Abstract
A plethora of human pathogens invade and/or colonize mucosal surfaces. Elaboration of strong, protective immune responses against those pathogens by mucosal vaccination, however, is hampered by endogenous regulatory systems in the mucosae that dampen responses to foreign antigens (Ag). To overcome those natural barriers, mucosal adjuvants must be employed. Using a mouse mucosal immunization model and AgI/II, a weak immunogen from Streptococcus mutans, LT-IIc, a new member of the type II subgroup of the heat-labile enterotoxin family, was shown to have potent mucosal adjuvant properties. In comparison to mice intranasally immunized only with AgI/II, co-administration of AgI/II with LT-IIc enhanced production of Ag-specific IgA antibodies in the saliva and vaginal fluids and Ag-specific IgA and IgG in the serum. Secretion of IL-2, IL-6, IL-17, IFN-γ, and TNF-α was enhanced in cultures of AgI/II-stimulated splenic cells isolated from mice that had received LT-IIc as a mucosal adjuvant. In contrast, secretion of IL-10 was suppressed in those cells. This pattern of cytokine secretion suggested that LT-IIc stimulates both Th1 and Th2 immune responses. In contrast to LT-IIa and LT-IIb, the original members of the type II subgroup that also are mucosal adjuvants, LT-IIc dramatically enhanced secretion of IL-1α and IL-1β in peritoneal macrophages that had been co-cultured with LPS. Furthermore, the B pentameric subunit of LT-IIc augmented uptake of Ag by bone marrow-derived dendritic cells to levels that exceeded those attained by use of LPS or by the B pentamers of LT-IIa or LT-IIb. These data confirmed that LT-IIc is a strong mucosal adjuvant with immunomodulatory properties that are distinguishable from those of LT-IIa and LT-IIb and which has immunomodulatory properties that may be exploitable in vaccine development.
Keywords: heat-labile enterotoxin, mucosal immunology, adjuvant, IL-1β, IL-17, IFN-γ
INTRODUCTION
The oral/gastrointestinal, nasopharyngeal, respiratory, and vaginal mucosae are continuously confronted with antigenic substances from the environment. The vast majority of those substances are harmless and, in most cases, the mucosae are unresponsive to those antigens (Ag) or respond only in a muted manner (15). The various mucosae, however, represent natural sites for entry of many human pathogens. Protection against those pathogens requires the use of vaccines to evoke strong pathogen-specific, protective immune responses on the mucosae. Unfortunately, the same endogenous immunoregulatory systems in the mucosae that suppress immune responses to harmless environmental Ags also suppress immune responses to potentially protective Ags. To overcome or bypass those natural immune barriers in the mucosae, mucosal adjuvants are required (5, 7, 28, 29).
Members of the A1B5 family of oligomeric ADP-ribosylating heat-labile enterotoxins (HLT) (14) have been shown to be potent systemic and mucosal adjuvants (5, 18). Based on similarities and differences in genetic, biochemical, and immunological characteristics, the family is divided into two major subfamilies (5, 14). Cholera toxin (CT) produced by Vibrio cholerae and heat-labile enterotoxin (herein referred to as LT-I) produced by enterotoxigenic Escherichia coli are members of the type I HLT subfamily. The members of the type II subfamily (LT-IIa and LT-IIb), are distinguishable antigenically and structurally from the members of the type I subfamily (10, 11, 14).
A compendium of research has demonstrated that the distinctive immunomodulatory activities of LT-IIa, LT-IIb, CT, and LT-I are dependent upon the enterotoxins’ binding patterns for gangliosides, a family of sugar-containing lipids that are embedded in the plasma membrane of eukaryotic cells (34). Binding of an enterotoxin to its ganglioside receptor(s), directly or indirectly, triggers responses that regulate specific immune functions of T cells, B cells, and/or (DC) cells (1, 2, 24). The types of responses observed in each of those cells are determined by the ganglioside(s) to which each of the HLT binds (5). CT and LT-I bind with high affinity to GM1 (although LT-I also has affinity for an unknown glycoprotein) (32). CT also binds with low affinity (in descending order) to GM2, GD1a, GM3, GT1b, GD1b, and asialo-GM1 (16). LT-IIa binds specifically, in descending order of avidity, to gangliosides GD1b, GM1, GT1b, GQ1b, GM2, GD1a, and GM3 (8). LT-IIb binds avidly only to GD1a and to GM2 and GM3 with much lower affinity (8).
