Summary
Background
In many differentiated cells microtubules are organized into polarized noncentrosomal arrays, yet few mechanisms that control these arrays have been identified. For example, mechanisms that maintain microtubule polarity in the face of constant remodeling by dynamic instability are not known. Drosophila neurons contain uniform polarity minus-end-out microtubules in dendrites, which are often highly branched. As undirected microtubule growth through dendrite branch points jeopardizes uniform microtubule polarity, we have used this system to understand how cells can maintain dynamic arrays of polarized microtubules.
Results
We find that growing microtubules navigate dendrite branch points by turning the same way, towards the cell body, 98% of the time, and that growing microtubules track along stable microtubules towards their plus ends. Using RNAi and genetic approaches, we show that kinesin-2, and the +TIPS EB1 and APC, are required for uniform dendrite microtubule polarity. Moreover, the protein-protein interactions and localization of Apc2-GFP and Apc-RFP to branch points suggests these proteins work together at dendrite branches. The functional importance of this polarity mechanism is demonstrated by the failure of neurons with reduced kinesin-2 to regenerate an axon from a dendrite.
Conclusions
We conclude that microtubule growth is directed at dendrite branch points, and that kinesin-2, APC and EB1 are likely to play a role in this process. We propose that is recruited to growing microtubules by +TIPS, and that the motor protein steers growing microtubules at branch points. This represents a newly discovered mechanism to maintain polarized arrays of microtubules.
Introduction
Most cells in multicellular organisms contain polarized noncentrosomal microtubule arrays. In interphase mammalian cultured cells, microtubules are nucleated at the centrosomal microtubule organizing center (MTOC), and plus ends grow towards the cell periphery. However, in many differentiated cells, minus ends are not focused at a centrosomal MTOC. In epithelial cells, a major population of microtubules has minus ends focused at the apical side and plus ends at the basal side. In muscle cells, minus ends spread out around the nuclear envelope, and neurons have perhaps the simplest and most strikingly polarized noncentrosomal microtubule arrays [1, 2]. The mechanisms that organize these noncentrosomal microtubule arrays are poorly understood.
Neurons have two types of processes that extend from the cell body: axons and dendrites. Dendrites primarily receive signals from other neurons or the environment, and axons send signals to other neurons or output cells. One basic difference between axons and dendrites is the arrangement of microtubules. In axons microtubules are arranged into an overlapping array of uniform polarity plus-end-out microtubules [3-7]. In dendrites of cultured mammalian neurons microtubules have mixed orientation near the cell body [7, 8]. In dendrites of Drosophila neurons 90-95% of microtubules have minus ends distal to the cell body [9-12]. As the dendritic array in Drosophila is very simple, and extremely different from a centrosomal array, we used it as a model system to identify mechanisms that organize polarized noncentrosomal microtubules.
It is not known how uniform dendrite microtubule polarity is established or maintained. Models for generating the plus-end-out axonal microtubule array focus on sliding of microtubules by motor proteins. In mammalian neurons, microtubules are thought to be nucleated in the cell body at the centrosome [13], then released from the centrosome and transported down the axon in the correct orientation by motors including dynein [14, 15]. Models to account for mixed orientation microtubules in dendrites of cultured neurons have also been proposed. In this case, the kinesin MKLP1 has been proposed to transport minus-end-out microtubules into dendrites along plus-end-out microtubules [16]. In the current study we identify a new mechanism that is required for uniform microtubule polarity in dendrites. As it uses conserved, generally expressed proteins, it could play a role in maintaining microtubule polarity in many other cell types.
Results
Directed growth of microtubules at dendrite branch points
In branched dendrites with minus-end-out microtubules (Figure 1A), undirected microtubule growth threatens uniform microtubule polarity. If microtubule plus ends extend from a distal dendrite through a branch point into the other distal dendrite, then a plus-end-out microtubule will be present in the recipient dendrite (Figure 1B, right diagram). We therefore assayed microtubule growth through dendrite branch points by visualizing growing plus ends with neuronally expressed EB1-GFP. EB protein dynamics have been successfully used to image growing microtubules in both Drosophila and mammalian neurons [8, 9, 17]. In all cases the tagged EB proteins bind primarily to the growing plus ends of microtubules.
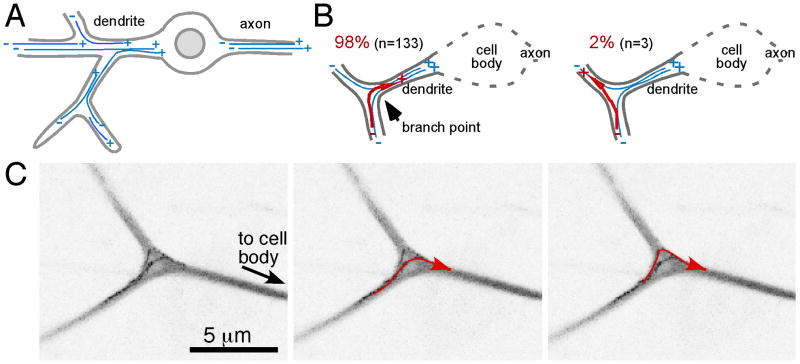
Figure 1. Microtubules grow towards the cell body at dendrite branch points.
The layout of stable microtubules in da neurons is shown in A. B. Growing microtubules (red) turn either into a distal dendrite (left) or towards the cell body (right). Quantitation above the diagrams is based on analysis of EB1-GFP dynamics as in C. C. EB1-GFP was expressed in da neurons with elav-Gal4, and movies of growing microtubules in larval da neurons were acquired with a confocal microscope. For this figure, 16 frames were projected into a single image with a maximum projection method and the image lookup table was inverted. Paths of two microtubules can be seen, and are highlighted in red. See also Movie S1.
