Abstract
Hypoxic respiratory and cardiovascular responses in mammals are mediated by peripheral chemoreceptor afferents which are relayed centrally via the solitary tract nucleus (NTS) in dorsomedial medulla to other cardiorespiratory-related brainstem regions such as ventrolateral medulla (VLM). Here, we test the hypothesis that peripheral chemoafferents could also be relayed directly to the Kölliker-Fuse/parabrachial complex in dorsolateral pons, an area traditionally thought to subserve pneumotaxic and cardiovascular regulation. Experiments were performed on adult Sprague-Dawley rats. Brainstem neurons with axons projecting to the dorsolateral pons were retrogradely labeled by microinjection with choleras toxin subunit B (CTB). Neurons involved in peripheral chemoreflex were identified by hypoxia-induced cFos expression. We found that double-labeled neurons (i.e., immunopositive to both CTB and cFos) were localized mostly in the commissural and medial subnuclei of NTS and to a lesser extent in the ventrolateral NTS subnucleus, VLM and ventrolateral pontine A5 region. Extracellular recordings from the commissural and medial NTS subnuclei revealed that some hypoxia-excited NTS neurons could be antidromically activated by electrical stimulations at the dorsolateral pons. These findings demonstrate that hypoxia-activated afferent inputs are relayed to the Kölliker-Fuse/parabrachial complex directly via the commissural and medial NTS and indirectly via the ventrolateral NTS subnucleus, VLM and A5 region. These pontine-projecting peripheral chemoafferent inputs may play an important role in the modulation of cardiorespiratory regulation by dorsolateral pons.
Keywords: hypoxia, pons, cFos, solitary tract nucleus, respiration, ventrolateral medulla
1. Introduction
The mammalian ‘pneumotaxic center’, comprising mainly the Kölliker-Fuse nucleus (KFN) and medial parabrachial nucleus (MPBN) and extending to the lateral parabrachial nucleus (LPBN) in the dorsolateral pons (dl-pons) (Ezure and Tanaka, 2006, Song et al., 2006), is traditionally thought to play a critical role in the inspiratory off-switch mechanism (Cohen et al., 2004) or in maintaining eupneic breathing (St-John et al., 2004, Smith et al., 2007). In recent years, there is increasing evidence that the pneumotaxic center may also modulate the respiratory and cardiovascular responses to hypoxia and hypercapnia. For example, pneumotaxic neurons exhibited excitatory response to hypoxia (Erickson et al., 1994, Teppema et al., 1997, Berquin et al., 2000, Bodineau and Larnicol, 2001, Poon and Song, 2004) whereas lesions of the dl-pons blunted the respiratory responses to hypoxia and hypercapnia (Mizusawa et al., 1995, Song and Poon, 2009a, Song and Poon, 2009b) and augmented the carotid sympathetic chemoreflex response (Koshiya et al., 1994). In addition, electrical or chemical stimulations at loci within the LPBN and KFN caused dramatic increase in arterial blood pressure while evoking changes in breathing pattern (Lara et al., 1994, Dawid Milner et al., 2003).
The solitary tract nucleus (NTS) in caudal dorsomedial medulla provides the first central synaptic relay to various visceral afferents such as those from carotid chemoreceptors and baroreceptors, and pulmonary stretch receptors (Housley et al., 1987, Finley et al., 1992, Polson et al., 1995, Gozal et al., 2000, Marchenko and Sapru, 2000, Ezure, 2004). Neural tracing studies have shown that this structure projects heavily to all dl-pontine pneumotaxic subnuclei (Saper and Loewy, 1980, Herbert et al., 1990b, Otake et al., 1992, Ezure, 2004, Song and Poon, 2004, Kubin et al., 2006). Some of these axonal projections have been shown to originate from NTS relay neurons that receive pulmonary stretch receptor afferents and probably carotid baroreceptor afferents (Jhamandas et al., 1992, Ezure and Tanaka, 1996, Ezure, 2004). However, no data are hitherto available to establish that hypoxia-activated NTS neurons that relay peripheral chemoreceptor afferents also project to the dl-pons. Alternatively, the dl-pons may also receive peripheral chemoreceptor afferents indirectly via other brainstem areas that are activated by NTS relay neurons, such as the ventrolateral medulla (VLM) and ventrolateral pons/A5 region (Otake et al., 1992).
In this study, we sought to identify the axonal projections of hypoxia-excited neurons in respiratory-related brainstem structures to the dl-pontine pneumotaxic center. We used two complementary neural tracing approaches to corroborate our findings: neuronal double-labeling and antidromic activation of hypoxia-excited brainstem neurons. Preliminary data have been published in abstract form (Song et al., 2005).
2. Experimental Procedures
Experiments were conducted on 19 adult male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) weighing 290-330 g. Surgical procedures were performed under sterile conditions. All experimental protocols had been reviewed and approved by the MIT Committee on Animal Care in accordance with published guidelines.
2.1. Double-labeling experiments
2.1.1. Animal preparation
After injections with atropine sulphate (0.05 mg/kg, s.c.) to reduce tracheal secretions and with Buprenex (0.03 mg/kg, s.c.) for analgesia, the rat was anesthetized with pentobarbital at initial dosage of 50 mg/kg (i.p.). Surgical anesthesia was affirmed if a pinch with a clamp at the hind paw caused no withdrawal reflex. Throughout the experiment, the level of anesthesia was accessed every 15 min and a supplemental dose of pentobarbital (1/10 of initial dosage, i.p.) was given when necessary. Body temperature was kept at 37 ± 0.5 °C with a temperature controller (CWE, TC-831).
