Abstract
Purpose
To determine how primary human trabecular meshwork (HTM) cells are influenced by their interaction with nanopatterned substrates.
Methods
HTM cells from several individuals were grown on planar or anisotropically ordered nanopatterned surfaces. Microscopy was used to measure cellular elongation and alignment. Cells were also incubated with 10−7 M dexamethasone for comparison to control cells. Quantitative PCR for myocilin and versican isoforms was performed in addition to Western blots of myocilin and αB-crystallin.
Results
Cells on anisotropically ordered nanopatterned substrates aligned with the surface nanopatterns and displayed actin filaments that were parallel to the patterned ridges and grooves. The cells became more elongated on the nano-grooved surfaces compared with the planar control cells. Myocilin mRNA and protein levels increased when HTM cells were plated onto 400-nm pitch surfaces. With some HTM cells, myocilin increased to a greater extent when untreated cells were plated on nanosurfaces compared with the cells grown on planar surfaces with dexamethasone. The V0 and V1 isoforms of versican had increased expression on patterned surfaces.
Conclusions
Nanopatterned surfaces containing biomimetic length scale features clearly influenced cellular behavior of HTM cells. Increased mRNA and protein levels of myocilin were observed when cells were grown on 400-nm pitch surfaces, suggesting that the reduction of myocilin mRNA when cells are plated onto flat tissue culture plastic is an artifact of a nonphysiologic culture environment that lacks appropriate topographic cues.
The trabecular meshwork of the human eye is believed to be the tissue that regulates intraocular pressure.1 Human trabecular meshwork (HTM) cells line the trabeculae of the inner areas of the outflow pathway and also are present in the cribriform area. The HTM cells can be cultured in vitro for several passages, but some characteristic properties of the cells, such as myocilin mRNA expression, are downregulated when the cells are cultured on normal tissue culture plastic.2 One possibility for this loss of myocilin could be the absence of appropriate topographic cues normally provided to the cells in vivo. The in vivo basement membrane on the trabeculae and the extracellular matrix at the cribriform region are the surfaces with which HTM cells interact. In contrast to flat plastic surfaces, basement membranes have been shown to have a topographically rich composition at the nanoscale level so that when cells interact with their physical environment, they receive thousands of topographic cues from the surfaces on which they grow.3– 6 Immunomicroscopy7 and scanning electron microscopy8 studies suggest that the trabecular meshwork cells interact with features on the nanometer-scale level. The phenotypic and molecular changes in cells grown on nanosurfaces demonstrate that the topographic features on which the cells are grown profoundly influence them.5,9 –12 These topographic cues are missing on the flat tissue culture plastic normally used to culture HTM cells.
It is now possible to fabricate surfaces that have features in the nanometer range to study the interaction of biochemical and physical cues that cells may obtain from their surrounding milieu. These surfaces can finally be produced in large enough quantities for a variety of assays that allow identification of molecular and biochemical properties of cells. Currently, several nanoscale patterns for cell growth can be fabricated.13,14 Synthetic extracellular matrix surfaces are commercially available; however, these are not of uniform topography (Donaldson, Minneapolis, MN). One of the patterns that has been studied is an anisotropic array of ridges and grooves.15–18 The distance between the ridges is termed pitch and is composed of the width of one ridge plus one groove (Fig. 1). The depth of the groove has been shown to be important in directing contact guidance for cells. Loesberg et al.19 reported a threshold of 35 nm of depth to be necessary before a cell is influenced by the substrate pattern, whereas our laboratory found threshold depth to be cell-type dependent and to range from 75 to 150 nm.20 In the study reported herein, all the surfaces had depths of 300 nm. A biomimetic surface can have a variety of pitches as well as differences between the size of the grooves and the ridges. The surfaces used for this study, however, used pitches that each had equal dimensions for the grooves and ridges.
Figure 1.
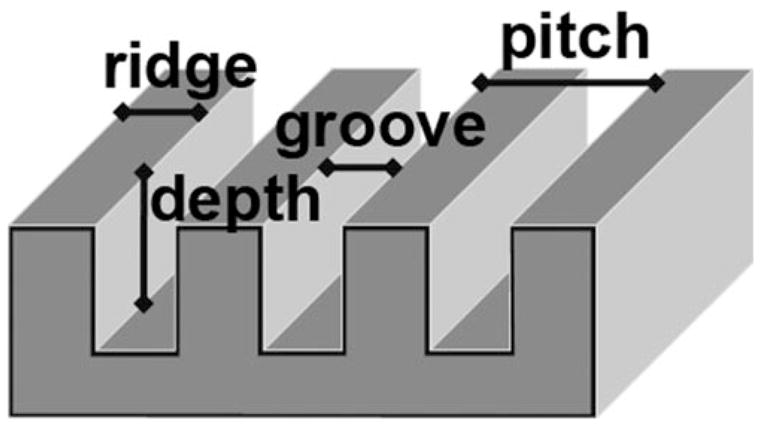
Schematic diagram of a patterned surface. Pitch is defined as the sum of the distance of one ridge and one groove. The patterned surfaces used in this report had equivalent dimensions for the grooves and ridges. The depth for all the patterned surfaces was 300 nm.