In Drosophila, EB1-GFP dynamics can be visualized in the branched dendritic arborization (da) neurons of the larva. In these neurons the dendritic tree is sandwiched between the cuticle and epithelial cells and so is essentially two-dimensional, which is ideal for microscopic analysis [18, 19]. All of our live imaging experiments were performed in the dorsal cluster of da neurons.
When microtubules that extended through branch points were assayed, 98% grew towards the cell body (Figure 1B). The path of a growing microtubule could curve gently through the branch point, or a microtubule plus end could make a sharp turn (Figure 1C and Movie S1). As the behavior of growing microtubules at branch points is not random, microtubule growth must be directed by an active mechanism.
Plus ends of growing microtubules track stable microtubules
To understand how microtubule growth could be directed through dendrite branch points, we observed sequential microtubules growing through the same branch. Microtubules frequently followed exactly the same route through a branch point (Figure S1 and Movie 2). We hypothesized that growing microtubules might be recognizing some structure that passed through the branch and served as a track.
For a track to direct microtubules reproducibly towards the cell body, it would need to have an intrinsic polarity. The most obvious long polarized structures in cells are microtubules. To determine whether microtubule growth could be guided by an existing microtubule, we visualized stable microtubules using a protein trap transgenic line that contains the GFP coding sequence inserted into the endogenous Drosophila tau gene [9] together with growing microtubules labeled by EB1-RFP. EB1-RFP labels growing microtubule plus ends, but also localizes to other larger structures that move much more rapidly through dendrites, or do not move at all, and are not seen with EB1-GFP; we think these other structures represent nonspecific labeling.
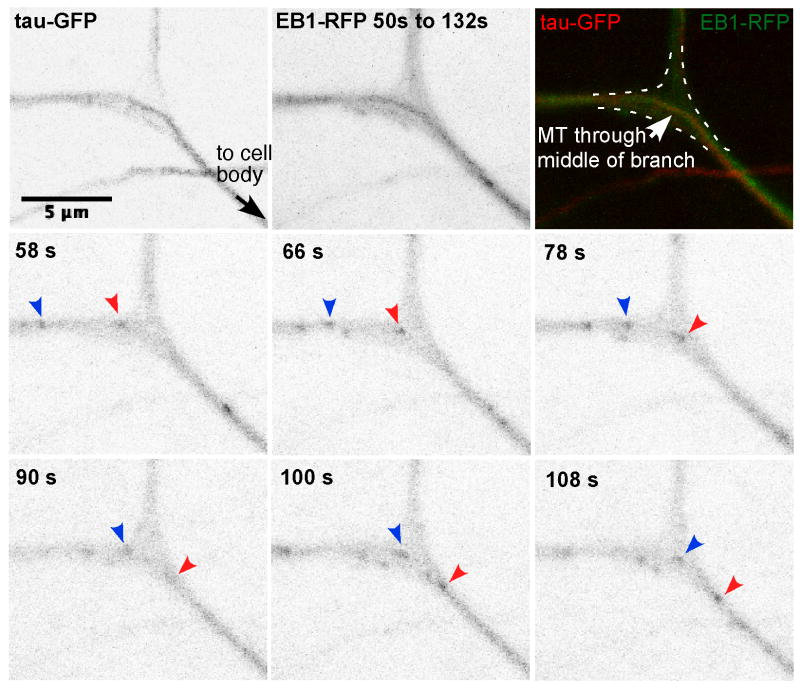
When we performed two-color live imaging of animals expressing tau-GFP and EB1-RFP, we observed microtubule plus ends coinciding with stable microtubules (Figure 2 and Movies S3 and S4). The tau-GFP signal was low and susceptible to photobleaching, but as its pattern was stable over time, we used a single frame to ascertain the layout of stable microtubules. Particularly informative were large branch points with distinctive patterns of stable microtubules running through them. In these cases we could clearly see that the EB1-RFP followed along the stable microtubule track.
Figure 2. Growing microtubule plus ends track along stable microtubules through dendrite branch points.
Movies of da neurons in larvae expressing EB1-RFP (arrows) and tau-GFP were acquired with a confocal microscope. A maximum projection of frames from 50s to 132s is shown (top middle panel). Superposition of the EB1-RFP projection and tau-GFP image shows that the microtubule plus end in the branch follows the path of the middle microtubule track labeled with tau-GFP (top right panel). See also Figure S1 and Movies S2, S3 and S4.
The kinesin-2 motor is required for uniform microtubule polarity in dendrites
As growing microtubule plus ends in dendrites can use stable microtubules as tracks and travel towards their plus end (Figure 1B), kinesins that walk towards microtubule plus ends are good candidates to mediate directed growth. For a kinesin to play this role, it would need to interact with the growing plus end. Khc73, a kinesin-3 family member, has been shown to localize to microtubule plus ends in Drosophila neuroblasts [20]. In mammalian cells a kinesin-2 (KIF3) subunit, Kap3, can interact with the microtubule plus end-binding protein (+TIP) adenomatous polyposis coli (APC) [21]. We therefore tested kinesin-2 and Khc73 for a role in directed microtubule growth.
Kinesin-2 is a heterotrimeric kinesin with two different motor subunits, named Klp64D and Klp68D in Drosophila, and an accessory subunit, Kap3. Using RNAi, we reduced levels of each of the kinesin-2 subunits or Khc73 in da neurons expressing EB1-GFP. To perform cell type-specific RNAi, UAS-hairpin RNAs were expressed under control of a neuronal Gal4 driver, as was EB1-GFP. A UAS-Dicer2 transgene was also included, as this can increase RNAi effectiveness in Drosophila neurons [22].