The animal's head was fixed in a stereotaxic frame (KOPF 1430, David Kopf Instrument, Tujunga, CA) using ear bars but without breaking the animal's ear drums. A small area of the dorsal scull around the lambda was exposed and a hole was drilled at the lambda level on left side at 2.3 mm lateral to the midline sagittal seam. The brain surface was exposed by making a small “×” cutting on the dura and removing the pia. To record respiratory movement, a plethysmographic voltage-resistor transducer was tied around the animal's chest and connected to an amplifier (CyberAmp 380, Axon Instruments, Union City). The signal was recorded on a Dell computer through an analog-to-digital interface (LabView, National Instruments, Austin, TX).
Microinjection
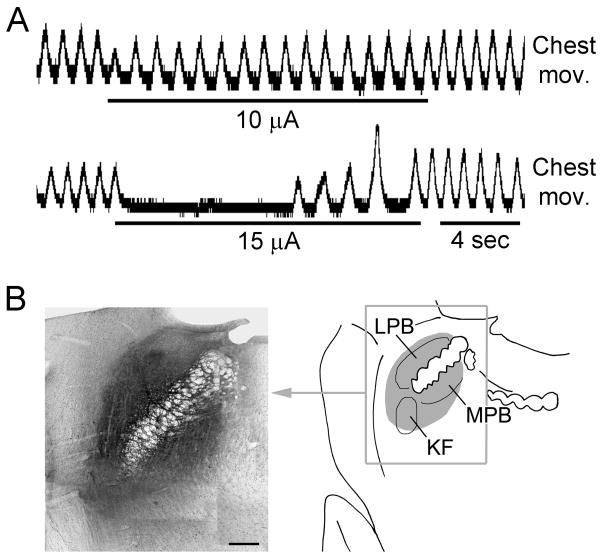
Glass micropipettes were fabricated from borosillica pipettes (O.D. 1.5 mm, I.D. 0.86 mm) on a Sutter P-87 puller (Sutter Instrument, Novato, CA). The tip of the micropipette was broken to an O.D. of 10-20 μm. Cholera toxin subunit B (CTB) was purchased from List Biological Laboratories Inc. (Campbell, CA) and dissolved in artificial cerebrospinal fluid (ACSF) at 0.5%. The micropipette was backfilled with the CTB solution and inserted stereotaxically into the dl-pons (14 animals) at stereotaxic coordinates of 2.3 - 2.4 mm lateral from the midline, -0.2 mm (caudal) - 0.1 mm (rostral) to the lambda, and at a depth of 7.5-8.0 mm from lambda surface (Paxinos and Watson, 1986). A thin silver wire was inserted into the micropipette to touch the CTB solution and was connected to a Master-8 electronic stimulator (A.M.P.I., Jerusalem, Israel). Electrical stimulation (80 Hz, pulse duration 0.1 ms) was delivered through the micropipette in order to search for an optimal tip position where the lowest stimulation intensity could induce inhibitory respiratory responses, such as decrease in inspiratory amplitude and prolongation of expiratory phase (Fig. 1A). The threshold intensity for such inhibitory response was 15-30 μA. In most animals such low-intensity loci were found with 1-2 penetrations at. Repeated penetrations were avoided to minimize damage. Microinjections were made by applying pressure pulses (10 Psi, pulse duration 0.3 sec) to the micropipette with a BH-2 microinjector (Harvard Apparatus, Holliston, MA). The volume of injection was calculated from the distance of movement of the meniscus inside the micropipette. In each animal, only one injection of 10-30 nl was made. The micropipette was left at the injection site for 10 min before being withdrawn slowly. Then the dura was moved back to cover the brain surface and the hole on skull was sealed with bone wax. The wound was cleaned and sutured. After the animal woke up, it was returned to the Institute's animal facility to recover. Another dose of Buprenex (0.03 mg, s.c.) was given at 8 hours from the initial dosage, and b.i.d. for 2-3 post-surgery days until the animal showed no sign of pain. Ringer's solution (5-10 ml) was given subcutaneously if the rat showed signs of dehydration. The surgical wound was checked daily and the animal was euthanized if found infected.
Fig. 1.
Microinjection of CTB into respiratory-related areas in dorsolateral pons. A: Electrical stimulation at the site of CTB microinjection depressed respiratory movement. B: The left panel is a montage photo of a section across the injection site. The right panel is a schematic drawing to show the area covered by CTB. In this animal, a total volume of ∼30 nl of CTB solution was injected, which infiltrated all the three major pneumotaxic structures including the Kölliker-Fuse nucleus (KFN), medial parabrachial nucleus (MPBN) and lateral parabrachial nucleus (LPBN).
