographic or Holter monitoring to determine their actual heart rhythm. It is heartening to see similar numbers obtained with more aggressive follow-up and a stricter definition of recurrent atrial tachyarrhythmias. It also is important to remember that in the current era, we are operating on a sicker cohort of patients with higher incidences of congestive heart failure and other comorbidities, who underwent more complex concomitant operations. Because of the difficulty and time-consuming nature of the traditional cut-and-sew Cox-Maze III procedure, principally low-risk patients were historically offered concomitant surgery.
There were three risk factors for recurrent atrial arrhythmias following the Cox-Maze IV procedure using our multivariant logistic regression analysis. These included left atrial size, failure to anatomically isolate the entire posterior left atrium, and early atrial tachyarrhythmias (Table 3). Increasing left atrial size was a significant risk factor for failure (Figure 2). This agrees with previous reports in the literature.12-14 This is not surprising as our laboratory has shown that as atrial surface area increases, there is a higher incidence of inducible atrial fibrillation.15 In this study, the probability of recurrent tachyarrhythmias exceeded 50% once left atrial size was over 8 cm. While it has been our policy to perform a left atrial reduction in left atria ≥ 7 cm, this added procedure obviously was not successful in preventing recurrence. Patients with large left atria need to be adequately counseled on their high risk for failure. In this group, the value of concomitant atrial fibrillation ablation remains unclear and surgeons should be cautious adding the Cox-Maze procedure, if there is any chance that it may increase perioperative risk. Our data does suggest the need for either a more aggressive left atrial reduction or a more extensive ablation lesion set in this patient population.
Another risk factor for recurrence of atrial tachyarrhythmias was the failure to anatomically isolate the entire posterior left atrium. Our initial version of the Cox-Maze procedure involved only one inferior connecting lesion between the pulmonary vein isolations, thus leaving most of the posterior left atrium in continuity with the rest of the left atrium.4 In 2005, we began to isolate the entire posterior left atrium by making two connecting lesions, one between the left and right inferior pulmonary veins and one between the superior pulmonary veins. This significantly increased our drug-free success rates in a prior report.6 In this larger series of patients, this still remained a significant risk factor for recurrence. It is our present recommendation that all patients undergoing surgical ablation for atrial fibrillation have their entire posterior left atrium anatomically isolated. This finding in surgical patients agrees with data from the electrophysiology laboratory, in which wide-area circumferential ablation involving most of the posterior left atrium has been shown to be more effective than targeting only the pulmonary veins. 3, 16-17
A final risk factor for failure was patients who experienced early tachyarrhythmias. This does not agree with a prior report from our group, but it is intuitive that these patients would have a higher failure rate.18 Early ATAs were managed aggressivelyfirst with chemical than electrical cardioversion if drug therapy was unsuccessful. Cardioversion was usually performed between one and four weeks postoperatively. The high incidence of early ATAs in this group is likely due to a number of reasons. All of the patients had preoperative AF, usually of long duration, and had already developed the appropriate substrate required to sustain this arrhythmia. Also, previous work from our laboratory has shown that early postoperative AF is due to myocardial inflammation.19 The multiple atrial ablations likely cause a robust inflammatory response. It is not known whether a more aggressive attempt to prevent early ATAs would improve late success, but this seems unlikely. It is more probable that early ATAs may be a marker of a more advanced pathology of the atrial substrate and this would logically make these patients more prone to late recurrence.
Numerous factors were not found to be associated with late failure on univariate analysis, including gender, age, NYHA class, type of bipolar device, type or duration of atrial fibrillation, ejection fraction, failed catheter ablation or postoperative pacemaker implantation. It is interesting that in this study that preoperative atrial fibrillation duration was not shown to be a significant predictor of late failure. This contradicts our prior report evaluating mainly the Cox-Maze III procedure.5 This may be a result of patients being referred earlier for atrial fibrillation ablation, as indicated by a significantly lower median duration of atrial fibrillation in this series (3.7 years) compared to our previous report (5 years). It may be also due to the fact that in patients with concomitant structural heart disease, who comprised of 66% of the patients in this series, but only 41% of patients in our prior report, the effect of prolonged duration of atrial fibrillation is overwhelmed by the electrical remodelling and atrial dilatation secondary to the congestive heart failure and valvular heart disease. 5
The study has several limitations. While follow-up has improved considerably over time, there are still patients who did not have 24 hour holter monitoring, and thus the actual failure rate may be underestimated. 20 However, compared to previous reports in the literature, this patient cohort has been well monitored, and no patient was lost to follow-up. Another limitation of this report was that the precise mechanism of atrial tachyarrhythmia recurrence was not defined in the majority of patients. The question remains unanswered whether this was due to technical difficulty in performing a complete Cox-Maze lesion set or to inherent atrial pathology. Since we have been performing this procedure for over two decades, it is unlikely that the failures were due to an inappropriately performed lesion set. It is possible that there were recurrences due to bridging of cardiac conduction over the linear scars formed by the bipolar devices, but this phenomenon has not been demonstrated in patients. It also is possible that some of the lines of ablation were not actually transmural, since only the efficacy of pulmonary vein isolation was tested. In the future, our hope is to perform non-invasive electrocardiographic imaging to better define the mechanisms of failure to allow us to develop strategies to improve surgical success rates.