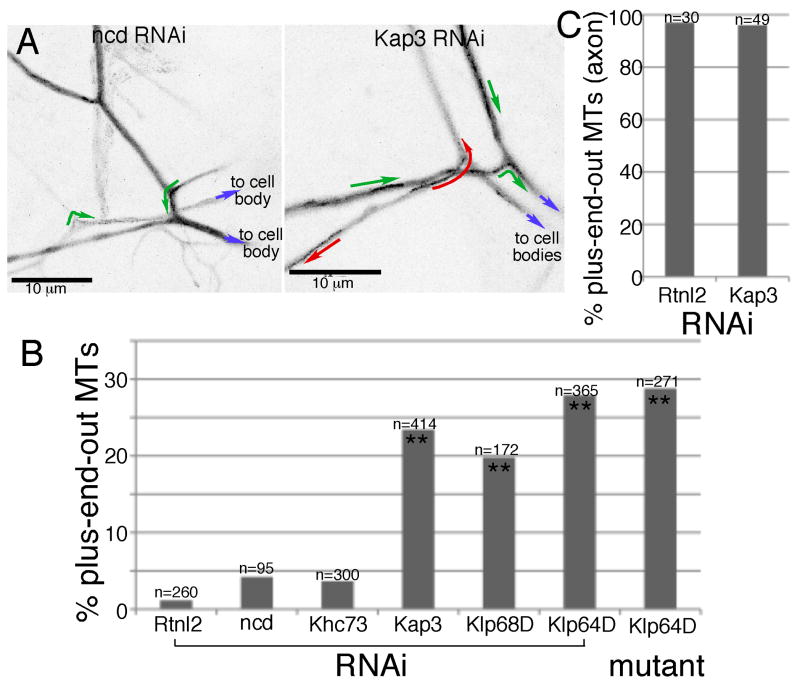
If directed growth of microtubules at branch points requires either of these kinesins, then microtubule polarity in the dendrite should change when their levels are reduced. Movies of EB1-GFP dynamics in dorsal cluster neurons were used to assay microtubule orientation by scoring the direction of movement of EB1-GFP comets. In control (Rtnl2) RNAi experiments, 1.2% of comets moved away from the cell body representing plus-end-out microtubules, and the rest moved towards the cell body at the plus ends of minus-end-out microtubules. Rtnl2 RNAi was used as a control because we have never observed any phenotypes when we compare cells expressing this RNAi to cells expressing no RNAi hairpin. In dendrites in which any of the three kinesin-2 subunits were targeted by RNAi, the number of plus-end-out microtubules was increased to 20-28% (Figure 3 and Movies S5 and S6). To analyze the specificity of the phenotype, we analyzed microtubule orientation in proximal axons of neurons expressing control or Kap3 RNAi hairpins. In both conditions microtubules were almost exclusively plus-end-out (Figure 3C).
Figure 3. Reduction of kinesin-2 subunits increases the proportion of plus-end-out microtubules in dendrites.
Cell-type specific RNAi was performed in neurons using the elav-Gal4 panneuronal driver and UAS-hairpin lines from VDRC; neurons also expressed dicer2 and EB1-GFP. A. Maximum value projections of EB1-GFP in da neuron dendrites. Green or red arrows indicate the direction of individual EB1-GFP comets. Blue arrows indicate the position of the cell body. B. The direction of EB1-GFP comet movement was quantitated in larvae expressing different RNAi hairpins and in Klp64D mutant animals (genotype elav-Gal4/UAS-EB1-GFP; Klp64Dk1/Klp64Dk5). n's indicate number of EB1-GFP comets assayed in each genotype. Double stars indicate statistically significant difference from control Rtnl2, p<0.0001 using Fisher's exact test. C. Microtubule orientation in control and Kap3 RNAi axons was assayed as above with EB1-GFP. Quantitation was performed as in dendrites. See also Figure S2 and Movies S5 and S6.
To confirm the RNAi results, we also assayed microtubule orientation in Klp64D mutants. We used a combination of hypomorphic Klp64D alleles, Klp64Dk1/Klp64Dk5, and assayed EB1-GFP dynamics in this background. Previous phenotypic characterization of Klp64D alleles determined that animals with this mutant combination noted defects only in ciliated sensory neurons [25]. As in the RNAi experiments, we found that the proportion of plus-end-out microtubules was increased to 29% in Klp64D mutant animals (Figure 3B). In addition, we used a different assay to test whether kinesin-2 might have a role in microtubule organization in dendrites of central neurons (see Figure S2). All experiments were consistent with a specific role for kinesin-2 in controlling dendrite microtubule polarity.
Dendrite branch angle correlates with the severity of kinesin-2 phenotype
Having shown that kinesin-2 is required for uniform microtubule polarity in dendrites, we wished to determine whether the geometry of dendrite branch points might also be important for polarity. Four classes of da neurons are present in the larval body wall, and each has its own distinctive branching pattern [19]. One of these cells, the class I neuron ddaE, has a dendrite with branch angles closer to right angles than other dendrites. We measured the turns growing microtubules would need to make in this ddaE comb-like dendrite compared to dendrites of class II-IV neurons. We found that in class II-IV neurons the turn angle towards the cell body was much smaller than away from the cell body, meaning that the geometry of the branch favored growth towards the cell body. In the comb dendrite these two angles were much more similar (Figure 4A). If directed growth at dendrite branch points contributes to microtubule polarity in dendrites, then we should observe stronger polarity defects in the comb dendrite when kinesin-2 levels were reduced.