2.1.2. Hypoxia challenge
To prevent non-specific expression of c-Fos that might be evoked by handling stress, rats were accustomed to the handling with daily mock experiments for 3-5 days before receiving the actual hypoxia challenge. After a survival period of 10-14 days, rats were put into a 7.8-liter airtight chamber that was continuously ventilated with humidified N2 balanced 8% O2 at a flow rate of 1 liter/min. The animal was kept in the chamber for 2-2.5 hrs. To assess possible c-Fos expression secondary to changes in systemic blood pressure during hypoxia, in 3 additional rats the tail blood pressure was monitored using a non-invasive blood pressure monitor (CODA Monitor, Kent Scientific Corp., Torrington, USA).
2.1.3. Immunohistology
At the end of the hypoxia challenge, the rats were deep-anesthetized with urethane (2 g/kg, i.p.) and transcardially perfused with 300 ml of heparinized PBS followed by 300 ml of chilled 4% paraformaldehyde in PBS. The brains were immediately removed and post-fixed in 4% paraformaldehyde overnight at 4°C, and subsequently cut into 40-μm coronal sections on a vibratome or freezing microtome.
All immunohistological procedures were performed at room temperature. For cFos immunohistology, brainstem sections were first incubated in rabbit anti-cFos polyclonal antibody (K-25, sc-253, lot # B2208, Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:4000 dilution in PBS-T for 48 hrs and rinsed (3×10 min). This highly specific and widely-used anti-cFos antibody was raised against a peptide mapping within an internal region of cFos of human origin, affinity purified, and fully characterized using Western blot analysis. Sections were then incubated in biotin-conjugated goat anti-rabbit IgG (B8895, Sigma-Aldrich, St. Louis, MO) at 1:500 dilution for 3 hrs, rinsed, and incubated in ABC reagent (standard ABC kit, Vector Laboratories Inc., Burlingame, CA) at 1:200 dilution for 1 hr. After the above processing, sections were developed with Ni-intensified DAB (DAB 0.05%, NiCl2 0.1%, H2O2 0.0015%, in 0.05 M TBS) methods.
For immunohistological visualization of CTB, sections were thoroughly rinsed and incubated in 0.3% H2O2 for 1 hr to quench any remaining peroxidase from the cFos immunohistology. Then the sections were incubated in goat anti-choleragenoid (List Biological Laboratories, Inc.) at 1:10,000 dilution for 48 hrs and rinsed, incubated in biotin-conjugated horse anti-goat IgG (List Biological Laboratories, Inc.) at 1:250 dilution for 3 hrs, rinsed, and then incubated in ABC reagent. Sections were developed with DAB method (DAB 0.05%, H2O2 0.0015%, in 0.05 M PBS).
2.1.4. Control experiments
Two naïve rats and 3 rats recovered from microinjection (CTB-control) were not challenged with hypoxia. Instead, they stayed in the same 7.8-liter airtight chamber and were ventilated with humidified medical air for 2-2.5 hrs. They were handled, perfused and processed identically as other animals for the cFos and CTB immunohistology.
2.1.5. Data analysis
After immunohistological processing, sections were mounted onto gelatin coated glass slide, oven dried, cleared in xylene and cover-slipped. cFos immunopositive neurons and CTB retrogradely labeled neurons were observed and plotted with a bright-field microscope (BH-2, Olympus, Center Valley, PA) equipped with a drawing tube. Photomicrographs of selected areas were taken with a Nikon digital camera, inputted into a Dell PC and edited with Photoshop CS2 (Adobe Systems Inc., San Jose, CA).
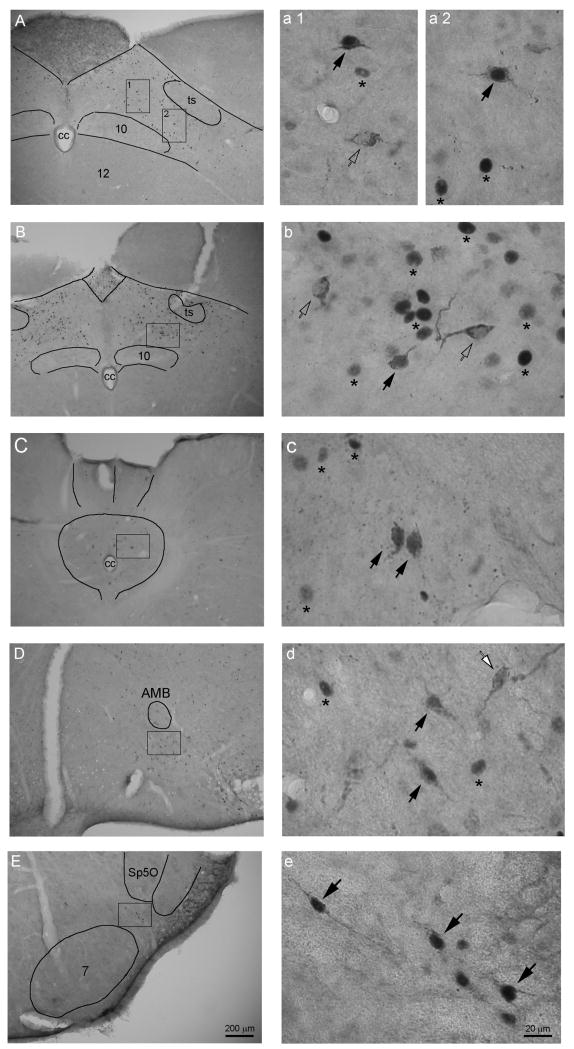
In our double-labeling method, cFos immunopositive neurons were only stained for their dark purple nuclei and the CTB retrogradely labeled neurons had somata filled with brown granules and empty nuclei. Double-labeled neurons can be easily identified for their dark-purple nuclei and granule-filled somata (Fig. 2).
