Abstract
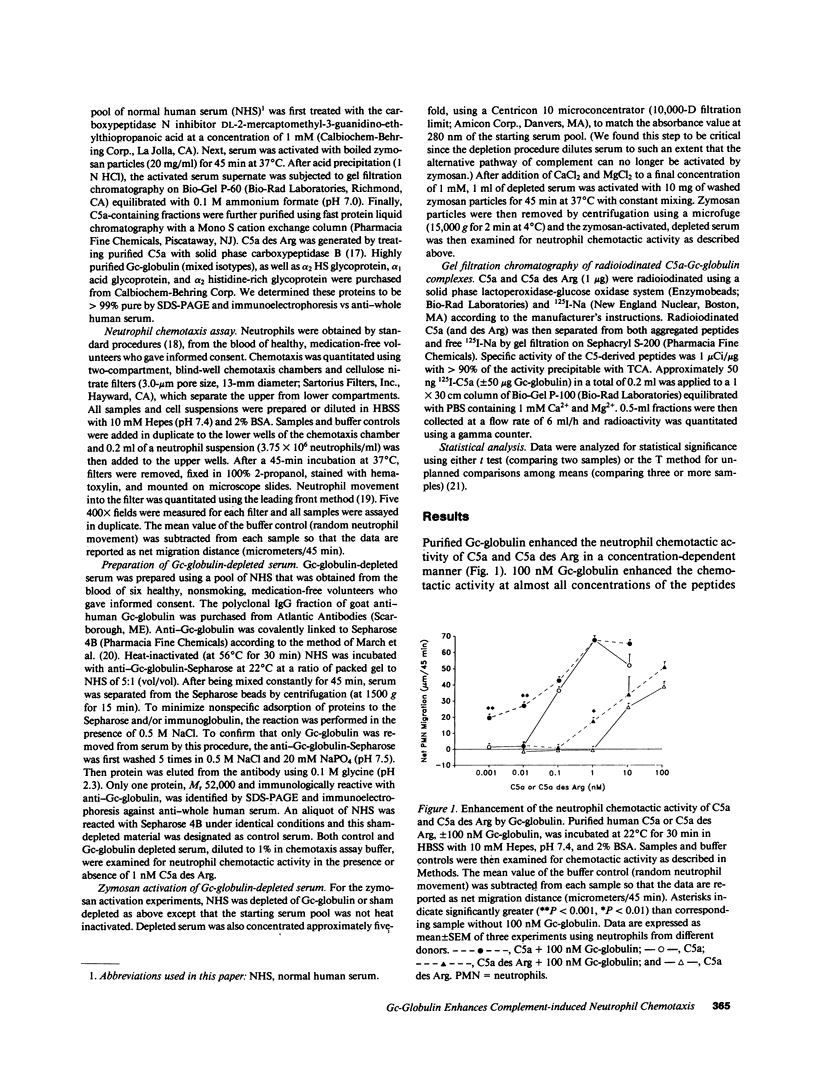
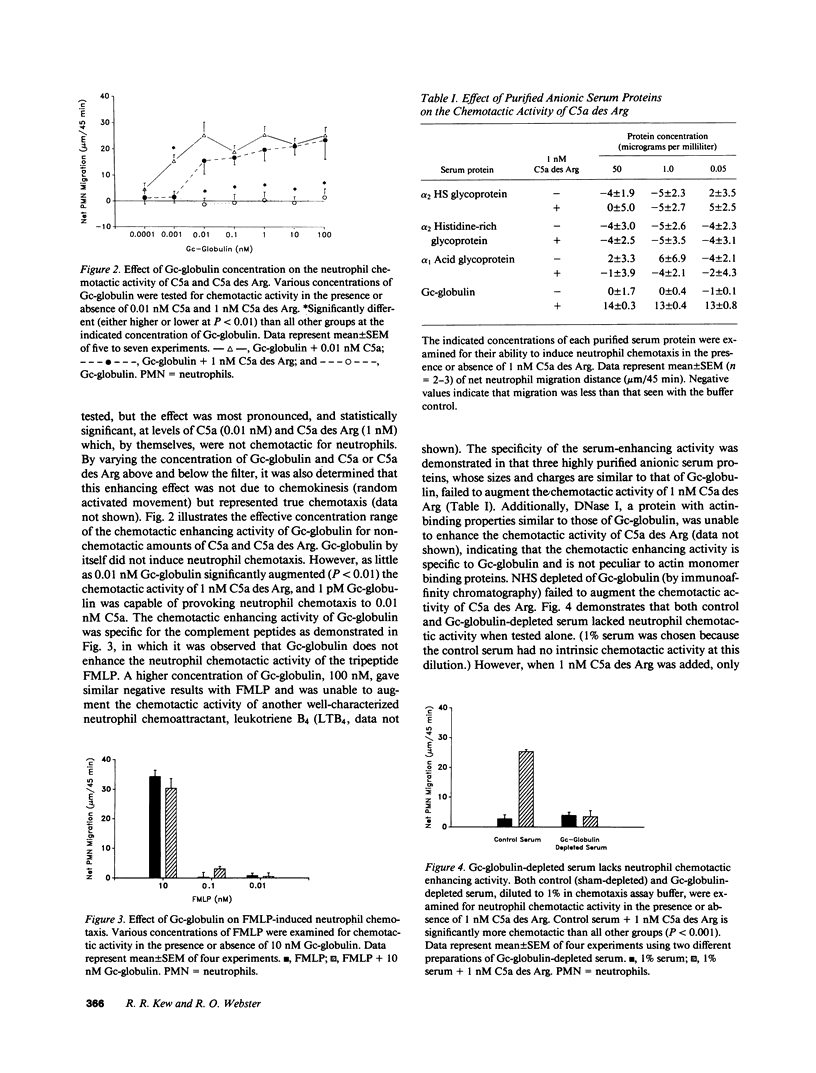
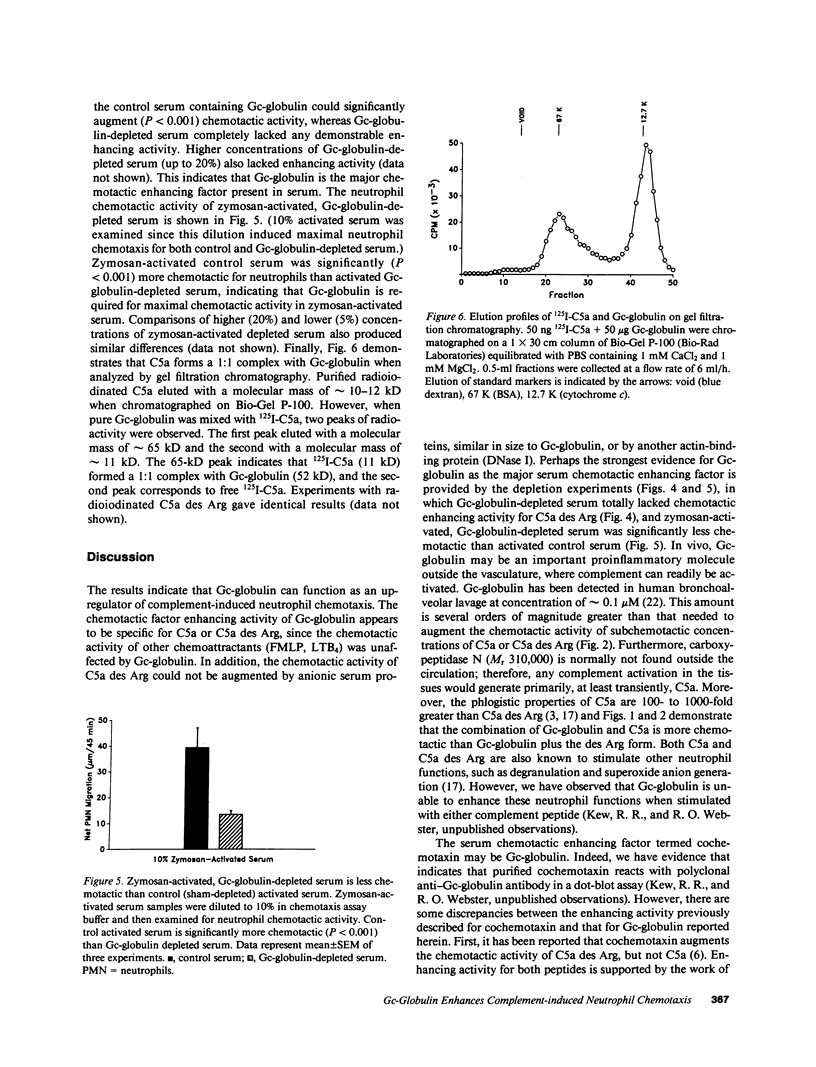
Several serum proteins have been shown to be important in modulating leukocyte chemotaxis and inflammation. We investigated the possibility that the multifunctional serum protein Gc-globulin (vitamin D-binding protein) may also enhance the neutrophil chemotactic activity of complement-derived peptides. Purified Gc-globulin by itself did not induce chemotaxis of human neutrophils. However, as little as 0.01 nM Gc-globulin greatly enhanced the neutrophil chemotactic activity of C5a and its derivative, C5a des Arg over a wide concentration range. The effect was most pronounced at nonchemotactic doses of C5a (0.01 nM) and C5a des Arg (1 nM). Gc-globulin was unable to augment the neutrophil chemotactic activity of FMLP and leukotriene B4. This enhancing activity was not due to a nonspecific effect of anionic proteins since other purified serum proteins, of similar size and charge as Gc-globulin (alpha 1 acid glycoprotein, alpha 2 HS glycoprotein, alpha 2 histidine-rich glycoprotein), could not increase the chemotactic activity of C5a des Arg. Serum depleted of Gc-globulin by immunoaffinity chromatography totally lacked chemotactic enhancing activity for C5a des Arg. Gc-globulin-depleted serum activated with zymosan also had significantly less chemotactic activity than control- (sham-depleted) activated serum. Finally, radioiodinated C5a or C5a des Arg formed a 1:1 complex with purified Gc-globulin when analyzed by gel filtration chromatography. These results indicate that Gc-globulin is the major chemotactic enhancing factor in serum and may function as an up-regulator of the chemotactic activity of C5-derived peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B. The neutrophil. Int Arch Allergy Appl Immunol. 1985;76 (Suppl 1):13–20. doi: 10.1159/000233730. [DOI] [PubMed] [Google Scholar]