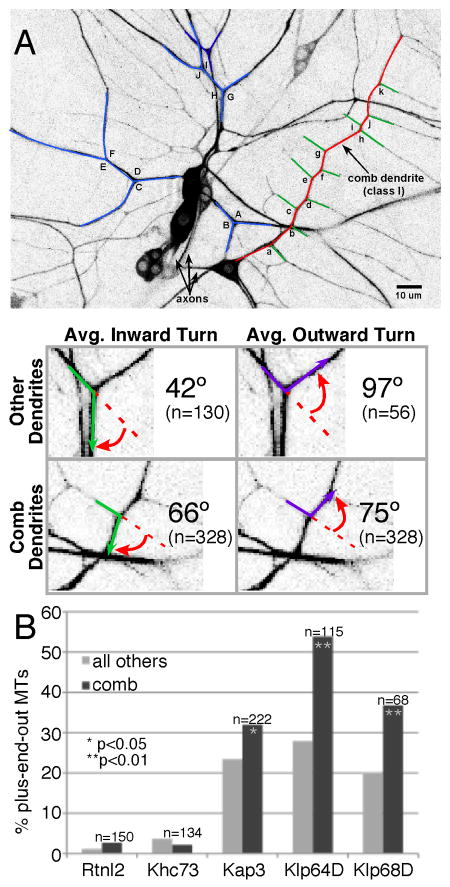
Figure 4. Dendrite branching angle determines severity of kinesin-2 phenotype.
A. Highlighted examples of class I (red & green) and non class I (blue) neurons are shown. Red indicates the main trunk of the class I neuron. The angle a growing microtubule (green and purple arrows) would be required to turn at dendrite branch point (red arrow) was measured in the comb dendrite of ddaE, class I da neuron, and all other da neurons. B. RNAi and EB1-GFP analysis was performed as in Figure 3, except that EB1-GFP direction was quantitated in the main trunk of the ddaE dendrite only (dark bars in graph). For comparison, the light bars are data from other dendrites shown in Figure 3. p values (Fisher's exact test) indicate level of significance for the difference between the comb and other data for a particular RNAi knock down. In all cases, phenotypes were significantly stronger in comb dendrites. See also Figure S3, and Movies S7 and S8.
We performed RNAi to reduce kinesin-2 subunits and focused on the comb dendrite of the class I neuron ddaE. More plus-end-out microtubules were present in the comb dendrite (Figure 4B dark bars) than in other dendrites (Figure 4B light bars) when each of the kinesin-2 subunits was targeted. In fact, microtubule orientation was completely randomized in the comb dendrite when Klp64D was targeted (Figure 4B and Movies S7 and S8). These results offer an explanation for why only 20-28% of microtubules are plus-end-out in dendrites of class II-IV neurons with reduced levels of kinesin-2: the branch angles in these dendrites most likely also help to channel growing microtubules towards the cell body.
Despite this large change in microtubule polarity, overall dendrite shape and branch number were not changed in ddaE (Figure S3A and B). We also analyzed the speed of EB1-GFP comet movement in control and Kap3 RNAi dendrites, and found this to be the same in both conditions (Figure S3C). To make sure that movements in both directions were consistent with microtubule plus end growth, we separated speed data in Kap3 RNAi dendrites into groups based on direction of comet movement. Both groups had very similar rates of movement (Figure S3C, right), and are consistent with rates of microtubule plus end growth stimulated by msps/XMAP215 [23] which participates in microtubule growth in Drosophila neurons [24]. Thus kinesin-2 specifically affects microtubule polarity and not other parameters of microtubule growth
+TIP proteins are required to maintain uniform microtubule polarity in dendrites
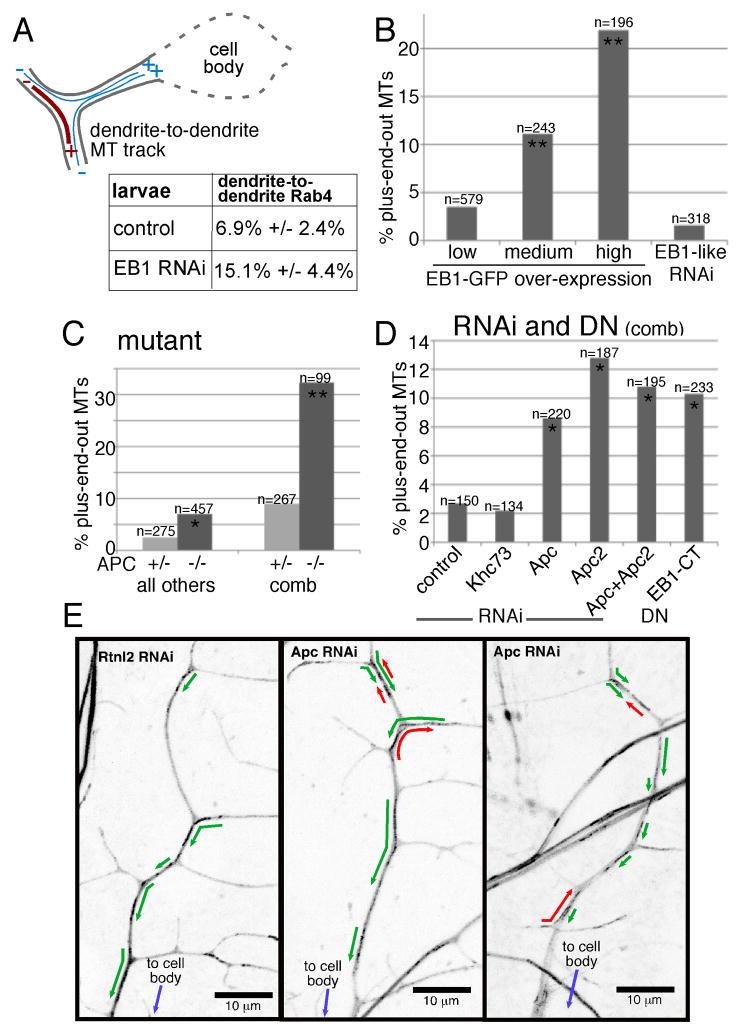
To identify proteins that could mediate an interaction between kinesin-2 and growing microtubule plus ends, we reduced levels of +TIP proteins in da neurons and assayed microtubule orientation. The +TIP EB1 is proposed to be a core plus end binding protein that mediates interaction of other proteins with growing plus ends [26-29], and is likely to be involved in most microtubule plus end behaviors. To assay microtubule polarity without relying on EB1, we used a previously developed assay that is based on patterns of membrane (Rab4-RFP) transport at dendrite branch points [9]. Microtubules that extend from one distal dendrite into another at a branch point (bridging microtubules) must have a plus end out in one dendrite and a minus end out in the other, meaning that one of the dendrites has mixed polarity (Figure 5A). In control RNAi experiments in da neurons, 6.9% of Rab4-labeled particles moved from one distal dendrite into the other, most likely on bridging microtubules. The remainder moved from dendrite to cell body or vice versa. When EB1 was targeted by RNAi, the number of Rab4 particles moving between dendrites doubled to 15.1% (Figure 5A). These results are consistent with an increased number of dendrites with mixed polarity in neurons depleted of EB1.