Fig. 2.
Photomicrographs showing cFos immunopositive neuronal nuclei (stars), CTB labeled neurons (white arrows), and cFos-CTB double-labeled neurons (black arrows) in NTS (A-B, medial subnucleus; C, commissural subnucleus), ventrolateral medulla (D), and caudal A5 (E). Photos in the right column (a-e) are large magnifications of the square areas in corresponding left-column photos. Because the cFos protein existed only in the nucleus and the CTB only in neuronal plasma, in unstained sections under bright-field microscope the cFos immunopositive neurons are visible only from their purple colored nuclei (dark dots in B/W photos); and the CTB labeled neurons are those that are filled with brown-granular CTB immunoreaction products in somata and dendrites but not in nuclei (visible for their somata and dendrite in B/W photos). Therefore CTB labeled neurons that contain purple (dark) nuclei are identified as cFos-CTB double-labeled neurons. AMB, ambiguus nucleus; cc, central canal; Sp5O, spinal trigeminal nucleus; ts, solitary tract; 7, facial nucleus; 10, dorsal motor nucleus of vagus; 12, hypoglossal nucleus.
Since the NTS is anatomically well-defined and its commissural and medial subnuclei contained the highest density of retrogradely labeled neurons, we selected 10 sections cut around (with 5 rostral and 5 caudal to) the level of obex in each animal for counting and comparing the densities of CTB retrogradely labeled and double-labeled neurons. Double-labeled neurons in other areas (mainly in VLM regions surrounding AMB) in each of these 10 sections were also counted and averaged, but their percentages among retrogradely labeled neurons were not calculated because the VLM is not an anatomically distinct structure and its borders are not well defined. No counting was made in the A5 region as only a few double labeled neurons were observed at the level of facial nucleus.
2.2. Antidromic activation experiments
In this part of the study, neuronal discharges were recorded from commissural and medial NTS and tested for antidromic activation to electrical stimulation at dl-pons. Rats (290-310 g, male, n=3) were anesthetized with urethane at an initial dose of 1.5 g/kg (i.p.). Supplemental dose (1/10 of the initial dose) was given when a noxious stimulus (clamp at hind paw) caused withdrawal response, changes in respiratory rhythm, blood pressure and heart rate. Other animal procedures were as previously described (Song and Poon, 2009a). For electrical stimulation of dl-pons, a tungsten microelectrode was inserted to the dl-pons as described in the first part of this study. The tungsten electrode was then fixed to the scull with dental cement. Then the rat's head was flexed 30° downward to facilitate the exposure of dorsal medulla through the atlanto-occipital foramen. Dura and pia were carefully removed to expose the obex. Unit discharge was recorded with a high-impedance (5 MΩ) tungsten microelectrode. Recording area was 0-1.5 mm from midline and 0-2 mm caudal from obex at a depth of 0.5-1.5 mm. During recording the dl-pons was continuously stimulated. Antidromic activation of NTS unit discharge was confirmed by the collision test as previously described (Gang et al., 1991).
3. Results
3.1. CTB injection site
In all animals the CTB, as indicated by the DAB reaction products, diffused extensively to cover the dl-pontine area including KFN, external MPBN, and the external and central subnuclei of LPBN. The center of the injection sites was difficult to identify; nevertheless, the densest deposit of DAB reaction products occurred at the KF or external LPBN in most of the animals. The diameters of the CTB diffusions were 300-500 μm. (Fig. 1B)
3.2. Single labeling
Previous studies have shown that injections of retrograde tracers into the dl-pons labeled neurons in medullary respiratory-related structures such as ventrolateral medulla, and NTS (Kalia, 1977, King, 1980, Herbert et al., 1990a). In the present study, we observed retrogradely labeled neurons (i.e. CTB immunopositive neurons) in ventrolateral medullary areas ventral to the rostral compact AMB (rcAMB), areas of and surrounding AMB-retroAMB, and in the region immediately medial to the ventral tip of spinal trigeminal nucleus. Retrogradely labeled neurons were also observed in the contralateral dl-pons, the ipsilateral vl-pontine area including A5, spinal trigeminal nucleus, and a few in the retrotrapezoid nucleus and parapyramidal nucleus.
Retrogradely labeled neurons were observed at all levels of NTS in almost all of its subnuclei: however, the highest densities of labeled neurons were observed in the medial and commissural subnuclei at levels around the obex (10 - 70 neurons/40-μm section). Going rostrally beyond the obex, more and more labeled neurons were observed in the ventral, lateral, and ventrolateral subnuclei.
Hypoxia challenge has been found to evoke the expression of cFos protein in respiratory-related brainstem structures (Erickson et al., 1994, Teppema et al., 1997, Bodineau and Larnicol, 2001). In the present study, almost identical distribution patterns as described in previous reports were observed for hypoxia-evoked cFos immunopositive neurons. For brevity, no further details will be given here. As a measure of possible c-Fos expression secondary to blood pressure changes during hypoxia, in three additional rats mean arterial blood pressure was found to decrease moderately from a resting value of 128.2±1.6 mmHg (mean±SE) to 100.1±2.6 mmHg after one hour of hypoxia and return to pre-hypoxia value within 5-10 minutes after the cessation of hypoxia.