Recently, a new HLT was cloned from a strain of enterotoxigenic E. coli that was obtained from the fecal material of a diarrheic ostrich (27). Comparative studies of the predicted amino acid sequences demonstrated that the new HLT produced by an E. coli colonizing a non-mammalian host was distantly related to CT or LT-I, but closely related to LT-IIa and LT-IIb (31). The amino acid sequences of the catalytic A polypeptide of the new HLT were very similar to the amino acid sequences of the A polypeptides of LT-IIa and LT-IIb (79% and 72%, respectively). Major divergence in amino acid sequences between LT-IIa, LT-IIb, and the new HLT, however, was observed in the B polypeptides that bind the HLT to their cellular receptors. Specifically, the B polypeptide of the new HLT exhibited only 53% amino acid sequence similarity to the B polypeptide of LT-IIa and only 54% amino acid sequence similarity to the B polypeptide of LT-IIb (31). Notably, the B polypeptide of the new HLT had no detectible amino acid sequence similarity to the B polypeptides of CT and LT-I. Thus, the new enterotoxin, designated LT-IIc, was assigned as the third member of the type II subfamily of HLT.
The divergence in the amino acid sequences of the LT-IIc B polypeptide suggested that LT-IIc likely had binding affinities for gangliosides that would be different from those of LT-IIa, LT-IIb, CT, or LT-I. In fact, LT-IIc bound strongly to GD1a, GM1, GM2, and GM3, but had no detectible affinity for GD1b, GT1b, or GQ1b (31). Since the distinctive immunomodulatory properties of LT-IIa, LT-IIb, CT, and LT-I are dependent upon their capacity to bind to different gangliosides (2, 5, 24), it was hypothesized that distinctive immunomodulatory properties would be conferred upon LT-IIc by its distinctive ganglioside-binding properties. Experiments reported herein supported that model and provide additional evidence for the roles of gangliosides in immunomodulation.
MATERIALS AND METHODS
Cloning and purification of a His-tagged LT-IIc
Cloning of recombinant His-tagged LT-IIc holotoxin has been described (31). To engineer a recombinant His-tagged version of the B- subunit of LT-IIc (LT-IIc-B5), The EcoRI/XhoI fragment of pJCH6.2 which encodes the B polypeptide of LT-IIc was ligated into pBluescript II SK- (Stratagene, La Jolla, CA) at the EcoRI/XhoI sites to produce pHN51 which was introduced into E. coli DH5αF’Kan (Life Technologies, Inc., Gaithersburg, MD). Expression of recombinant LT-IIc was induced by addition of isopropyl-β-D-thiogalactoside to the culture medium. LT-IIc was extracted from the periplasmic space and purified to homogeneity by nickel affinity chromatography and gel filtration chromatography (Sephacryl-100; Pharmacia, Piscataway, NJ,) using an ÄKTA-FPLC (Pharmacia)(28). LT-IIa and LT-IIb and the respective B pentamers were purified using established methods (12).
Lipopolysaccharide
All purified recombinant enterotoxins were analyzed for potential contamination by endotoxin using a quantitative Limulus amoebocyte lysate assay kit (Charles River Endosafe, Charleston, SC). All enterotoxins preparations were essentially free of lipopolysaccharide (< 0.03 ng/μg protein).
Mucosal immunization model
A well-established mouse mucosal immunization model was employed (23, 28). Unanesthetized female BALB/c mice (The Jackson Laboratory, Bar Harbor, Maine) of approximately 8 weeks of age were immunized by the intranasal (i.n.) route with various combinations of Ag and enterotoxin. Groups of 6 mice were immunized 3× at 2-week intervals with 10 μg of AgI/II, a streptococcal Ag (33), alone or in combination with 1.0 μg of LT-IIb or 1.0 μg of LT-IIc. Immunizations were administered in a standardized volume (10.0 μl) that was applied slowly to both external nostrils at 5 μl/nostril. Animal experiments were approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
Collection of secretions and sera
Samples of serum, saliva, and vaginal washes were collected from individual mice at a point one week before the initial immunization (pre-immune) and at two weeks after the third immunization (28). Mucosal secretions and serum samples were stored at −70°C.
Antibody analysis
Levels of Ag-specific IgA and Ag-specific IgG antibodies in saliva, sera, and vaginal washes were determined by ELISA (28).
Cytokine assays
Single cell suspensions of splenic cells were isolated from immunized mice at a time point 63 days after the initial immunization when the primary anti-AgI/II response had significantly declined (23). Splenic cells were plated in triplicate at a concentration of 107 cells/well in 6-well tissue culture plates (Nunc, Roskilde, Denmark) and stimulated for 4 days in the presence of AgI/II (5 μg/ml). Culture supernatants were collected after centrifugation and stored at −70°C. Cytokine levels in culture supernatants were determined using a mouse Th1/Th2/Th17 cytokine BD cytometric bead array kit, a DB FACSArray bioanalyzer, and FCAP array software (BD Biosciences, San Jose, CA).