Figure 5. EB1 and APC are required to maintain uniform microtubule polarity in dendrites.
A. Rab4-RFP labeled membranes that moved smoothly through a branch point were classified into two categories: moving from dendrite-to-dendrite or between the cell body and dendrite (table). Error bars indicate standard deviation; the difference between the EB1 RNAi and wild-type was statistically significant using an unpaired t-test (p<0.05). B. EB1-GFP was expressed in neurons at low levels with elav-Gal4, at medium levels with one copy of 109(2)80, and high levels with two copies of 109(2)80. Direction of EB1-GFP movement in dendrites was quantitated. The differences between medium and high EB1-GFP expression and low expression were statistically significant (**) using a Fisher's exact test (p<0.0001). See also Movie S9. Also included are results from RNAi targeting EB1-like, CG32371 performed as in Figure 3. C. EB1-GFP was expressed with elav-Gal4 in animals homozygous and heterozygous for the double mutant combination Apc2N175K, ApcQ8 (APC in table). The difference between the double mutant and heterozygote was statistically significant in a Fisher's exact test in the comb dendrite (p<0.0001, **) and other dendrites (p<0.05, *). D. EB1-GFP, Dicer2 and hairpin RNA or EB1-CT expression was driven with elav-Gal4. The direction of comet movement was scored in the main trunk of the ddaE comb dendrite as in Figure 4. Compared to control (rtnl2 RNAi) neurons the indicated conditions were significantly different (p<0.05, *). E. Maximum value projections of EB1-GFP in da neuron dendrites in larvae in which Rtnl2 or Apc were targeted by RNAi are shown. Green or red arrows indicate the direction of EB1-GFP comets.
To confirm a role for EB1 in dendrite microtubule orientation, we overexpressed EB1-GFP in da neurons, and used direction of comet movement to assay microtubule orientation. Many +TIPs, including CLIP proteins and APC, are recruited to growing microtubules by binding to the C-terminus of EB1 [28]. Addition of GFP to the C-terminus of EB proteins can block these interactions [30]. In most experiments, EB1-GFP was expressed at low levels in all neurons using one copy of elav-Gal4. To drive higher levels of expression, we used one or two copies of the stronger 109(2)80 Gal4 driver which expresses in a subset of da neurons. At very high expression levels, some EB1-GFP accumulated along the length of the microtubule as well as at the tip (Movie 9), but we were still able to assay direction of comet movement. At the normal low expression level, 4.8% of EB1-GFP comets moved away from the cell body, representing plus-end-out microtubules. At moderate expression levels, with one copy of 109(2)80, this was increased to 10.8%, and at very high levels this was increased further to 18.1% (Figure 5B). As an additional method to determine whether EB1 contributes to dendrite microtubule polarity, we expressed a C-terminal fragment of EB1 (EB1-CT) in da neurons; a similar region of mammalian EB1 has been shown to have dominant negative (DN) activity [31, 32]. In the comb dendrite of the ddaE cell, EB1-CT expression increased the number of plus-end-out microtubules (Figure 5D). These results are consistent with a role for EB1 in maintaining uniform dendritic microtubule polarity.
In mammals the major neuronal EB family member is EB3. In Drosophila, EB1 is the protein most similar to both mammalian EB1 and EB3, so Drosophila EB1 most likely fulfills the functions of both EB1 and EB3. There are, however, additional genes that have sequence similarity to EB1 in the Drosophila genome [33]. We targeted the most similar of these EB1-like genes, CG32371, by RNAi, and the results were similar to control (Figure 5B).