3.3. Double labeling
3.3.1. Double-labeled neurons in NTS
Neurons that were cFos-CTB double-labeled (i.e., immunopositive to both cFos and CTB) were observed mainly in the NTS commissural and medial subnuclei, and to a lesser extent in NTS ventrolateral subnucleus, at levels around and caudal to the obex. They were small ovoid neurons with large nuclei and one stem dendrite at each end (Fig. 2 A-C, a-c).
Because the degree of c-Fos expression as well as the actual amount and loci of CTB injections varied from animal to animal, the percentage of double-labeled neurons also varied considerably. In NTS at the level of obex, 10-40% of all CTB-labeled neurons (mostly in medial and commissural subnuclei) were double-labeled for cFos, comparing sharply to the 0-1% in control animals that were not subjected to hypoxia challenge. The number of double-labeled neurons decreased with the levels of sections going rostrally beyond the obex.
3.3.2. Double-labeled neurons in other brainstem regions
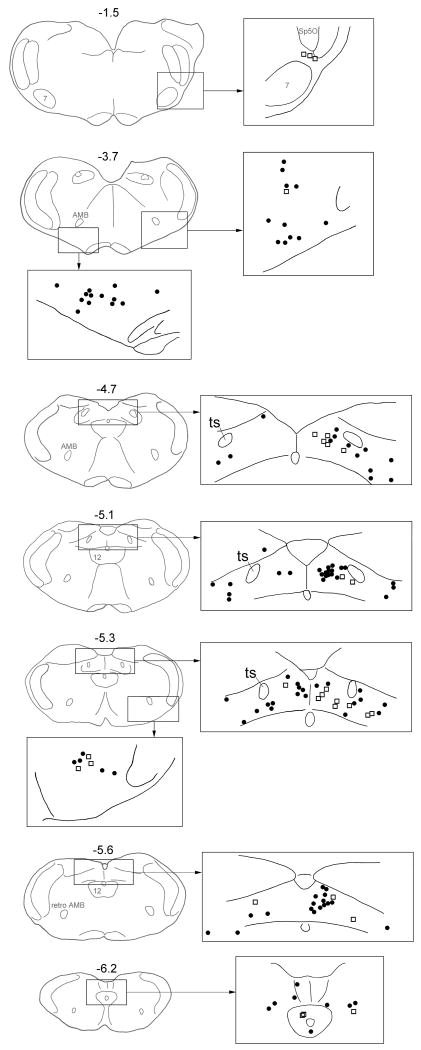
Besides NTS, double-labeled neurons were observed in ventrolateral medulla in areas surrounding AMB-retroAMB. They are small to medial bipolar or multipolar neurons. In sections cut around the level of obex, 1-5 neurons were observed in each 40-μm section. The numbers decreased with the levels of sections going rostrally or caudally. Like CTB single-labeled neurons, the distribution of double-labeled neurons was bilateral with ipsilateral dominance (Fig. 3).
Fig. 3.
Distribution of CTB single-labeled neurons (black dots) and cFos-CTB double-labeled neurons (open squares) in NTS (levels -4.7 – -6.2), ventrolateral medulla (level -5.3) and ventrolateral pons/A5 (level -1.5) of a representative animal. Each camera lucida drawing is based on observation of a single 40-μm section. Numbers are the distance (mm) from the interaural level.
A few double-labeled neurons were observed in regions where A5 located at the level of facial nucleus. They are small bipolar neurons and are exclusively ipsilateral.
3.3.3. Control animals
In the 3 control rats that were injected with CTB at dl-pons but not challenged with hypoxia, the CTB retrogradely labeled neurons exhibited similar distribution pattern as in other animals but only a few scattered c-Fos positive neurons were observed in the brainstem respiratory-related structures; even fewer were double-labeled (0-1% in NTS at obex level and not confirmed in VLM and A5). No CTB positive neuron was observed in naïve rats that were not injected with CTB, confirming that the anti-CTB antibody used in this study was highly selective.