Primary cell isolation, culture, and cytokine induction
Thioglycollate-elicited macrophages isolated from the peritoneal cavities of female BALB/c mice (3) were cultured in complete RPMI 1640 (Invitrogen/Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS)(Gibco), 2 mM L-glutamine, 10 mM HEPES (Gibco), 100 units/ml penicillin G, 100 μg/ml streptomycin (Gibco), and 0.05 mM 2-mercaptoethanol (Gibco). Mouse macrophages (2×106/well) were stimulated with 1 μg/ml ultrapure LPS (InvivoGen, San Diego, CA) for 4 hrs before being treated for 20 hrs with 2.0 μg/ml of LT-IIa, LT-IIb or LT-IIc. In comparison to medium-only control treatments, none of the experimental treatments significantly affected cell viability. Supernatants of mouse macrophages were collected and stored at −70°C until being analyzed for interleukin 6 (IL-6), interleukin 1α (IL-1α), and interleukin 1β (IL-1β) using commercial cytokine specific ELISA kits (eBioscience, San Diego, CA).
Antigen uptake by bone marrow derived DC (BMDC)
Uptake of Ag by BMDC was measured using a standard protocol (17). FITC-labeled-chicken OVA (FITC-OVA) (Invitrogen-Molecular Probes) was employed as the model Ag. Individual 100 μl aliquots containing 2×105 BMDC were incubated for 10 min at 37°C with 5 μg/ml of LT-IIa-B5, 5 μg/ml of LT-IIbB5, 5 μg/ml of LT-IIc-B5, 1 μg/ml of LPS (positive control), or PBS (untreated control). FITC-OVA (0.2 mg/ml) was added to the BMDC and the cells incubated for 10 min at 37°C. Ag uptake by BMDC was terminated by washing the cells 3× with ice-cold PBS containing 2% FBS. Cells were resuspended in FACS buffer (PBS containing 2% bovine serum albumin and 0.1% sodium azide), stained with APC-conjugated anti-CD11c (Biolegend, San Diego, CA), washed in FACS buffer, and resuspended in FACS buffer containing 0.25 μg/ml of 7-AAD (Calbiochem, San Diego, CA) to distinguish living from dead cells. FITC fluorescence of the CD11c+ and 7-AAD− cells was determined using a FACScalibur flow cytometer (Becton-Dickinson) and CellQuest software (Becton-Dickinson). Fluorescence values were reported as mean fluorescence intensity (MFI).
RESULTS
Enhanced production of Ag-specific IgA and IgG
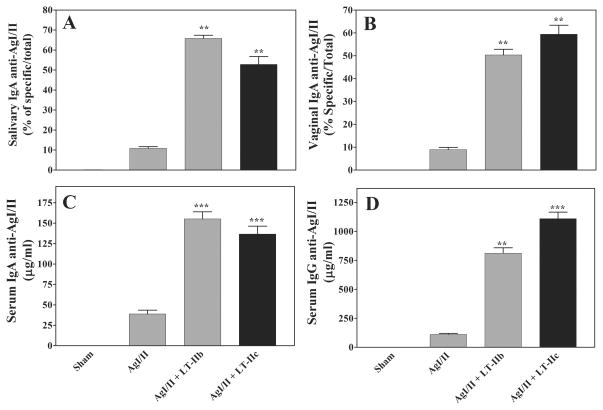
The striking similarity in protein structure and amino acid homology between LT-IIc, LT-IIa, and LT-IIb (31) strongly suggested that LT-IIc had the potential to behave as a mucosal adjuvant (27). To test this hypothesis, mice were immunized intranasally with a poor antigen (Ag)[AgI/II, a surface protein of Streptococcus mutans (33)] in the absence or presence of LT-IIc. LT-IIb, an established mucosal adjuvant, was employed as a positive control (23, 28). In comparison to mice immunized solely with AgI/II, mice administered AgI/II in the presence of LT-IIc augmented production of Ag-specific IgA in proximal (oral) (Fig 1A) and distal (vaginal) mucosal secretions (Fig. 1B). The levels of anti-AgI/II IgA enhanced by use of LT-IIc were comparable to those elicited by LT-IIb. Similar patterns of enhanced Ag-specific immune responses were also observed in the serum of mice receiving LT-IIc as a mucosal adjuvant. In comparison to mice that had been intranasally immunized only with AgI/II, levels of Ag-specific IgA (Fig. 1C) and Ag-specific IgG (Fig. 1D) were significantly elevated in the serum of mice that had received LT-IIc. Again, comparable levels of antibodies were detected in the serum of mice administered LT-IIb as a mucosal adjuvant. These data strongly demonstrated that LT-IIc was a potent mucosal adjuvant that could enhance both mucosal and systemic immune responses. Further experiments were performed to distinguish the mechanisms by which LT-IIc evoked those responses.
Figure 1. Intranasal co-administration of LT-IIc enhances Ag-specific mucosal and systemic immune responses to AgI/II.