The purpose of this study was to determine whether HTM cells would be influenced by nanoscale topography compared with flat surfaces. The effect of growth conditions was investigated using several parameters. The alignment and elongation of the HTM cells were parameters that could be compared on a variety of different pitches. At the molecular level, the influence of nanoscale cues on myocilin expression was determined, since data suggest this protein is secreted by HTM cells.21,22 In addition, isoforms of the protein versican appear to be altered when cells are grown in vitro23 and have recently been shown to be influenced by mechanical stretching.24 Changes in versican isoforms, which possess variable numbers of chondroitin sulfate– binding domains, have the potential to influence glaucoma, since it has been reported that chondroitin sulfate increases with disease.25 Therefore, the influence of topography on the versican isoforms was also investigated.
Materials and Methods
HTM Cells
HTM cells were obtained from corneal buttons that were unsuitable for transplant. The corneal buttons were provided by the Heartland Lions Eye Bank (St. Louis, MO). All work was conducted according to the tenets of the Declaration of Helsinki. Previous work has shown that corneal buttons are a useful source for HTM cells and when the buttons are kept in preservative (Optisol, Chiron Optics, Irvine, CA), the HTM cells remain viable for several weeks.26 The trabecular meshwork was dissected from the corneal button. The cells were removed from the meshwork as previously described27 and placed in DMEM/F-12 medium with 20% fetal bovine serum added (Invitrogen, Carlsbad, CA). The cells were all initially plated onto standard tissue culture plastic. All cells were used before the 6th passage. Because there can be variation in the properties of the trabecular meshwork cells from one donor to another,16 a minimum of three HTM cultures from different donors were used for each parameter that was measured.
Fabrication of Surfaces
The cellular substrates were fabricated as previously reported.15 Briefly, nanogrooved substrate designs were created by patterning a photoresist on the surface of a silicon wafer via lithography. The photoresist is exposed to radiation through a mask, and the exposed regions (for a positive tone resist) undergo chemical changes such that this material can be selectively removed by dissolving it with a developer solvent. The silicon that is not protected by the remaining photoresist can be etched to different depths by reactive ion etching to create three-dimensional relief structures. The remaining photoresist can be stripped to reveal a nanostructured surface composed of controlled grooves and ridges. The silicon surfaces are used as masters in soft lithographic processes to make replicas of the original relief structures. For example, the master can be molded into polydimethyl-siloxane (PDMS) stamps, and these stamps in turn can be molded into polyurethane.28,29 Optical adhesive (no. 81; Norland Products Inc., Cranbury, NJ) was used to create the final patterned surfaces for all cell experiments. The result was an optically clear polyurethane surface with the same pitch and depth dimensions as the silicon master. Three-square-centimeter nanogrooved surfaces of 400-nm pitch (equal ridge and groove widths) with grooves having a 300-nm depth were used for the molecular and biochemical studies. Control flat surfaces were made by spin coating tissue culture plates with the same polyurethane. Coverslips with six parallel ridge-and-groove patterns ranging from a 400-nm through 4000-nm pitch were used to study the effect of feature scale on elongation and orientation. This “six-pack” has 2-mm2 patterned regions with control planar regions separating the patterned ones.
Measurement of Alignment and Orientation
HTM cells were plated on the six-packs, fixed after 24 hours, and then analyzed as has previously been described.15 Briefly, cells were plated at 30,000 cells per six-pack so that most of the cells were isolated from one another. The cells were incubated overnight and then fixed in 4% paraformaldehyde for 20 minutes. After washing with phosphate-buffered saline, the cells were permeabilized with 0.5% Triton X-100 for 5 minutes and then washed again. TRITC-labeled phalloidin and 4′,6′-diamino-2-phenylindole (DAPI) were used for fluorescent staining of the actin filaments and the cell nucleus. Images of the cells on all the surfaces including the planar areas were taken (Axiovert 200M microscope; Carl Zeiss Meditec, Inc., Thornwood, NY) and analyzed with accompanying software (KS300; Carl Zeiss Meditec, Inc.) that measures the long and short axes of the cell as well as the orientation in relation to the alignment of the ridges. Any cell with a ratio of the long axis to the short axis ≥1.3 was considered elongated. Any cell within 10° of the orientation of the long axis of the ridges was considered aligned. Cells within 10° of perpendicular to the orientation of the long axis of the ridges were considered perpendicular. HTM cells from three different individuals were assessed for orientation and elongation; in general, 60 to 150 cells were measured for each cell on each surface. Qualitative images were acquired with the (Axiovert 200M; Carl Zeiss Meditec, Inc.) outfitted with fluorescence and 40× (1.3 NA) and 63× (1.4 NA) objectives. z-Stacks of the cells were taken with the 63× objective at 0.25- to 0.3-μm intervals and subjected to iterative deconvolution with the Zeiss deconvolution software module. Mosiac images for very long cells were acquired with the 40× objective (guided by MosiaX software; Carl Zeiss Meditec, Inc.).