3.4. Antidromic activation of NTS neuronal discharge
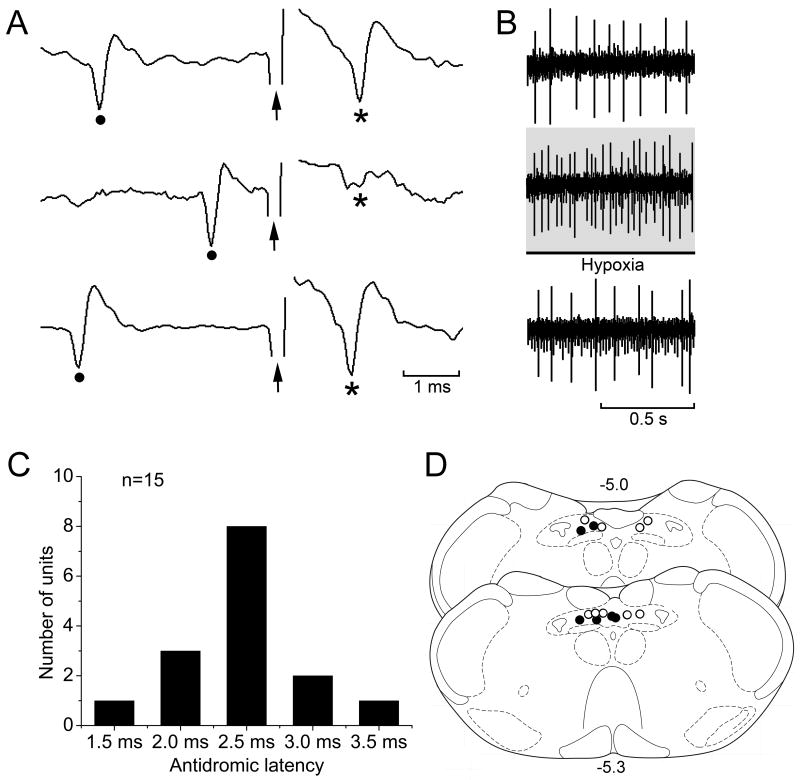
A total of 15 units that were recorded from medial and commissural NTS were antidromically activated by electrical stimulation at the dl-pons (Fig. 4A, D). All these units showed tonic (non-respiratory modulated) discharges and 6 of them showed excitatory response to brief hypoxia challenge (Fig. 4B, D). The antidromic latencies were 1.5-3.5 ms (Fig. 4C).
Fig. 4.
Electrical stimulation at the dorsolateral pons antidromically activated a neuron recorded from medial NTS. A: Upper trace, a stimulus pulse (arrow) following a spontaneous action potential (dot) evoked an antidromic action potential (*) when the time lapse between them exceeded the antidromic latency, defined as the time from the beginning of stimulus pulse to the peak of the evoked antidromic action potential. Middle trace, collision test; when the stimulus pulse was too close to the preceding spontaneous action potential (within a time window equal to or shorter than antidromic latency) the stimulus pulse failed to evoke an antidromic action potential. Lower trace, the antidromic action potential reappeared when the stimulus was sufficiently delayed from the preceding spontaneous action potential. B: This unit was activated by hypoxia challenge. C: Antidromic latencies of all the 15 NTS units. D: Distribution of antidromic units (circles) and hypoxia-activated antidromic units (dots) in NTS medial and commissural subnuclei.
4. Discussion
The major finding of this study was that some hypoxia-excited neurons in the commissural-medial NTS and to a lesser extent, ventrolateral NTS, ventrolateral medulla and A5 have axons projecting to dl-pontine pneumotaxic center (Fig. 5). The hypoxia-excited neurons were identified by the immunohistological visualization of cFos, which is expressed in neurons upon excitatory stress (Sagar et al., 1988, Bullitt, 1990). Although the underlying mechanisms linking neuronal excitation and the expression of cFos is not totally clear, the opening of glutamate NMDA receptors has been demonstrated to be the initial step in triggering cFos expression in NTS (Gozal et al., 1999). The afferent synaptic transmission of peripheral chemoreflex at the NTS second-order relay neurons is mediated in part by NMDA receptors (Ohtake et al., 1998, Gozal et al., 2000, Marchenko and Sapru, 2000). Upon exposure to hypoxia, which is the natural stimulus to peripheral chemoreceptors of the carotid bodies, these neurons undergo afferent excitation thus the expression of cFos. The present study demonstrated a similar distribution pattern of hypoxia-activated cFos immunopositive neurons in NTS and other brainstem respiratory structures as reported in previous studies (Erickson et al., 1994, Teppema et al., 1997, Bodineau and Larnicol, 2001). We believe that most of the cFos immunopositive neurons in NTS (especially those in the medial and commissural subnuclei) were second-order relay neurons of the peripheral chemoafferents and those in other brainstem structures were downstream higher-order neurons, although possible activation of baroafferent-sensitive neurons in these areas secondary to hypoxia-induced hypotension (Li et al., 1994, Graham et al., 1995) cannot be ruled out.
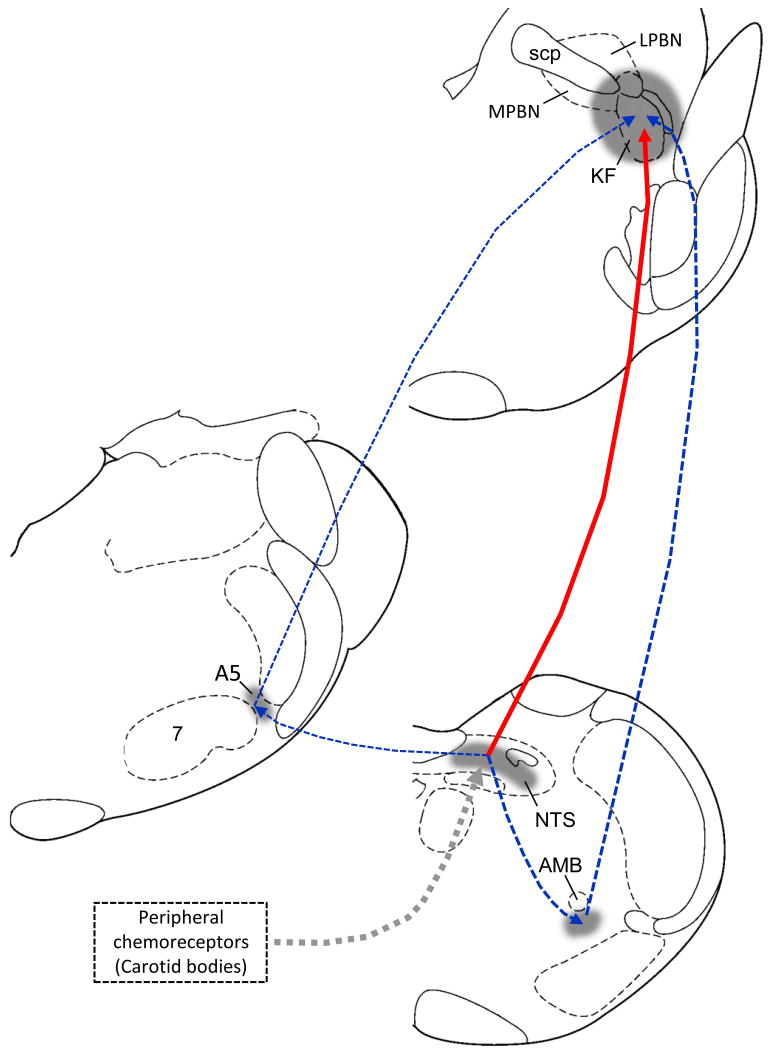
Fig. 5.