BALB/c mice were immunized intranasally on days 1, 14, and 28 with 10 μg of AgI/II in the presence or absence of 1 μg of LT-IIb or LT-IIc as a mucosal adjuvant. Samples were obtained at day 42 at the time of peak Ag-specific antibody response. Samples were measured for: (A) salivary IgA, (B) vaginal IgA, (C) serum IgA, and (D) serum IgG. Data are reported as arithmetic means; error bars denote one standard error of the mean (n = 5 or 6). Key: statistically different from mice immunized only with AgI/II at P<0.01 (**) and P<0.001 (***).
Induction of memory responses
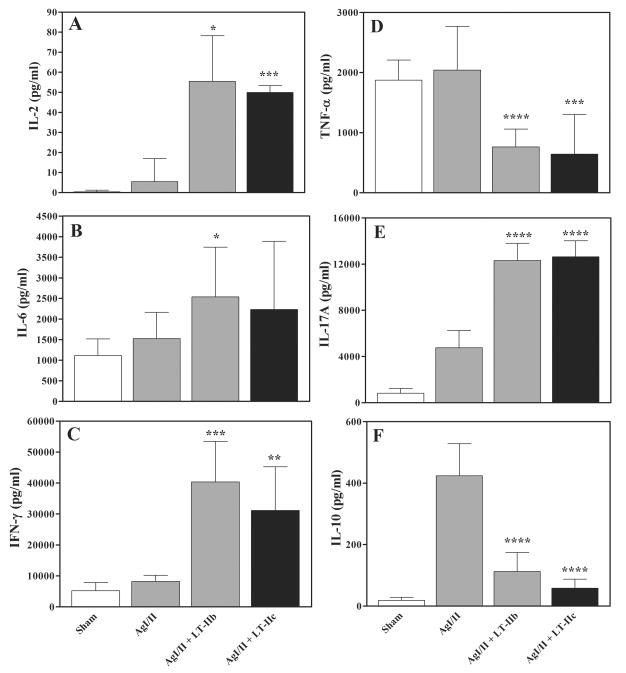
LT-IIa, LT-IIb, CT, and LT-I have the capacity to establish robust memory T-cell responses to co-administered Ags (23, 28, 29). To evaluate the capacity of LT-IIc to establish memory responses, cultures of splenocytes obtained from intranasally immunized mice after decay of the primary immune responses and stimulated with AgI/II were measured for secretion of IL-2, IL-6, IL-10, IL-17, INF-γ, and TNF-α. Splenic cells obtained from mice administered LT-IIc as a mucosal adjuvant elaborated significantly higher levels of IL-2, IL-6, IFN-γ, and IL-17 after application in vitro of AgI/II than did splenic cells obtained from mice immunized only with AgI/II (Fig. 2). In contrast, use of LT-IIc as a mucosal adjuvant suppressed secretion of TNF-α and IL-10. A similar pattern of cytokine responses was observed in splenic cells obtained from mice that had been administered LT-IIb as a mucosal adjuvant. These data indicated that LT-IIc was a potent enhancer of memory responses.
Figure 2. Production of cytokines by splenic cells.
Splenic cells were isolated from intranasally immunized mice at a time point 63 days after the initial immunization. Cultured splenic cells were stimulated in vitro for 4 days with AgI/II (5μg/ml). Culture supernatants from the stimulated splenic cells were measured for: (A) IL-2, (B) IL-6, (C) IFN-γ, (D) TNF-α, (E) IL-17, and (F) IL-10. Data are reported as arithmetic means; error bars denote one standard deviation of the mean (n = 5–6). Key: statistically different from mice immunized only with AgI/II at P<0.05 (*), P<0.01 (**), and P<0.001 (***).
Enhanced production of IL-1α and IL-1β
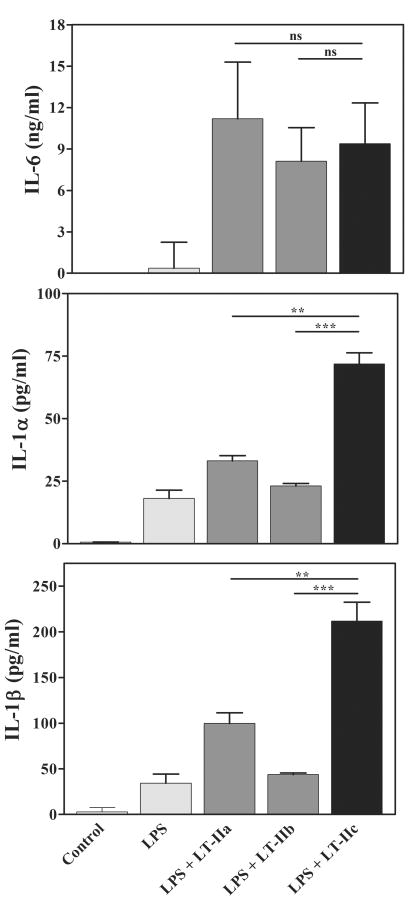
Secretion of many cytokines by murine and human immunocompetent cells is stimulated by treatment of the cells with either LT-IIa or LT-IIb (1, 12, 13, 21, 24, 30). To determine if LT-IIc also stimulates macrophages, LPS-activated peritoneal macrophages of naïve mice were treated in vitro with LT-IIc and evaluated for secretion of IL-6, IL-1α, and IL-1β. As demonstrated in prior studies (1), LT-IIa and LT-IIb enhanced secretion of IL-6 by LPS-treated peritoneal macrophages (Fig. 3). Treatment of the cells with LT-IIc promoted secretion of amounts of IL-6 similar to the amounts secreted by cells treated with either LT-IIa or LT-IIb.