Real-Time PCR
To obtain adequate amounts of mRNA, we grew ~250,000 cells in 6-cm dishes on surfaces that measure 2.8 × 3.0 cm. For each data point, cells from two or three surfaces were pooled. The mRNA was extracted from the cells after they had been grown on either planar or 400-nm pitch surfaces for 4 days. This period was used so that parallel cultures, either untreated or treated with 10−7 M dexamethasone, could be harvested to assess any possible interaction of dexamethasone and topographic cueing on myocilin expression. The mRNA was extracted with RNeasy kits according to the manufacturer’s protocol (Qiagen, Valencia, CA). The amount of mRNA was measured, and 75 ng was used with the one-step kit for real-time PCR (TaqMan; Applied Biosystems, Inc., [ABI] Foster City, CA). Individual reactions with the real-time PCR machine (StepOne; ABI) were performed with a total volume of 10 μL. The reverse transcription reaction was performed for 20 minutes at 50°C followed by PCR enzyme activation for 10 minutes at 95°C. Forty cycles of 60°C for 1 minute followed by 95°C for 15 seconds were performed. The reference mRNA was the 18S ribosomal RNA. At least three reactions were run for each sample. The mean ± SD was determined for each reaction and a Student’s t-test was used to determine statistical significance. To assess the levels of mRNA of the versican isoforms, 1 μm of mRNA from the cells was reverse transcribed into cDNA (Retroscript kit; Ambion, Austin, TX), according to the protocol supplied. The relative levels of each of the versican isoforms were determined (Power SYBR green PCR Master Mix kit; ABI) with 75 ng of cDNA and primers that had been reported previously to be characteristic of each of the isoforms.23 The PCR cycles were the same as the myocilin reactions. The samples were performed in triplicate and then normalized to the V0 isoform from the control planar sample. The planar V0 isoform was assigned a value of 1.0. The 18S ribosomal DNA was the reference DNA for these samples. The mean ± SD was determined for each reaction, and a Student’s t-test was used to determine statistical significance. The difference (n-fold) between the planar value and the value on the 400-nm pitch surfaces for each condition was also determined by dividing the mRNA from the 400-nm pitch surface by the planar value.
Western Blot
Larger area surfaces (2.8 × 3 cm) were also used to obtain sufficient protein for Western blots. Cells grown on planar surfaces and 400-nm pitch surfaces were treated with either ethanol (carrier) or dexamethasone dissolved in ethanol, as described earlier. The cells were lysed at the 10-day time point with buffer (M-Per; Pierce, Rockford, IL) containing 0.002% protease inhibitor cocktail III (1:500 dilution; Calbiochem, San Diego, CA). Protein concentration was assessed using the Bradford protein assay (Pierce). Samples of 10 μg were electrophoresed on a 10% polyacrylamide gel (Invitrogen) and the protein transferred to nitrocellulose membranes as previously described.27 The antibody to myocilin was made in chick,30 the antibody to αB-crystallin was made in rabbit (Assay Designs, Ann Arbor, MI) and the antibody to actin was made in rabbit (Abcam, Cambridge, MA). A chemiluminescent detection method was used with peroxidase-linked antibodies to chick or rabbit in conjunction with a chemiluminescence kit (Lumi-GLO; Kirkegaard and Perry, Gaithersburg, MD). The banding pattern obtained on the x-ray film was quantified (ImageQuant software; GE Healthcare, Piscataway, NJ).
Results
HTM cells, when grown individually, tend to send out processes in an apparent attempt to associate with other cells to form a more compact organization. The cells were plated on the six-packs at a concentration in which cells would generally not be in contact with one another (30,000 cells per 35-mm dish that contained one six-pack). This concentration was selected to eliminate the effects of cell-to-cell contact when assessing elongation and orientation on the surfaces. Approximately 80% of the single HTM cells on the planar surface were deemed elongated, according to the criteria (Fig. 2). This value approached 100% on any of the patterned surfaces so that, based on this measurement alone, there was a tendency toward increased elongation on the patterned surfaces. On the planar surfaces, the cell orientation was random with about the same number of cells parallel to a consistent arbitrary axis as perpendicular to it. On the patterned surfaces, even at a 400-nm pitch, there was an ~50% alignment of the cells with the ridges. This value increased to approximately 80% on the larger pitch surfaces.
Figure 2.