Schematic drawing showing the pathways from hypoxia-excited NTS neurons to dl-pons either directly or via the VLM or A5 regions. Solid line (red): direct projection; dashed lines (blue): secondary projections; dashed thick line (half-tone): afferents from peripheral chemoreceptors to NTS. 7, facial nucleus; A5, catecholamine neurons group A5; AMB, ambiguous nucleus; scp, superior cerebellar peduncle.
In this study, pontine-projecting medullary neurons were labeled using CTB which was pre-injected into dl-pons. The CTB, when combined with immunohistological detection, is highly sensitive in labeling both neurons and axonal terminals. We observed similar distribution patterns of retrogradely labeled neurons and anterogradely labeled axonal terminals in the brainstem as reported in earlier neurotracing studies on the efferent and afferent connections of dl-pons (Kalia, 1977, Fulwiler et al., 1984, Herbert et al., 1990a). Accordingly, double-labeled neurons – cFos immunopositive neurons that were also positive to CTB – were deemed primarily pontine-projecting chemoafferent relay neurons. We found that most double-labeled neurons were localized in commissural and medial NTS. Therefore, we conclude that peripheral chemoafferents were forwarded to the pneumotaxic center mainly through the direct pathway from second-order relay neurons in commissural and medial NTS, although indirect pathways via higher-order relay neurons in ventrolateral NTS where dorsal respiratory neuron group resides, VLM where ventral respiratory neuron group resides, and A5 that mediates the post-hypoxic respiratory frequency decline (Dick et al., 2000) were certainly also involved. The existence of this direct NTS-pontine chemoafferent pathway was further supported by our electrophysiological experiments, in which electrical stimulation at the dl-pons antidromically activated some hypoxia-excited neurons in commissural and medial NTS. The tonic, non-respiratory modulated firing pattern of these hypoxia-excited neurons is characteristic of second-order NTS neurons receiving carotid chemoafferent inputs (Marchenko and Sapru, 2000).
In previous studies, we have shown that lesions of the LPBN in anesthetized rats depress the peripheral chemoreflex response mainly by attenuating the shortening of the expiratory duration during hypoxia, without appreciable changes in all other inspiratory or expiratory hypoxic-response components and post-hypoxic frequency decline (Song and Poon, 2009a). On the other hand, our preliminary work indicated that suppression of excitatory synaptic neurotransmission in KFN blocked the post-hypoxic frequency decline but largely sparing the acute hypoxic responses in inspiratory and expiratory durations (Song et al., 2009). The present demonstration of direct axonal projections from hypoxia-excited NTS neurons to the pneumotaxic center provides a mechanistic explanation of these pontine-mediated effects from the afferent side of the peripheral chemoreflex response. Presumably, these pontine nuclei may relay the NTS-mediated peripheral chemoafferent information to separate inspiratory and expiratory rhythm generating circuits via parallel and segregated neural pathways, as proposed in a recent model of the peripheral chemoreflex (Young et al., 2003, Song and Poon, 2009a, Song and Poon, 2009b). In addition, these pontine nuclei may also integrate peripheral chemoafferent information conveyed indirectly via axonal projections from respiratory-related and hypoxia-excited neurons in the ventrolateral NTS, ventrolateral medulla and A5, as revealed herein. Further studies are needed to delineate the relative contributions of these direct and indirect peripheral chemoafferent inputs to the pneumotaxic center in modulating the inspiratory and expiratory components of the hypoxic respiratory response.
The dl-pons, besides its well-known respiratory function, also participates in the control of cardiovascular functions. Many neurons in the dl-pons exhibit modulations by both arterial pulse pressure and respiratory activity and such neurons could contribute to cardiorespiratory coupling (Dick et al., 2009). The NTS-pontine pathway (as well as the VLM-pontine and A5-pontine pathways) presently described may also forward peripheral chemoafferents further downstream to modulate hypoxic cardiovascular response, presumably via projections of hypoxia-activated KFN neurons to the rostral VLM (Hirooka et al., 1997, Guyenet et al., 2001, Song et al., 2004). In addition, we found that hypoxia caused moderate decrease in mean arterial blood pressure (from 128.2±1.6 mmHg to 100.1±2.6 mmHg, n=3), which could evoke cFos expression in baroreflex-related neurons in NTS and VLM through disinhibition as reported in previous studies (Li et al., 1994, Graham et al., 1995, Dampney et al., 2003). The NTS-pontine and VLM-pontine pathways revealed in this study could include projections from those baroreflex-related neurons.