As Kap3 and EB1 have both been reported to bind to APC in mammals, we also wished to test a role for APC in organizing dendritic microtubules. In Drosophila there are two APC proteins, Apc and Apc2. They have overlapping roles in the nervous system [34], so we generated double mutant larvae homozygous for Apc2N175K, ApcQ8 that expressed EB1-GFP in neurons. In dendrites of class II-IV da neurons the number of plus-end-out microtubules was significantly elevated in mutants (Figure 5C). The phenotype was not as strong as when kinesin-2 was targeted, perhaps because of the stability of maternally loaded APC proteins. When we examined the phenotype in the comb-shaped dendrite of the class I ddaE cell, the percentage of plus-end-out microtubules increased in double mutants dramatically, from 9% in the heterozygote to 32% in the homozygote (Figure 5C). This increase in phenotype strength in the comb dendrite was similar to that seen with kinesin-2 RNAi.
We used neuron-specific RNAi to determine whether APC proteins have a cell-autonomous role in controlling dendrite microtubule polarity. Targeting either Apc or Apc2 by RNAi, or both, resulted in an increase in plus-end-out microtubules in the comb dendrite (Figure 5D). We conclude that EB1, Apc and Apc2 are all required to maintain uniform microtubule polarity in dendrites.
An interaction network links kinesin-2 to +TIPs
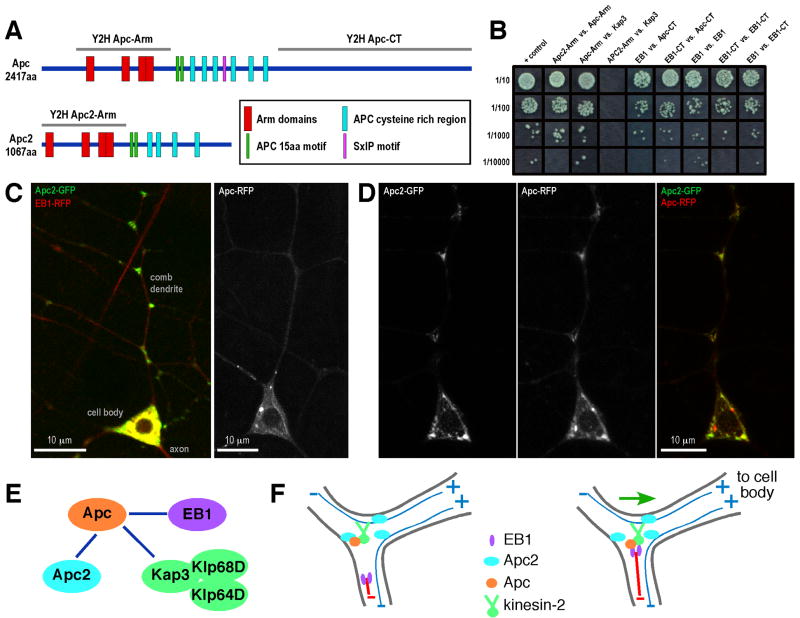
Having shown that kinesin-2, EB1, Apc and Apc2 are all required for dendrite microtubule polarity, we wished to determine whether any of them might physically interact. Mammalian Kap3 was previously reported to interact with the N-terminal region of APC that contains Armadillo repeats (ARM domain) [21]. We found that the ARM domain of Drosophila Apc, but not Apc2, could interact with Drosophila Kap3 in a two-hybrid assay (Figure 6B). We also found that the ARM domains of Apc and Apc2 could interact with one another, and the C-terminus of Apc interacted with the C-terminus of EB1. In addition, Apc and Klp68D contain the newly identified SxIP motif which can mediate interactions of proteins with EB1 [29]. The interactions identified by two-hybrid analysis place Apc at the center of a network that links kinesin-2 to EB1 (Figure 6E).
Figure 6. Interactions between kinesin-2 and +TIPs, and localization of Apc2-GFP to dendrite branch points.
A. Schematics of domains in the Drosophila Apc and Apc2 proteins are shown. The Arm domain region of both proteins and the C-terminal domain of Apc were used for two-hybrid analysis, as was full-length Drosophila Kap3, full length EB1, and the C-terminal domain of EB1. B. Results of the two-hybrid analysis. C. Apc2-GFP and EB1-RFP (for background) were expressed in class I da neurons with 221-Gal4 in the cell in the left panel. Apc-RFP was expressed alone in the cell in the right panel. The cell body and comb dendrite of ddaE are shown. D. Apc-RFP and Apc2-GFP were expressed together in ddaE. E. A summary of interactions based on two-hybrid analysis is shown. F. Model: See discussion. Growing microtubule is shown in red, stable microtubules in blue.
Apc2 localizes to dendrite branch points and can recruit Apc
We previously found GFP-tagged Apc2 localized to dendrites in Drosophila neurons [10]. In da neurons Apc2-GFP was highly concentrated at branch points (Figure 6C). It was also present in the cell body and sometimes in proximal axons as previously reported [10, 24]. We conclude that Apc2 contains a signal for localization to dendrite branch points.
To test whether Apc2 could recruit other proteins to dendrite branch points, we expressed Apc2-GFP together with Apc-RFP. Apc-RFP when expressed on its own localized diffusely throughout the entire neuron, with some concentration on structures that look like microtubules (Figure 6C). When Apc-RFP was expressed in neurons with Apc2-GFP it colocalized to large spots in the cell body, and was enriched at dendrite branch points (Figure 6D). We conclude that Apc2 contains a signal that targets it to dendrite branch points to which it can recruit Apc.
Kinesin-2 is required for regeneration of an axon from a dendrite
As well as determining how kinesin-2 might contribute to dendrite microtubule polarity, we wanted to test the physiological importance of directed microtubule growth. General neuronal defects were not observed when kinesin-2 levels were reduced, so we wanted to test whether phenotypes would arise under conditions that challenge neuronal polarity. Regeneration of an axon from a dendrite is a type of regeneration that requires polarity reversal and is conserved from Drosophila to mammals [35, 36].
