Abstract
When the DNA of a cell is damaged, cell cycle progression is arrested and cell cycle-specific transcription is inhibited. However, cell cycle-specific transcription is required for eventual recovery from the DNA damage-induced arrest. Here we discuss recent findings that demonstrate how transcription is fine-tuned during the DNA damage response and how this controls the capacity to recover from a DNA damage arrest in G2 phase.
Key words: DNA damage, checkpoint recovery, recovery competence, FoxM1, Wip1, mitotic entry, G2/M
The Cell Cycle Engine
Cyclin dependent kinases (Cdks) phosphorylate hundreds of targets in cells to regulate cell cycle progression.1 An important aspect of this regulation relies on the activation of cell cycle specific transcriptional programs, which ensure periodical expression of a great variety of genes. Cdk activity is primarily regulated by association with Cyclins. Generally, the activation of a Cyclin-Cdk complex triggers the expression of a different Cyclin.2 In early S-phase, Cyclin E containing complexes induce the expression of Cyclin A, which subsequently induces the expression of Cyclin B in G2. Activation of Cyclin B in complex with Cdk1 ultimately leads to mitotic entry.3 In this sense, a feed-forward mechanism ensures sequential expression of different Cyclin proteins, which leads to a general increase of Cdk activity as cells progress through the cell cycle. In addition, Cdk activity also stimulates transcription and inhibits degradation of other positive regulators of Cdk activity (see below). By controlling both their own activity and distinct transcriptional programs, Cdks both maintain and define a cell cycle state.
Transcription in G2
In mammalian cells different transcription factors have been shown to regulate the expression of G2 genes. The forkhead transcription factor FoxM1 regulates the expression of genes that promote Cdk activity and progression into mitosis such as Cyclin A2, Cyclin B1, Plk1, Cdc25 phosphatases and Cdk1.4 Moreover, the FoxM1 target Plk1 itself influences FoxM1 transcriptional activity, indicating that feedback mechanisms amplify G2-specific transcription.5 A similar set of G2 genes have been shown to be direct targets of B-Myb in association with LIN-9.6 Moreover, expression of the mitotic cyclins Cyclin A2 and Cyclin B1 is regulated by the trimeric transcriptional activator NF-Y, which recognizes CCAAT-boxes in the promoter of those genes.7 Importantly, the main G2-specific transcription factors (FoxM1, B-Myb and NF-Y) are all activated through phosphorylation by Cyclin-Cdk complexes,8–10 indicating that Cyclin-Cdk dependent mechanisms regulate temporal transcription dynamics during the cell cycle. Although not all parts of the cell cycle-regulated transcriptional program depend on Cdk activity,11 the positive feedback between Cdk activity and the G2 transcriptional program suggests an elegant mechanism for coordination of cell cycle progression.
Transcription during the G2 DNA Damage Checkpoint Response
Upon DNA damage, proliferating cells activate signalling pathways to arrest the cell cycle and enable DNA repair. Different checkpoint components are activated depending on the type of genotoxic stress and the phase of the cell cycle. In response to DNA double strand breaks (DSBs), probably the most deleterious type of DNA lesion, ATM and ATR kinases are activated to initiate a checkpoint response. In the G2 phase of the cell cycle the ultimate targets of the DNA damage checkpoint are Cyclin A-Cdk1/2 and Cyclin B-Cdk1 complexes, which are inhibited to prevent entry into mitosis in the presence of damaged DNA.12 Since Cdk promotes the transcriptional activity of FoxM1, B-Myb and NF-Y,8–10 transcription of large parts of the G2-specific program is expected to be reduced during a DNA damage-induced checkpoint arrest in G2. Moreover, induction of the tumor suppressor p53 contributes to the maintenance of the G2 arrest.13 Activation of p53 induces the expression of the Cdk inhibitor p21, which leads to further reduction in Cdk activity. Furthermore, p53 actively represses the expression of G2-genes, such as Cyclin B and Cdk1, through direct binding to their promoters.14,15 Thus, the DNA damage checkpoint blocks cell cycle progression by both inhibiting Cdk activity and repressing G2-specific transcription.
Retaining Competence to Recover from a DNA Damage Response
As described above, Cdks and the G2 transcription program enhance each other through a positive feedback loop. The question therefore arises whether they can be re-initiated if both are lost? Together, Cdk activity and the G2 transcriptional program characterize the G2 state, necessary for progression into mitosis. So how can a G2 cell maintain its competence to resume the cell cycle in G2 phase during an ongoing DNA damage response that represses both Cdk activity and G2-specific transcription?
Recent work from our lab demonstrated that a minimal level of Cdk activity needs to be present throughout a DNA damage-induced arrest to allow for the eventual resumption of cell division.16 Complete inhibition of Cdk activity during a G2 arrest results in a significant reduction in transcription of mitotic Cyclins and Plk1. Even if Cdk inhibition is reversed and the DNA damage checkpoint is silenced, these cells do not regain the expression of G2-specific proteins and fail to enter mitosis.16 This suggests that full inhibition of Cdk activity during a DNA damage-induced arrest renders cells unable to enter mitosis due to very low levels of mitotic inducers. Therefore, DNA-damaged cells need to retain sufficient levels of Cdk activity to maintain the transcription of G2-specific genes and retain the competence to recover from a G2 arrest. So, how can Cdk activity maintain the expression of G2-specific proteins during an ongoing DNA damage response?