*Research Highlights.
Hypoxia evokes cFos expression in NTS and ventrolateral medullary neurons.
Injecting CTB at dorsolateral pons labels neurons in NTS and ventrolateral medulla.
Some neurons in NTS and ventrolateral medulla are double-labeled for cFos and CTB.
Antidromic stimulation at dorsolateral pons activates hypoxia-excited NTS neurons.
NTS neurons forward peripheral chemoreceptor afferents to dorsolateral pons.
Acknowledgments
This work was supported by National Institutes of Health grants HL093225 (GS), HL067966 and HL072849 (CSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Larnicol N. Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience. 2001;108:643–653. doi: 10.1016/s0306-4522(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol. 2004;143:127–140. doi: 10.1016/j.resp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol. 2003;23:597–616. doi: 10.1023/A:1025080314925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S. A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res. 2003;982:108–118. doi: 10.1016/s0006-8993(03)03005-1. [DOI] [PubMed] [Google Scholar]
- Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol. 2009;168:76–85. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Contralateral projections of barosensitive neurons of the nucleus tractus solitarii. Neurosci Lett. 1996;219:37–40. doi: 10.1016/s0304-3940(96)13169-4. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gang S, Mizuguchi A, Aoki M. Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir Physiol. 1991;85:329–339. doi: 10.1016/0034-5687(91)90072-q. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol. 2000;121:209–221. doi: 10.1016/s0034-5687(00)00129-8. [DOI] [PubMed] [Google Scholar]
- Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett. 1999;262:93–96. doi: 10.1016/s0304-3940(99)00065-8. [DOI] [PubMed] [Google Scholar]
- Graham JC, Hoffman GE, Sved AF. c-Fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst. 1995;55:92–104. doi: 10.1016/0165-1838(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Schreihofer AM, Stornetta RL. Regulation of sympathetic tone and arterial pressure by the rostral ventrolateral medulla after depletion of C1 cells in rats. Ann N Y Acad Sci. 2001;940:259–269. doi: 10.1111/j.1749-6632.2001.tb03682.x. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990a;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990b;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80:1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Housley GD, Martin-Body RL, Dawson NJ, Sinclair JD. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience. 1987;22:237–250. doi: 10.1016/0306-4522(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH. Influence of nucleus tractus solitarius stimulation and baroreceptor activation on rat parabrachial neurons. Brain Res Bull. 1992;28:565–571. doi: 10.1016/0361-9230(92)90104-6. [DOI] [PubMed] [Google Scholar]
- Kalia M. Neuroanatomical organization of the respiratory centers. Federation Proc. 1977;36:2405–2411. [PubMed] [Google Scholar]
- King G. Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol. 1980;191:615–638. doi: 10.1002/cne.901910408. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Role of the pons in the carotid sympathetic chemoreflex. Am J Physiol. 1994;267:R508–518. doi: 10.1152/ajpregu.1994.267.2.R508. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol. 1994;477(Pt 2):321–329. doi: 10.1113/jphysiol.1994.sp020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Sapru HN. Different patterns of respiratory and cardiovascular responses elicited by chemical stimulation of dorsal medulla in the rat. Brain Res. 2000;857:99–109. doi: 10.1016/s0006-8993(99)02377-x. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489(Pt 3):877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J Comp Neurol. 1992;324:365–378. doi: 10.1002/cne.903240307. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Polson JW, Potts PD, Li YW, Dampney RA. Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla after sustained hypertension in conscious rabbits. Neuroscience. 1995;67:107–123. doi: 10.1016/0306-4522(95)00034-g. [DOI] [PubMed] [Google Scholar]
- Poon C, Song G. Distinct responses of respiratory neurons in rostrolateral pons to vagal stimulation and brief hypoxia. Soc Neurosci Abs. 2004;145:4. [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, MacDonald SM, Poon CS. Projections of hypoxia-activated or lung Inflation-activated medullary neurons to dorsolateral pons in rats. Soc Neurosci Abs. 2005;639:635. [Google Scholar]
- Song G, Poon C. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2009a;165:1–8. doi: 10.1016/j.resp.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009b;165:9–12. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS. Suppression of excitatory transmission in Kölliker-Fuse nucleus reverses initial shortening of inspiration during hypoxia and attenuates post-hypoxic frequency decline. Soc Neurosci Abs. 2009;572 [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Zhuang D, Poon CS. Anterograde tracing of bilateral axonal projections from dorsolateral pons to respiratory-related brainstem regions in rats. Soc Neurosci Abs. 2004;661:1. [Google Scholar]
- St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Young DL, Eldridge FL, Poon CS. Integration-differentiation and gating of carotid afferent traffic that shapes the respiratory pattern. J Appl Physiol. 2003;94:1213–1229. doi: 10.1152/japplphysiol.00639.2002. [DOI] [PubMed] [Google Scholar]