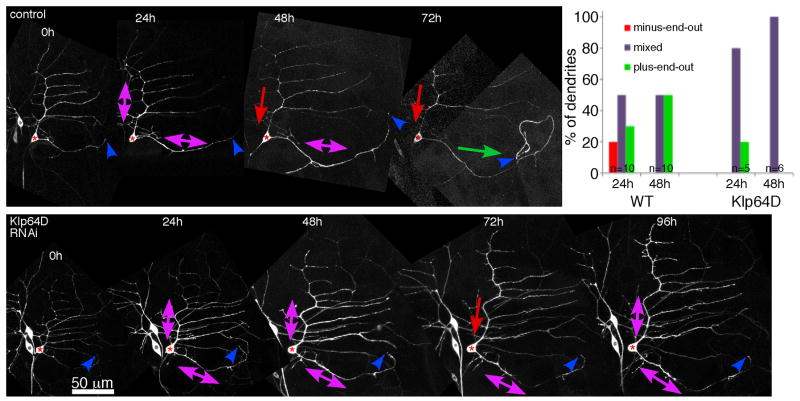
We have previously shown that axon removal induces mixing of microtubule polarity throughout the dendritic tree [35]. If a single dendrite takes on the axonal plus-end-out microtubule polarity, and the remainder return to minus-end-out polarity, then growth of the process with axonal polarity is initiated [24]. We expressed an RNAi hairpin to target Klp64D and performed axon removal in vivo with a pulsed UV laser as previously described [24]. In control animals that do not express RNAi hairpins about 2/3 of ddaE neurons reverse polarity of a single dendrite and initiate tip growth (Figure 7 and [24]). None of the six Klp64D RNAi neurons tested initiated normal tip growth (Figure 7). Four did not grow at all, and two had short new regions that were first seen at 96 hours. We quantitated microtubule orientation in the dendrite closest to the site of the axon as it is most often this dendrite that is converted to an axon [24]. In control neurons, this dendrite acquired axonal plus-end-out polarity about 50% of the time by 48h after injury. In Klp64D RNAi neurons this dendrite remained mixed (Figure 7). Thus in Klp64D RNAi neurons, the failure to establish uniform polarity in a dendrite correlated with failure to initiate axon regeneration from a dendrite.
Figure 7. Kinesin-2 is required to initiate growth from the tip of a dendrite after axon removal.
Axons of ddaE neurons (red stars) expressing EB1-GFP were severed close to the cell body at time 0 with a pulsed UV laser. Neurons were then imaged briefly every 24 hours to determine whether they initiated growth from the tip of a dendrite, and to evaluate dendrite microtubule polarity (arrows). Normal cells (top panel) initiated growth from the tip (blue arrowheads) of the dendrite nearest the site of the axon between 48 and 72 hours more than 50% of the time. Neurons expressing Klp64D hairpins (lower panel) never initiated growth from this dendrite tip (blue arrowheads) by 48 or 72 hours. 2/6 had very short regions of growth by 96 hours. Scale is the same in both panels. The graph shows quantification of microtubule orientation in the dendrite closest to the site of the axon.
Discussion
A model for directed microtubule growth in dendrites
Using Drosophila dendrites as a model system, we demonstrate that growing microtubule plus ends almost always turn towards the cell body at branch points, and that they track stable microtubules through branches. Kinesin-2, EB1 and APC are all required to maintain microtubule polarity and are linked in an interaction network.
Based on these results, we propose a model for directed growth of microtubules in dendrites. Apc2 most likely contains a branch localization signal because Apc2-GFP localizes well to dendrite branches even when overexpressed (Figure 6C). Localization of Apc2 to dendrite branch points can recruit Apc to the branch (Figure 6D). We show that Apc can interact with the Kap3 subunit of kinesin-2, and so could increase concentration of the motor near the branch. EB1-GFP does not concentrate at branches, so we propose that a growing microtubule plus end coated with EB1 is transiently linked to kinesin-2 as it passes through the branch, through the interaction between Apc and the EB1 tail. SxIP motifs in Apc and Klp68D could also contribute to this interaction. As both Kap3 and the SxIP motif in Klp68D are in the kinesin-2 tail, the motor domain would be free to walk along a nearby stable microtubule towards the plus end and cell body (Figure 6F).
Even a very brief application of force pulling the growing microtubule towards the cell body should be sufficient to steer growth towards the cell body. Once the tip of the microtubule turns, growth would be constrained by the dendrite walls. The association of the growing plus end with stable microtubule would probably only need to be maintained over a distance of a micron. This model is consistent with the observations that kinesin-2 has shorter run lengths than kinesin-1 [37], and that individual EB1 interactions with the microtubule plus end persist less than a second [38].
Observations of plus end behavior in vivo also favor a model in which only transient interactions of the motor and microtubule plus end are involved, because we frequently see plus ends turning sharply as in Figure 1B. Stable microtubules do not accommodate such sharp turns, so the sharp turns of plus ends are not likely to occur while the plus end is tracking along a stable microtubule. Instead they likely represent a switch from a freely growing plus end to one that is following a track.