- Beebe D. P., Ward P. A., Spitznagel J. K. Isolation and characterization of an acidic chemotactic factor from complement-activated human serum. Clin Immunol Immunopathol. 1980 Jan;15(1):88–105. doi: 10.1016/0090-1229(80)90023-9. [DOI] [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Identification of classical anaphylatoxin as the des-Arg form of the C5a molecule: evidence of a modulator role for the oligosaccharide unit in human des-Arg74-C5a. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1833–1837. doi: 10.1073/pnas.78.3.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Gerard C., Kawahara M., Scheetz M. E., 2nd, Barton R., Briggs S., Koppel G., Russell S. Isolation of three separate anaphylatoxins from complement-activated human serum. Mol Cell Biochem. 1981 Dec 4;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Håkansson L., Venge P. The chemotactic activity of normal and complement-activated serum as measured by the leading-front method using a Boyden chamber. Scand J Immunol. 1984 Jan;19(1):63–73. doi: 10.1111/j.1365-3083.1984.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Smith D. B., Janmey P. A., Stossel T. P. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J Clin Invest. 1986 Sep;78(3):736–742. doi: 10.1172/JCI112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marder S. R., Chenoweth D. E., Goldstein I. M., Perez H. D. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985 May;134(5):3325–3331. [PubMed] [Google Scholar]
- Perez H. D., Chenoweth D. E., Goldstein I. M. Attachment of human C5a des Arg to its cochemotaxin is required for maximum expression of chemotactic activity. J Clin Invest. 1986 Dec;78(6):1589–1595. doi: 10.1172/JCI112751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Goldstein I. M., Chernoff D., Webster R. O., Henson P. M. Chemotactic activity of C5ades Arg: evidence of a requirement for an anionic peptide 'helper factor' and inhibition by a cationic protein in serum from patients with systemic lupus erythematosus. Mol Immunol. 