In conclusion, failure to isolate the entire posterior LA and increasing left atrial diameter were the two risk factors for failure that can be modified by surgeons. In patients with large left atrium, this study would suggest that efforts to improve left atrial reduction procedures, or perform more extensive lesion sets are warranted.
Acknowledgments
Funded in part from National Institutes of Health Grants R01 HL032257-21 and F32 HL082129-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Ralph J. Damiano, Jr. is a consultant for AtriCure, Inc., Medtronic, Inc., and ATS Medical, Inc. Dr. Richard Schuessler receives research funding from AtriCure, Inc., Medtronic, Inc., and Estech. Dr. Hersh Maniar is a consultant for nContact Surgical and Estech.
REFERENCES
- 1.Cox JL, Schuessler RB, D'Agostino HJ, Jr., et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–83. [PubMed] [Google Scholar]
- 2.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg. 2005;129:104–11. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–7. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Melby SJ, Zierer A, Lubahn JG, et al. Normal Quality of Life after the Cox Maze Procedure for Atrial Fibrillation. Innovations Phila Pa. 2008;3:142–6. doi: 10.1097/IMI.0b013e31819165d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melby SJ, Zierer A, Bailey MS, et al. A new era in the surgical treatment of atrial fibrillation: the impact of ablation technology and lesion set on procedural efficacy. Ann Surg. 2006;244:583–92. doi: 10.1097/01.sla.0000237654.00841.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaff HV, Dearani JA, Daly RC, Orszulak TA, Danielson GK. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:30–7. doi: 10.1016/s1043-0679(00)70014-1. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D., 3rd The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25–9. doi: 10.1016/s1043-0679(00)70013-x. [DOI] [PubMed] [Google Scholar]
- 11.Millar RC, Arcidi JM, Jr., Alison PJ. The maze III procedure for atrial fibrillation: should the indications be expanded? Ann Thorac Surg. 2000;70:1580–6. doi: 10.1016/s0003-4975(00)01707-0. [DOI] [PubMed] [Google Scholar]
- 12.Kamata J, Kawazoe K, Izumoto H, et al. Predictors of sinus rhythm restoration after Cox maze procedure concomitant with other cardiac operations. Ann Thorac Surg. 1997;64:394–8. doi: 10.1016/S0003-4975(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi J, Kosakai Y, Nakano K, Sasako Y, Eishi K, Yamamoto F. Improved success rate of the maze procedure in mitral valve disease by new criteria for patients' selection. Eur J Cardiothorac Surg. 1998;13:247–52. doi: 10.1016/s1010-7940(97)00328-x. [DOI] [PubMed] [Google Scholar]
- 14.Gillinov AM, Sirak J, Blackstone EH, et al. The Cox maze procedure in mitral valve disease: predictors of recurrent atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:1653–60. doi: 10.1016/j.jtcvs.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005;112:I7–13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 16.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 17.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Gleva MJ, Gamache MC, et al. Atrial tachyarrhythmias after the maze procedure: incidence and prognosis. Circulation. 2004;110:II164–8. doi: 10.1161/01.CIR.0000138400.44799.65. [DOI] [PubMed] [Google Scholar]
- 19.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–8. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 20.Edgerton JR, McClelland JH, Duke D, et al. Minimally invasive surgical ablation of atrial fibrillation: six-month results. J Thorac Cardiovasc Surg. 2009;138:109–13. doi: 10.1016/j.jtcvs.2008.09.080. discussion 14. [DOI] [PubMed] [Google Scholar]