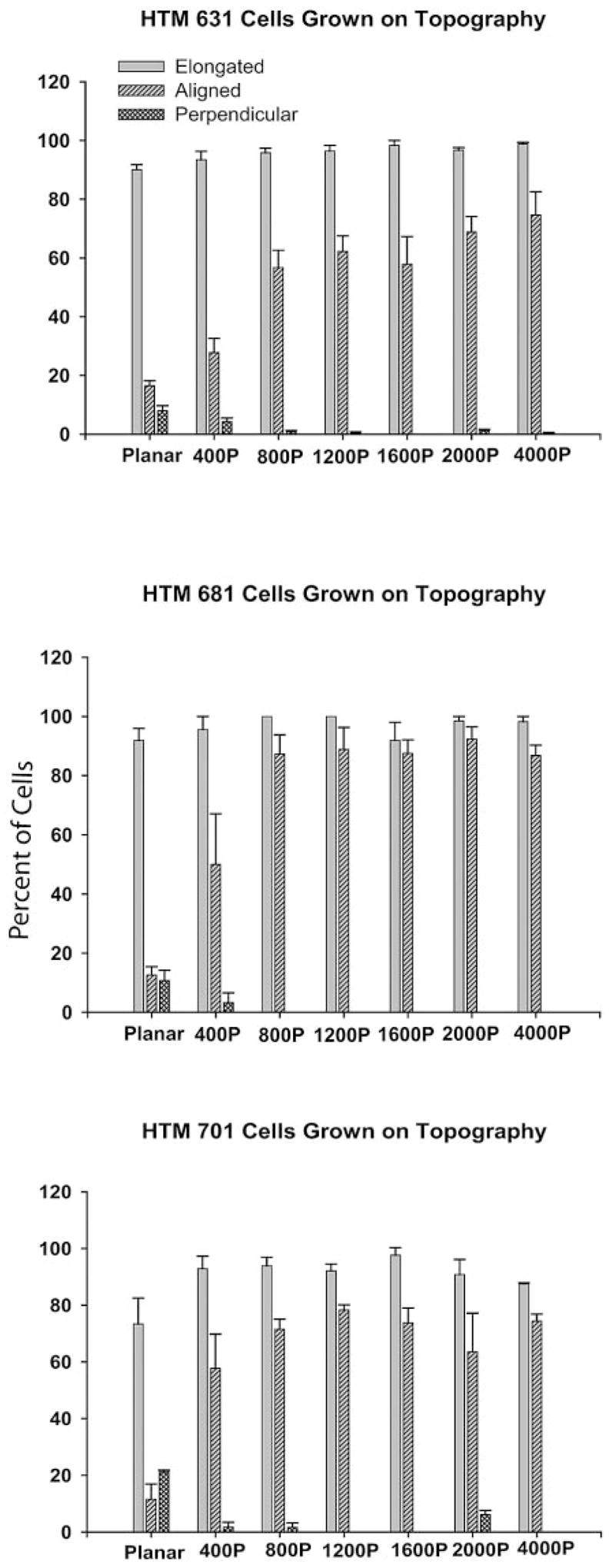
Analysis of HTM 631, 682, and 701 cells grown on planar surfaces and on six-packs. The cells were stained with TRITC-labeled phalloidin, and then the number of cells that were elongated, oriented in the direction of the grooves and ridges, or perpendicular to the grooves and ridges were measured. Besides the flat surface, cells were grown on 400-, 800-, 1200-, 1600-, 2000-, and 4000-nm pitch surfaces. Elongation was generally very high, even on the planar surfaces. The cells were orienting to the ridges even at 400-nm pitch and the alignment increased to around 80% on the other pitched surfaces (±SEM).
Qualitative analysis of HTM cells on flat and patterned surfaces showed a gross difference in the actin cytoskeleton. HTM cells stained with TRITC-phalloidin on flat surfaces revealed actin filaments extending in all directions (Fig. 3A), whereas the same cells on a 400-nm pitch surface have F-actin filaments that are strongly aligned with the grooves and ridges (Fig. 3B). Although the quantitative analysis indicated that the HTM cells were elongated and aligned to the patterned surfaces, visualization of cells revealed the extent of some of the elongation (Fig. 3C). Cells would often be observed reaching nearly 1 mm in length with the actin filaments parallel to the ridges of the patterned surfaces.
Figure 3.
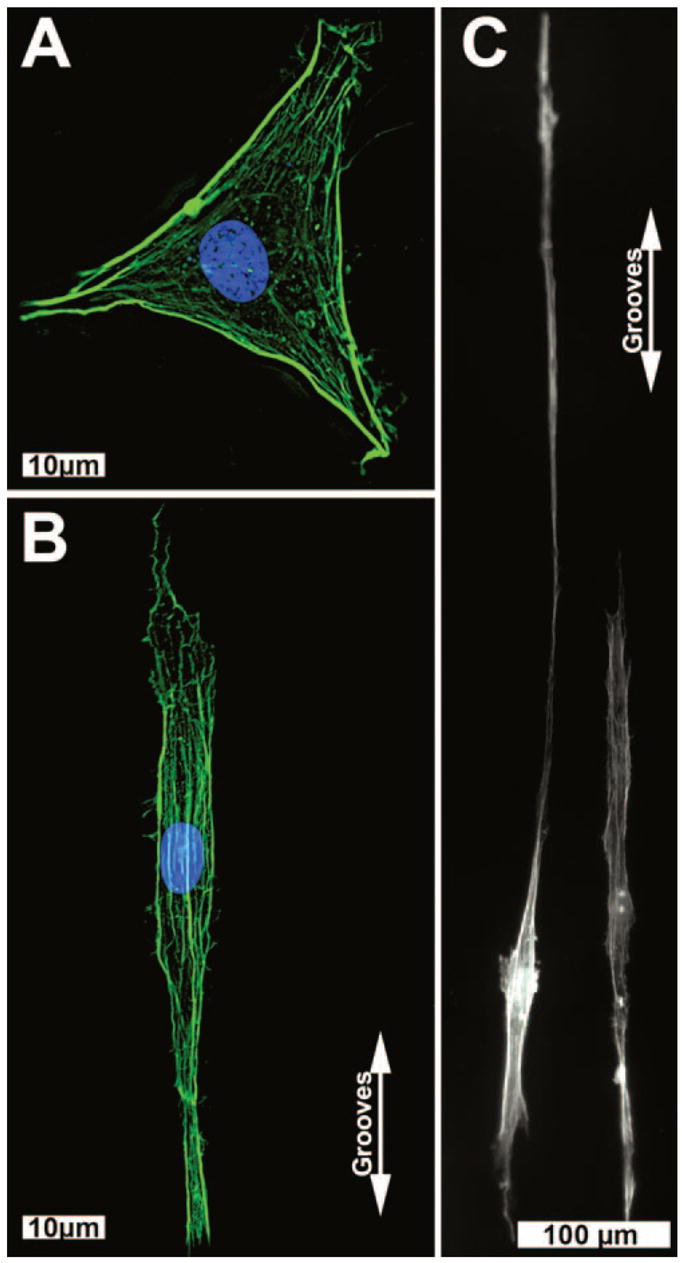
Representative images of HTM cells grown on planar surfaces (A) or 400-nm pitch (B, C). The actin filaments (green) were in a random pattern on the flat surface, but aligned with the grooves on the patterned surface. Blue: nuclei. Very elongated cells were commonly observed on the nanosurfaces (C) with cells sending out long processes along the patterned features.