Figure 3. Cytokine production by peritoneal macrophages.
Peritoneal macrophages from naïve mice were treated for 4 hrs with 1 μg/ml of LPS prior to addition of LT-IIa, LT-IIb or LT-IIc. After 20 hrs of incubation, the amounts of cytokines in the culture supernatants were determined. Data are reported as arithmetic means; error bars denote one standard error of the mean (n = 3). Key: statistical difference between groups denoted by crossbars at P < 0.01 (**), at P < 0.001 (***); ns, not significantly different between groups denoted by crossbars.
In contrast, LT-IIc was superior to either LT-IIa or LT-IIb in promoting secretion of the pro-inflammatory cytokines IL-1α and IL-1β. The capacity to elevate secretion of IL-1α and IL- 1β at levels greater than the levels promoted by either LT-IIa or LT-IIb indicated that LT-IIc had distinctive immunomodulatory properties to influence pro-inflammatory responses.
Enhanced uptake of Ag by BMDC
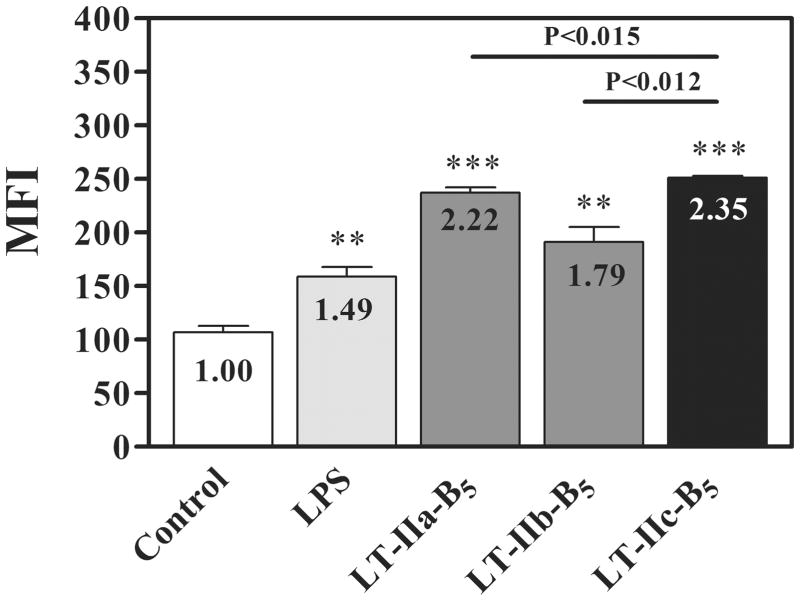
Immunization experiments have shown that the B pentamers of LT-IIa (LT-IIa-B5) (C.H.L, and T.D.C., unpubl. data) and LT-IIb (LT-IIb-B5), which can be recombinantly expressed and purified, exhibit strong mucosal adjuvant activity, although with less potency than their respective holotoxins (20). The adjuvant properties of LT-LT-IIa-B5 and LT-IIb-B5 are likely mediated, in part, by the ability of the pentamers to enhance uptake of Ag by DC, a property which is not shared by their respective holotoxins or by the B pentamers of either CT or LT-I (17). The capacity of LT-IIa-B5 and LT-IIb-B5 to enhance the uptake of Ag is dependent upon expression by DC of toll-like receptor 2 (TLR2) (17). LT-IIa-B5 and LT-IIb-B5 interact with TLR2 via a conserved hydrophobic motif [N-AMAA(I/V)LS-C] located at amino acid positions 68–74 in the B polypeptides (19). This motif is absent in the B polypeptides of CT and LT-I, and thus, these HLT lack the capacity to augment uptake of Ag by DC (17). Amino acid sequence alignments revealed the presence of a seven amino acid sequence (N-AMAAVLS-C) in the B polypeptides of LT-IIc (LT-IIc-B5) that were highly homologous to the amino acid sequences of the TLR2-interacting motif of LT-IIa-B5 and LT- IIb-B5 (31). These data suggested that LT-IIc-B5 had the capacity to augment uptake of Ag by BMDC using similar TLR2-dependent mechanisms. To test this model, BMDC were treated briefly with LT-IIc-B5, subsequently mixed with FITC-conjugated ovalbumin (FITC-OVA), and analyzed for fluorescence by flow cytometry as a measure of Ag uptake (17). LPS, a potent TLR4 agonist and a strong stimulant of Ag uptake (35), and LT-IIa-B5 and LT-IIb-B5 were used as positive controls. As expected, uptake of FITC-OVA was augmented in BMDC treated with LPS (Fig. 4). Uptake was enhanced to a higher degree by treatment of the cells with either LT-IIa-B5 or LT-IIb-B5. The highest levels of Ag uptake, however, were observed when BMDC were treated with LT-IIc-B5. LT-IIc-B5, therefore, of the four agents appeared to have the greatest stimulatory properties in respect to augmenting Ag uptake by BMDC.