1980 Feb;17(2):163–169. doi: 10.1016/0161-5890(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Goldstein I. M., Webster R. O., Henson P. M. Enhancement of the chemotactic activity of human C5a des Arg by an anionic polypeptide ("cochemotaxin") in normal serum and plasma. J Immunol. 1981 Feb;126(2):800–804. [PubMed] [Google Scholar]
- Petrini M., Emerson D. L., Galbraith R. M. Linkage between surface immunoglobulin and cytoskeleton of B lymphocytes may involve Gc protein. Nature. 1983 Nov 3;306(5938):73–74. doi: 10.1038/306073a0. [DOI] [PubMed] [Google Scholar]
- Petrini M., Galbraith R. M., Emerson D. L., Nel A. E., Arnaud P. Structural studies of T lymphocyte Fc receptors. Association of Gc protein with IgG binding to Fc gamma. J Biol Chem. 1985 Feb 10;260(3):1804–1810. [PubMed] [Google Scholar]
- Petrini M., Galbraith R. M., Werner P. A., Emerson D. L., Arnaud P. Gc (vitamin D binding protein) binds to cytoplasm of all human lymphocytes and is expressed on B-cell membranes. Clin Immunol Immunopathol. 1984 May;31(2):282–295. doi: 10.1016/0090-1229(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Svasti J., Kurosky A., Bennett A., Bowman B. H. Molecular basis for the three major forms of human serum vitamin D binding protein (group-specific component). Biochemistry. 1979 Apr 17;18(8):1611–1617. doi: 10.1021/bi00575a036. [DOI] [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. The heterogeneity of human Gc-globulin. J Biol Chem. 1978 Sep 25;253(18):6344–6345. [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980 Mar 25;255(6):2270–2272. [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Wissler J. H., Sorkin E., Stecher V. J. Regulation of serum-derived chemotactic activity by the lucotactic binary peptide system. Antibiot Chemother (1971) 1974;19:442–463. doi: 10.1159/000395446. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