The relative amount of myocilin mRNA was influenced by the growth of the cells on the nanosurfaces. Even in the least responsive group of HTM cells from a 68-year-old donor, growth of the cells on the 400-nm pitch resulted in a 1.8-fold increase in myocilin mRNA (Table 1). Cells on planar surfaces were responsive to dexamethasone, producing four times the amount of myocilin mRNA as the untreated planar cells. However, with cells grown on patterned surfaces in dexamethasone, there was an additional increase in mRNA expression. As has been reported previously, there can be variations in HTM cells from different donors31 and this can be seen with the values obtained by relative quantitative PCR for myocilin with this donor (HTM 681) and two others aged 63 and 68 (HTM 631 and 682; Table 1). HTM 631 and 682 cells were much more reactive to the patterned surfaces than were the HTM 681 cells and in both of those cases, the myocilin mRNA of the cells on the 400-nm pitch surfaces, even untreated, exceeded the amount of myocilin increase caused by the dexamethasone. From these data, the increases with dexamethasone on the 400 nm pitch surfaces were about the same magnitude compared with the planar as in the untreated conditions from the three different donors, suggesting that there was not a synergetic effect for the steroid and the patterned surface, but rather that the baseline for the comparison was higher with dexamethasone treatment. Thus, the increases between the planar and 400-nm pitch surfaces remained about the same, regardless of the corticosteroid treatment and showed that topography did not interfere with an HTM cell’s sensitivity to dexamethasone.
Table 1.
Relative Quantitative PCR for Myocilin mRNA
| HTM 631 | HTM 681 | HTM 682 | |
|---|---|---|---|
| Control Planar | 1.0 | 1.0 | 1.0 |
| Control 400 P | 15.7 ± 8.6 | 5.1 ± 0.4* | 1.8 ± 0.3* |
| Dex Planar | 2.4 ± 0.4* | 2.6 ± 0.7* | 4.2 ± 0.5* |
| Dex 400 P | 23.2 ± 1.1* | 12.7 ± 2.2* | 6.2 ± 1.5* |
Relative quantitation of the myocilin mRNA (±SD) for the HTM 631, 681, and 682 cells when grown on either planar or 400-nm pitch (P) surfaces under control conditions or with 10−7 M dexamethasone. When cells were grown on patterned surfaces, there were increases in the mRNA levels of myocilin.
Statistically significant difference from the myocilin mRNA from the control planar surfaces (Student’s t-test P < 0.05).
Because the increase in myocilin mRNA was extremely high in the HTM 631 cells, we performed Western blot analyses to determine whether the increase in mRNA would be seen in the protein concentration of myocilin (Fig. 4). The blots were scanned and corrected for protein loading using actin as the reference protein. The values obtained were normalized with the myocilin from the control planar sample, which was then assigned a value of 1.0. Very large increases in myocilin protein were noted with a 12-fold increase on the control 400-nm pitch. The effect of dexamethasone was sixfold, indicating that the HTM 631 cells were indeed responsive to this drug. The effect of nanotopography and corticosteroid raised the levels of myocilin to almost 20 times the level in the control planar cells. Western blot analysis was also performed with proteins from the HTM 682 cells, and there was a 4.6-fold increase in myocilin that was similar to the increase in mRNA between the control planar and the 400-nm pitch surfaces. In another primary culture from a 66-year-old donor, an increase in myocilin of 1.9-fold was measured by Western blot between the planar surface and the 400-nm one (data not shown).
Figure 4.
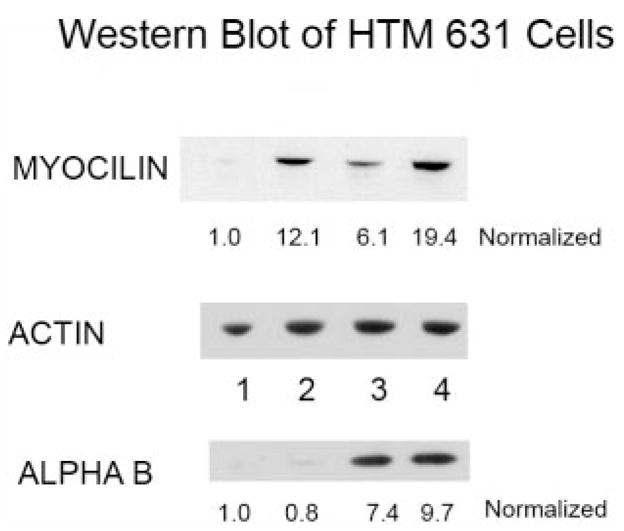
Western blot of myocilin, αB-crystallin, and actin for the homogenate of the HTM 631 cells. The actin blot was used to normalize the loading of the samples. The normalized values for each of the conditions: (1) control planar, (2) control 400-nm pitch, (3) dexamethasone-treated planar, and (4) dexamethasone treated 400-nm pitch are listed below the Western blots. In each case, the value for the control planar was set at 1.0. Although the myocilin protein levels were increased substantially in cells grown on patterned surfaces, the αB-crystallin level was similar on both planar and 400-nm pitch. However, the amount of this crystallin increased with dexamethasone treatment on both surfaces.