Figure 4. Uptake of FITC-OVA by BMDC is enhanced by LT-IIc-B5.
BMDC were incubated at 37°C with 5 μg/ml of LT-IIa-B5, 5 μg/ml of LT-IIb-B5, 5 μg/ml of LT-IIc-B5, 1 μg/ml of LPS, or PBS (untreated). After 10 min, FITC-OVA (0.2 mg/ml) was added to the cultures. Ten min after addition of FITC-OVA, cells were washed with ice-cold PBS, stained with an APC-conjugated anti-CD11c mAb (BioLegend) and 7-AAD (Calbiochem) and the mean fluorescent intensity (MFI) of CD11c-positive, 7-AAD-negative cells was determined using flow cytometry. Data are reported as arithmetic means; error bars denote one standard error of the mean (n = 3). Data shown represents one of two independent experiments. Fold increases (MFI levels in treated cells/MFI levels in untreated cells) for each group of cells are denoted. Key: statistically different from untreated BMDC at P < 0.005 (**) and P < 0.0001 (***); statistical differences between the values for LT-IIc-B5 & LT-IIa-B5 and LT-IIc-B5 & LT-IIb-B5 are denoted above the crossbars.
DISCUSSION
When employed as mucosal adjuvants, all members of the type I and type II HLT subfamilies stimulate strong Th2-type immune responses to a co-administered Ag (23, 28, 29). The immune responses are usually long-lived due to establishment of memory T cells. Yet, it is clear that each member of the two families exert a unique immunomodulatory activity at the cellular level (1, 2, 24). The distinctive immune responses evoked by use of the HLT as mucosal adjuvants are likely dependent upon the gangliosides to which the enterotoxins bind on immunocompetent cells (5, 9). In fact, the situation is more complex. LT-IIb binds to gangliosides decorated with either N-acetylneuraminic acid (NeuAc) or N-glycolylneuraminic acid (NeuGc) (3). The LT-IIb(T13I) mutant of LT-IIb which binds preferentially to NeuGc and exhibits less affinity for NeuAc gangliosides have lost the capacity to intoxicate cells, yet retain most of the adjuvant properties of the wt LT-IIb in mice expressing only NeuAc (28, 30). It is feasible that other structural variances in particular gangliosides (e.g., different mixtures of NeuAc and NeuGc in di- and tri-sialo-gangliosides, length of the ceramide core, etc.) may influence binding and immunomodulation by HLT. Whether binding of an HLT to a particular ganglioside(s) (i) directly triggers immune signal transduction, (ii) indirectly positions the enterotoxins into locations in the membrane (e.g., lipid rafts) where secondary interactions evoke immune signal transduction, or routes the enterotoxins into specific pathways for internalization where the enterotoxin interacts with different cytoplasmic regulators has not been experimentally established.
LT-IIc exhibits ganglioside-binding properties that are distinguishable from those of LT-IIa, LT-IIb, CT, and LT-I (31). Using the established mouse mucosal immunization model, LT-IIc was shown to enhance Ag-specific mucosal and systemic antibody responses and to induce production of IL-2, IL-6, IFN-γ, and IL-17 by splenic cells. Furthermore, when used to treat BMDC in vitro, LT-IIc-B5 augmented uptake of Ag to levels unattainable by treatment of the BMDC with LPS, a powerful TLR4 agonist with a capacity to enhance uptake of Ag by DC (17, 35). It is likely, therefore, that these activities are stimulated by binding of LT-IIc and LT-IIc-B5 to specific gangliosides on the surfaces of one or more types of immunocompetent cells. To test this model, mutants of LT-IIc with altered or abrogated ganglioside-binding activities need to be engineered. Amino acid substitutions in the B polypeptides of LT-IIa or LT-IIb at amino acids threonine-13, threonine-14, or threonine-34 either abrogated binding of the HLT for gangliosides or altered the affinity of the HLT for binding to particular gangliosides (4, 6). Notably, threonines at each of those three amino acid positions are conserved in the B polypeptide of LT-IIc (31). It is feasible, therefore, that engineering threonine to isoleucine substitutions in the B polypeptides of LT-IIc will produce mutant LT-IIc holotoxins with the desired characteristics, i.e. alterations in ganglioside binding activities. These mutants will be invaluable in determining which, if any, of the gangliosides bound by LT-IIc are primarily responsible for this HLT’s immunomodulatory properties.