Directed growth and neuronal microtubule polarity
Directed growth of microtubules at dendrite branch points allows dendrites to maintain uniform minus-end-out polarity despite continued microtubule remodeling. This mechanism is also likely necessary to establish uniform microtubule polarity in branched dendrites, but probably cannot account for minus-end-out polarity on its own. We hypothesize that directed microtubule growth is used in concert with an unidentified mechanism to control microtubule polarity in dendrites. Transport of oriented microtubule pieces has been proposed to contribute to axon microtubule polarity [14] and a similar mechanism could play a role in dendrites. For example, a kinesin anchored to the cell cortex by its C-terminus could shuttle minus-end-out microtubule seeds into dendrites. Alternately, polarized microtubule nucleation sites could be localized within dendrites. Identifying directed growth as one mechanism that contributes to dendrite microtubule polarity should facilitate identification of the missing pieces of this puzzle.
+TIPs, kinesin-2 and microtubule polarity
Because kinesin-2, APC, EB1 and polarized microtubules are found in many cell types, directed growth of microtubules along stable microtubule tracks could be very broadly used for maintaining microtubule polarity. This is a newly identified function for both the +TIP proteins and kinesin-2. APC has previously been localized to junctions between a microtubule tip and the side of another microtubule at the cortex of epithelial cells, and sliding of microtubule tips along the side of microtubules was also seen in this system, but there was no association with a particular direction of movement or overall microtubule polarity [39].
As kinesin-2 has previously been shown to be enriched in tips of growing axons in cultured mammalian neurons [40], as has APC [41], it is possible that they could mediate tracking of growing microtubules along existing microtubule tracks in the growth cone. In fact, this type of microtubule tracking behavior has been observed in axonal growth cones under low actin conditions [42]. Thus directed growth of microtubules could also be important during axon outgrowth. Indeed, the same principle of directed growth by a motor protein connected to a growing microtubule plus end could be used to align microtubules in many circumstances.
Experimental Procedures
Drosophila stocks
Many useful stocks were obtained from the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center (VDRC), National Institute of Genetics, Japan (NIG-Fly), Wes Grueber, and Dan Eberl. For details on these stocks, as well as transgenic Drosophila created for this study, see Supplemental Information.
Live imaging of larvae
All imaging of da neurons was performed in intact larvae mounted on a dried agarose pad under a coverslip. Neurons were imaged using a confocal microscope, either a Zeiss LSM510 or Olympus FV1000. For details on age, genotypes, and specific assays see Supplemental Information.
Supplementary Material
Acknowledgments
We are very grateful to Daniel Eberl for Klp64D mutant flies, to Wes Grueber for Gal4 drivers for da neurons, as well as the Bloomington Drosophila Stock Center, VDRC and NIG-FLY. Wendy Hanna-Rose, Will Hancock and Erkan Tuzel participated in stimulating discussions and provided helpful comments for the manuscript. This work was funded by an American Heart Association Scientist Development Grant, a March of Dimes Basil O'Connor Starter Scholar Award, NIH R01 GM085115. MMR is a Pew Scholar in the Biomedical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 2.Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–4163. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 3.Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton PR, Paige JL. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci U S A. 1981;78:3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troutt LL, Burnside B. Microtubule polarity and distribution in teleost photoreceptors. J Neurosci. 1988;8:2371–2380. doi: 10.1523/JNEUROSCI.08-07-02371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 7.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone MC, Roegiers F, Rolls MM. Microtubules Have Opposite Orientation in Axons and Dendrites of Drosophila Neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and compartmentalization of Drosophila neurons. Neural Development. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 13.Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. J Cell Biol. 1993;122:349–359. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. doi: 10.1016/s0896-6273(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 15.Baas PW, Buster DW. Slow axonal transport and the genesis of neuronal morphology. J Neurobiol. 2004;58:3–17. doi: 10.1002/neu.10281. [DOI] [PubMed] [Google Scholar]
- 16.Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a microtubule-associated motor protein essential for dendritic differentiation. J Cell Biol. 1997;138:833–843. doi: 10.1083/jcb.138.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 20.Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, Akiyama T. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002;4:323–327. doi: 10.1038/ncb779. [DOI] [PubMed] [Google Scholar]
- 22.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 23.Howard J, Hyman AA. Growth, fluctuation and switching at microtubule plus ends. Nat Rev Mol Cell Biol. 2009;10:569–574. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- 24.Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global Upregulation of Microtubule Dynamics and Polarity Reversal during Regeneration of an Axon from a Dendrite. Mol Biol Cell. Old doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan KT. TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J Cell Biol. 2005;171:197–200. doi: 10.1083/jcb.200509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison EE. Action and interactions at microtubule ends. Cell Mol Life Sci. 2007;64:307–317. doi: 10.1007/s00018-007-6360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 29.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 30.Lomakin AJ, Semenova I, Zaliapin I, Kraikivski P, Nadezhdina E, Slepchenko BM, Akhmanova A, Rodionov V. CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Dev Cell. 2009;17:323–333. doi: 10.1016/j.devcel.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM, Hoogenraad CC, Dogterom M, et al. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol. 2009;184:691–706. doi: 10.1083/jcb.200807179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 33.Rogers SL, Rogers GC, Sharp DJ, Vale RD. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J Cell Biol. 2002;158:873–884. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akong K, McCartney BM, Peifer M. Drosophila APC2 and APC1 have overlapping roles in the larval brain despite their distinct intracellular localizations. Dev Biol. 2002;250:71–90. doi: 10.1006/dbio.2002.0777. [DOI] [PubMed] [Google Scholar]
- 35.Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 2010;21:767–777. doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomis-Ruth S, Wierenga CJ, Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Muthukrishnan G, Zhang Y, Shastry S, Hancock WO. The processivity of kinesin-2 motors suggests diminished front-head gating. Curr Biol. 2009;19:442–447. doi: 10.1016/j.cub.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur EL. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc Natl Acad Sci U S A. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol. 2005;7:463–473. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- 41.Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24:11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.