It has been reported that αB-crystallin increases in glaucomatous tissue and that it is influenced by heat shock and oxidative stress.2,32 To determine whether the patterned surfaces also influence the expression of this heat shock protein, we incubated the blots with antibody to αB-crystallin. Clearly, the αB-crystallin is increased with drug treatment, but unlike myocilin, little change in the amount of this protein was observed when the cells were grown on topographic surfaces (Fig. 4).
In all three of the HTM cells studied, the levels of the V0 and V1 isoforms of versican far exceeded the levels of the V2 and V3 isoforms (Fig. 5). On the topographically patterned nanosurfaces, there was an increase in the V0 and V1 isoforms, but with the V2 and V3 isoforms, it was more difficult to discern a pattern. In general, the level of mRNA expression of both the V2 and V3 isoforms in cultured cells is very low compared with that of the other isoforms, so that changes in these expression levels can be difficult to measure accurately. In HTM 682 control cells, there was around a three- to fivefold increase of the V0, V1, and V2 isoforms when the cells were grown on 400-nm pitch surfaces (Table 2). With these cells, there appeared to be about a twofold response to the patterned surface in all the isoforms after dexamethasone treatment. The mRNA levels of the V0 and V1 isoforms were approximately doubled in the HTM 631 and HTM 701 cells. Dexamethasone appeared to abolish any effect of topography with these cells. In general, on both the flat surfaces and the 400-nm pitch polyurethane surfaces, the incubation with dexamethasone reduced the mRNA expression, and the patterned surface appeared to have little effect on the expression, unlike the control cells incubated with just carrier.
Figure 5.
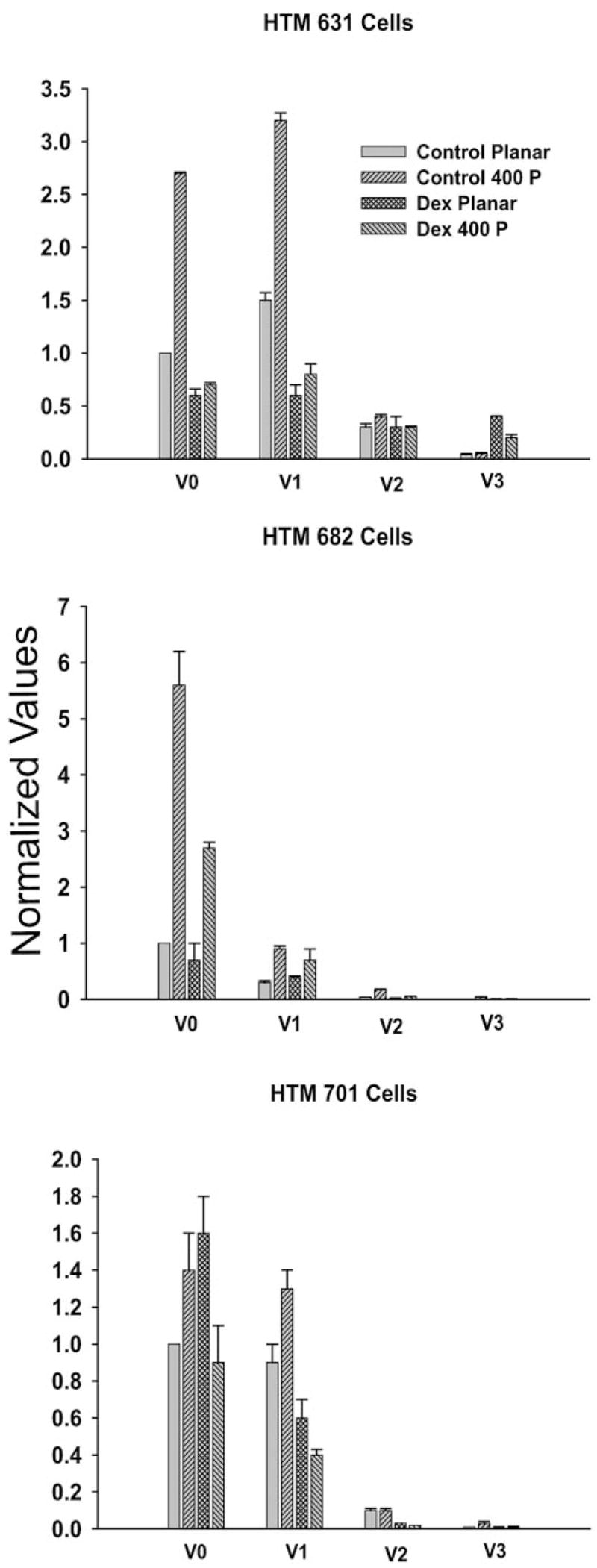
Levels of the mRNAs for the four versican isoforms for HTM 631, 682, and 701 cells. The cells were grown on either planar or 400-nm pitch surfaces with carrier (control or 10−7 M dexamethasone). In all cases, the V0 and V1 isoforms represented the bulk of the isoforms present in the cells. In the control cells, growth on 400-nm pitch increased the levels of mRNA for the V0 and V1 isoforms. Growth of the cells in dexamethasone appeared to blunt the effects of the patterned surfaces.