IL-1 is a major regulator of inflammatory responses. Experiments employing IL-1α/β−/− mice demonstrated that IL-1 is a major regulator of humoral immune responses. Two molecular forms of IL-1 are expressed in mice and humans (22), both which have the capacity to activate lymphocytes, monocytes, macrophages, and NK cells (26). Production of Ag-specific antibodies was reduced in IL-1α/β-deficient mice (25). While IL-1α and IL-1β are mutually inductive for several types of immune responses (25, 26), it is likely that IL-1β is more involved than IL-1α in stimulating T cell-dependent Ab production (25). Furthermore, IL-1 augments T cell-dependent antibody production by enhancing expression of CD40 ligand and OX40 on T cells (26).
Treatment of LPS-activated macrophages with LT-IIc induced IL-1α and IL-1β to levels that were greater than the levels induced by either LT-IIa or LT-IIb and dramatically higher than the levels induced by treatment of the cells with LPS. It is intriguing to speculate that the adjuvant properties of LT-IIc are mediated, in part, by this enhanced production of IL-1 by macrophages and/or by other Ag-presenting cells such as DC. Current experiments are focused on determining if treatment with LT-IIc induces production of IL-1α and IL-1β by circulating monocytes and by DC residing in the nasal lymphoid tissues and draining cervical lymph nodes. A critical experiment will be to employ IL-1α/β-deficient mice in mucosal immunization experiments to determine if IL-1α and IL-1β are essential for the mucosal and systemic adjuvant properties of LT-IIc.
Uptake and processing of Ag by Ag-presenting cells (APC) are critical steps in producing an immune response to an administered Ag. It is predicted, therefore, that any treatment which enhances uptake or processing of Ag by APC will also increase immune responsiveness to that Ag. Treatment with LT-IIa-B5 or LT-IIb-B5 significantly enhanced uptake and presentation of Ag by cultured BMDC (17). Furthermore, trafficking experiments demonstrated that migration of DC from the nasal-associated lymphoid tissue to the draining cervical lymph nodes was significantly elevated in mice receiving intranasal administration of LT-IIa-B5 (T.D.C. and C.H.L., in preparation), an effect which increased the numbers of DC in the CLN that would be available for presenting Ag to nodal T cells. Although the presence of a TLR2-interaction motif in the B polypeptides of LT-IIc suggested that treatment with LT-IIc-B5 would enhance uptake of Ag by BMDC, which was confirmed by in vitro experiments using BMDC, it was an unexpected finding that LT-IIc-B5 would exhibit an enhancing property that would exceed the enhancing properties of LT-IIa-B5, LT-IIb-B5, or LPS. One possibility to explain this finding is that BMDC express a greater numbers of ganglioside receptors for LT-IIc-B5 than does LT-IIa- B5 or LT-IIb-B5. Or, there may be secondary motifs in LT-IIc-B5 that are absent in LT-IIa-B5 or LT-IIb-B5 that interact with TLR2 in coordination with the major TLR2-interaction motif. To test those possibilities, LT-IIa-B5/LT-IIc-B5 and LT-IIb-B5/LT-IIc-B5 chimeras can be engineered. The ganglioside-binding patterns of those chimeras can be evaluated using a high-resolution, thin-layer chromatographic immunoblotting assay developed in our laboratory (3).
Each member of the type I and type II HLT promote a unique set of immune responses when employed as mucosal adjuvants. Thus, these enterotoxins are a potentially invaluable set of tools for driving immune responses into desirable directions, a concept which has powerful clinical implications. These experiments have begun to define the unique immunomodulatory properties of LT-IIc which will likely become a new tool for the mucosal adjuvant toolbox.
Acknowledgments
We would like to thank Dr. Tomomasa Yano for generously providing us with the isolates of E. coli obtained from ostriches. This work was supported by The National Institutes of Health research grant DE013833 (T.D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arce S, Nawar HF, Muehlinghaus G, Russell MW, Connell TD. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect Immun. 2007;75:1413–1423. doi: 10.1128/IAI.01367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arce S, Nawar HF, Russell MW, Connell TD. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect Immun. 2005;73:2718–2727. doi: 10.1128/IAI.73.5.2718-2727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenson CS, Nawar HF, Yohe HC, Castle SA, Ashline DJ, Reinhold VN, Hajishengallis G, Connell TD. Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I) Glycobiology. 2010;20:41–54. doi: 10.1093/glycob/cwp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell T, Holmes R. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell TD. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines. 2007;6:821–834. doi: 10.1586/14760584.6.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell TD, Holmes RK. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol Microbiol. 1995;16:21–31. doi: 10.1111/j.1365-2958.1995.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 7.Connell TD, Metzger D, Sfintescu C, Evans RT. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998;62:117–120. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidry JJ, Cardenas L, Cheng E, Clements JD. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infect Immun. 1997;65:4943–4950. doi: 10.1128/iai.65.12.4943-4950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guth BE, Pickett CL, Twiddy EM, Holmes RK, Gomes TA, Lima AA, Guerrant RL, Franco BD, Trabulsi LR. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986;54:587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guth BE, Twiddy EM, Trabulsi LR, Holmes RK. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986;54:529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect Immun. 2004;72:6351–6358. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes RK, Jobling MG, Connell TD. Cholera toxin and related enterotoxins of gram-negative bacteria. In: Moss J, Iglewski B, Vaughan M, Tu AT, editors. Bacterial toxins and virulance factors in disease. Vol. 8. Marcel Dekker, Inc; New York: 1995. pp. 225–255. [Google Scholar]
- 15.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuziemko GM, Stroh M, Stevens RC. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Nawar HF, Mandell L, Liang S, Hajishengallis G, Connell TD. Enhanced antigen uptake by dendritic cells induced by the B pentamer of the type II heat-labile enterotoxin LT-IIa requires engagement of TLR2. Vaccine. 2010;28:3696–3705. doi: 10.1016/j.vaccine.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S, Hajishengallis G. Heat-labile enterotoxins as adjuvants or anti-inflammatory agents. Immunol Invest. 2010;39:449–467. doi: 10.3109/08820130903563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, Connell TD, Hajishengallis G. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J Immunol. 2009;182:2978–2985. doi: 10.4049/jimmunol.0803737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang S, Hosur KB, Nawar HF, Russell MW, Connell TD, Hajishengallis G. In vivo and in vitro adjuvant activities of the B subunit of Type IIb heat-labile enterotoxin (LT-IIb-B5) from Escherichia coli. Vaccine. 2009;27:4302–4308. doi: 10.1016/j.vaccine.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 22.March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect Immun. 2000;68:281–287. doi: 10.1128/iai.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infect Immun. 2001;69:4486–4492. doi: 10.1128/IAI.69.7.4486-4492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae S, Asano M, Horai R, Iwakura Y. Interleukin-1 beta, but not interleukin-1 alpha, is required for T-cell-dependent antibody production. Immunology. 2001;104:402–409. doi: 10.1046/j.1365-2567.2001.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. IL-1 Enhances T Cell-Dependent Antibody Production Through Induction of CD40 Ligand and OX40 on T Cells. J Immunol. 2001;167:90–97. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Nardi AR, Salvadori MR, Coswig LT, Gatti MS, Leite DS, Valadares GF, Neto MG, Shocken-Iturrino RP, Blanco JE, Yano T. Type 2 heat-labile enterotoxin (LT-II)-producing Escherichia coli isolated from ostriches with diarrhea. Vet Microbiol. 2005;105:245–249. doi: 10.1016/j.vetmic.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Nawar HF, Arce S, Russell MW, Connell TD. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun. 2005;73:1330–1342. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawar HF, Arce S, Russell MW, Connell TD. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect Immun. 2007;75:621–633. doi: 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawar HF, Berenson CS, Hajishengallis G, Takematsu H, Mandell L, Clare RL, Connell TD. Binding to gangliosides containing N-acetylneuraminic acid is sufficient to mediate the immunomodulatory properties of the nontoxic mucosal adjuvant LT-IIb(T13I) Clin Vaccine Immunol. 2010;17:969–978. doi: 10.1128/CVI.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nawar HF, King-Lyons ND, Hu JC, Pasek RC, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a non-mammalian host. Infect Immun. 2010 doi: 10.1128/IAI.00730-10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlandi PA, Critchley DR, Fishman PH. The Heat-Labile Enterotoxin of Escherichia coli Binds to Polylactosaminoglycan-Containing Receptors in CaCo-2 Human Intestinal Epithelial Cells. Biochemistry. 1994;33:12886–12895. doi: 10.1021/bi00209a021. [DOI] [PubMed] [Google Scholar]
- 33.Russell MW, Bergmeier LA, Zanders ED, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm L. Structure and function of gangliosides. Plenum; New York, NY: 1980. [Google Scholar]
- 35.West MA, Wallin RPA, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced Dendritic Cell Antigen Capture via Toll-Like Receptor-Induced Actin Remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]