Table 2.
Versican mRNA Isoform Change in Cells on 400-nm Pitch Surfaces
| Cells | V0 | V1 | V2 | V3 |
|---|---|---|---|---|
| HTM 631 No Dex | 2.7* | 2.1* | 1.3* | 1.3 |
| HTM 631 Dex | 1.2* | 1.3 | 1.0 | 2.0* |
| HTM 682 No Dex | 5.6* | 3.0* | 4.3* | 4.0* |
| HTM 682 Dex | 3.9* | 1.8* | 2.5* | 1.3 |
| HTM 701 No Dex | 1.4* | 1.4* | 1.0 | 3.0* |
| HTM 701 Dex | 0.6* | 0.6* | 1.5 | 1.0 |
Changes (n-fold) in the versican mRNA isoform levels (±SD) when HTM 631, 682, and 701 were grown on 400-nm pitch topography compared with planar surfaces for each condition. These comparisons were determined by dividing the change on the 400-nm pitch surface by the planar value. There were increases in the V0 and V1 isoforms with the control cells without dexamethasone (Dex), but values for the cells grown with 10−7 M dexamethasone were more variable.
Statistically significant difference between the 400-nm pitch surface and planar surface mRNA (Student’s t-test P < 0.05).
Discussion
The data show that the provision of topographic cues has a profound effect on HTM cells. Not only do most cells elongate on these surfaces, but nearly all align with the anisotropic pattern. Perhaps more important, the levels of myocilin were increased when the cells were grown on the nanostructured substrates. It has been reported that myocilin mRNA is down-regulated when the cells are grown in culture on standard plastic ware.2 In contrast, the growth of cells on our patterned surfaces increased the expression levels of myocilin. This suggests that some of the HTM cell in vivo characteristics are retained when the cells are plated onto a substrate that presents topographic cues that approximate those encountered by the cells in vivo.
Many cellular behaviors have been shown to be modified by topographical cues.15,17,33,34 Proliferation, adhesion, and migration all change when cells interact with nanotopography. The topographic features present in vivo supply a cell with stimuli that influence these behaviors. Our data suggest that this would be a more advantageous way of culturing cells to get the expression of those proteins and traits that define cells in tissues. These physical signals are not present when cells are cultured on the standard flat surfaces typically used for tissue culture. The loss of these topographic cues may be the reason that distinctive mRNAs, such as myocilin, are downregulated or lost when cells are plated onto standard tissue culture plastic.
Not all proteins will be influenced by outgrowth on nanostructured surfaces, as can be seen with αB-crystallin. Although this protein was increased with dexamethasone on the polyurethane surfaces, no difference was observed whether or not the cells were presented with topographic cues. This result is not unexpected. Not every gene or protein is influenced in an identical manner. In other work in the laboratory on other cell types, multiple genes have been observed to change with topography, but most of the genome does not appear to be affected. Basic functions of the cell have consistently been shown to be altered by topography, such as proliferation and migration,16,29 but certain cellular processes probably are maintained in vitro, regardless of the topographical cues to support cell viability.
The data on the versican isoforms suggest that topography can influence expression of this extracellular matrix protein, but also suggest that the switch from flat to textured surfaces will not fully restore mRNA expression to what it is in vivo. The V1 isoform is the most prominent one in vivo, with the V2 also expressed at much higher levels than that seen in the cultured cells. Although the 400-nm pitch surfaces increased the levels of expression of at least the V0 and V1 isoforms, the levels of mRNA of the V2 isoform was much lower than either the V0 or the V1 forms. Thus, it appears that once the downregulation of the V2 isoform occurs when the cells are placed on tissue culture plastic, an upregulation to in vivo levels is not possible, even when the cells are provided with topographic cues. This switch may not have occurred because of the compliance of the polyurethane used in these studies. It is known that compliance or stiffness of the substratum also influences cellular behavior. The possibility exists that a surface that presents appropriate topographic and compliance cues would be optimal for the culture of cell in vitro.
These results have shown that topographical cues are important to HTM cells grown in tissue culture. Not only will cells align with a topographic pattern, but the levels of myocilin are influenced by these surfaces. Certain characteristic properties may be retained at a much higher level when the cell senses topographic cues from its growth surface. These cues may be extremely important in how the cell reacts to a stress or drug regimen, because the result seen on the flat tissue culture plastic may not reveal the true extent of a cellular reaction in vivo, which may explain, at least in part, why in vitro findings can fail to translate into a clinical setting.
Acknowledgments
The authors thank the Heartlands Lions Eye Bank for supplying the corneal buttons and Anna Kiyanova and Arinne Lyman for excellent technical assistance.
Supported by Grants 1R01EY016134-01A1 from the National Eye Institute, 5R01HL079012-02 from the National Heart, Lung, and Blood Institute, and DMR-9632527 from the National Science Foundation.
Footnotes
Disclosure: P. Russell, None; J.Z. Gasiorowski, None; P.F. Nealy, None; C.J. Murphy, None
References
- 1.Bill A. Editorial: The drainage of aqueous humor. Invest Ophthalmol. 1975;14:1–3. [PubMed] [Google Scholar]
- 2.Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- 3.Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea. 2000;19:57– 64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 5.Abrams GA, Bentley E, Nealey PF, Murphy CJ. Electron microscopy of the canine corneal basement membranes. Cells Tissues Organs. 2002;170:251–257. doi: 10.1159/000047929. [DOI] [PubMed] [Google Scholar]
- 6.Abrams GA, Murphy CJ, Wang ZY, Nealey PF. Bjorling DE Ultra-structural basement membrane topography of the bladder epithelium. Urol Res. 2003;31:341–346. doi: 10.1007/s00240-003-0347-9. [DOI] [PubMed] [Google Scholar]
- 7.Lütjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147. doi: 10.1016/0014-4835(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 8.Spencer WH, Alvarado J, Hayes TL. Scanning electron microscopy of human ocular tissues: trabecular meshwork. Invest Ophthalmol. 1968;7:651–662. [PubMed] [Google Scholar]
- 9.Andersson AS, Backhed F, von Euler A, et al. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials. 2003;24:3427–3436. doi: 10.1016/s0142-9612(03)00208-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim JH, Akaike T. Regulation of cell adhesion signaling by synthetic glycopolymer matrix in primary cultured hepatocyte. FEBS Lett. 2003;553:433– 439. doi: 10.1016/s0014-5793(03)01047-0. [DOI] [PubMed] [Google Scholar]
- 11.Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis AS. Morphologic and microarray analysis of human fibroblasts cultured on nanocolumns produced by colloidal lithography. Eur Cell Mater. 2005;9:1– 8. doi: 10.22203/ecm.v009a01. [DOI] [PubMed] [Google Scholar]
- 12.Dalby MJ, Gadegaard N, Wilkinson CD. The response of fibroblasts to hexagonal nanotopography fabricated by electron beam lithography. J Biomed Mater Res A. doi: 10.1002/jbm.a.31409. Published online July 23, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Dalby MJ, Yarwood SJ, Riehle MO, et al. Increasing fibroblast response to materials using nanotopography: morphologic and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp Cell Res. 2002;276:1–9. doi: 10.1006/excr.2002.5498. [DOI] [PubMed] [Google Scholar]
- 14.Fan YW, Cui FZ, Hou SP, et al. Culture of neural cells on silicon wafers with nano-scale surface topograph. J Neurosci Methods. 2002;120:17–23. doi: 10.1016/s0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karuri NW, Porri TJ, Albrecht RM, Murphy CJ, Nealey PF. Nano-and microscale holes modulate cell-substrate adhesion, cytoskeletal organization, and beta 1 integrin localization in SV-40 human corneal epithelial cells. IEEE Trans Nanobiosci. 2006;5:273–280. doi: 10.1109/tnb.2006.886570. [DOI] [PubMed] [Google Scholar]
- 17.Karuri NW, Liliensiek S, Teixeira AI, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley JD, Grunwald EW, Nealey PF, Murphy CJ. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials. 2005;26:3639–3644. doi: 10.1016/j.biomaterials.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Loesberg WA, Te RJ, van Delft FC, et al. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28:3944–3951. doi: 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Fraser S, Ting Y-H, Mallon KS, et al. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum rich and serum free media. J Biomed Mater Res A. doi: 10.1002/jbm.a.31519. Published online November 27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- 22.Cheng LE, Ueda J, Wentz-Hunter K, Yue BY. Age independent expression of myocilin in the human trabecular meshwork. Int J Mol Med. 2002;10:33– 40. [PubMed] [Google Scholar]
- 23.Zhao X, Russell P. Versican splice variants in human trabecular meshwork and ciliary muscle. Mol Vis. 2005;11:603– 608. [PubMed] [Google Scholar]
- 24.Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2007;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- 25.Knepper PA, Goossens W, Hvizd M, Palmberg PF. Glycosamino-glycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996;37:1360–1367. [PubMed] [Google Scholar]
- 26.Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756. doi: 10.1016/j.exer.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Tumminia SJ, Mitton KP, Arora J, et al. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 28.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 29.Franco M, Nealey PF, Campbell S, Teixeira AI, Murphy CJ. Adhesion and proliferation of corneal epithelial cells on self-assembled monolayers. J Biomed Mater Res. 2000;52:261–269. doi: 10.1002/1097-4636(200011)52:2<261::aid-jbm4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork–inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000;41:729–740. [PubMed] [Google Scholar]
- 31.Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895. doi: 10.1167/iovs.06-0036. [DOI] [PubMed] [Google Scholar]
- 32.Lütjen-Drecoll E, May CA, Polansky JR, et al. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525. [PubMed] [Google Scholar]
- 33.Teixeira AI, McKie GA, Foley JD, et al. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–3954. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J Biomed Mater Res A. 2006;79:185–192. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]