We found that FoxM1, one of the transcription factors driving the G2/M program, remains transcriptionally active during the G2 arrest and is essential for recovery after DNA damage.16 This indicates that FoxM1 is one of the Cdk targets that maintains the expression of mitotic Cyclins and Plk1 in damaged cells, to ensure that cells can re-enter the cell cycle once the checkpoint is silenced.16 Presumably, other G2 transcription factors whose activity is also regulated by Cyclin-Cdk complexes, such as B-Myb and NF-Y, may also contribute to maintain the expression of G2 genes. In fact, a recent report has showed that B-Myb, similar to FoxM1, is also required for recovery from a DNA damage-induced G2 arrest.17
In addition to transcription of G2-specific genes, Cdk activity may also regulate protein degradation during a checkpoint-dependent arrest. In an unperturbed G2 phase, the ubiquitin ligase APC/C-Cdh1 remains inactive due to Cdk-dependent phosphorylation of Cdh1.18 However, when Cdk activity is inhibited by the DNA damage response, APC/C-Cdh1 is reactivated, leading to increased degradation of G2-specific proteins.19,20 Therefore, it is likely that the minimal Cdk activity that remains during an ongoing DNA damage response is also required to prevent APC/C-Cdh1 activation and, consequently, degradation of its targets, such as mitotic Cyclins and Plk1.
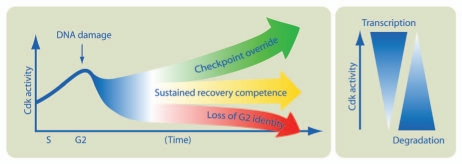
Taken together, this suggests that Cdk activity maintains the expression of G2-specific proteins during a DNA damage-induced arrest by both stimulating transcription and inhibiting degradation. If Cdk activity drops below a certain threshold, the expression of mitotic inducers, such as Cyclin A/B and Plk1, is strongly reduced. Reduced levels of these Cdk activators lead to even lower levels of Cdk activity. As a consequence, cells lose both Cdk activity and the G2 transcriptional program, both of which characterize a G2 state. Thus, a cell needs to fine-tune Cdk activity during a DNA damage-induced arrest in G2 so that it is low enough to induce a cell cycle arrest but high enough to maintain the G2 state (Fig. 1).
Figure 1.
Cdk activity needs to be balanced during a DNA damage arrest to enable resumption of the cell cycle once the damage is repaired. The DNA damage checkpoint in G2 blocks cell cycle progression by inhibition of Cdk activity. Whereas failure to limit Cdk activity during a checkpoint leads to premature cell cycle resumption, excessive inhibition leads to a loss of the G2 state. This is due to Cdk activity stimulating transcription and inhibiting degradation of multiple G2-specific proteins. These proteins both stimulate further Cdk activity and define the G2 state.
Transcriptional Repression and Recovery Competence
We recently identified the p53-induced phosphatase Wip1 as a crucial regulator of checkpoint recovery after a DNA damageinduced arrest in G2 phase.21 Although Wip1 can dephosphorylate multiple proteins involved in the DNA damage response,22 its effect on recovery does not seem to be mediated through regulation of checkpoint silencing. Instead, we found that Wip1 controls the cellular competence to recover from a DNA damage-induced arrest. Similar to what we observed after inhibition of Cdk activity during an ongoing arrest,16 Wip1-depleted cells did not retain sufficient levels of G2-specific proteins to enter mitosis once the DNA damage checkpoint was inactivated.21 We could show that Wip1 specifically counteracts p53 to maintain transcription of G2-specific genes during a DNA damage arrest in G2.
p53 can directly repress the transcription of large parts of the G2-specific transcriptional program.14,15 Our finding that Wip1 needs to counteract p53 to retain the expression of G2-specific proteins suggests that transcriptional repression of G2-specific genes needs to be balanced during an ongoing DNA damage response to allow for eventual recovery.
As mentioned above, Cdk activity and the G2-transcriptional program are heavily interconnected and amplify each other, and together, they define the G2 state. Our results demonstrate that maintenance of the G2 state during an ongoing DNA damage arrest requires a careful balance in the inhibition of Cdk activity and the repression of G2-specific transcription. By maintaining G2-specific transcription, proteins like FoxM1 and Wip1 are crucial for cellular competence to recover from a DNA damage arrest in G2.
p53 Oscillations
Although Wip1 can directly dephosphorylate p53, the exact mechanism by which Wip1 counteracts p53-dependent transcriptional repression is currently unclear. One interesting possibility involves regulation of p53 oscillations. p53 levels fluctuate during a DNA-damage response and Wip1 is a major determinant of both amplitude and duration of p53 oscillations.23,24
p53 oscillations have been suggested to provide a mechanism for a cell to regularly control whether DNA damage is repaired.24 Based on our observations, we propose that in addition the oscillations may be essential to maintain G2-specific transcription during an ongoing DNA damage response. By ensuring that p53 reaches points of low activity during the checkpoint arrest, reciprocal pulses of Cdk activity could allow induction of G2-specific transcription and inhibition of APC/C-Cdh1. As such, p53 oscillations could provide breathing pauses to allow a checkpoint-arrested cell to maintain sufficient amounts of Cdk activity to retain a G2 state.
Future Directions
Large parts of the regulation of checkpoint recovery competence remain unclear. How and when does a damaged cell lose its ability to re-start the cell cycle? Is it dependent on the magnitude and/or duration of checkpoint activation? Is it linked to the ability to repair the damage? Another important aspect that has not been addressed yet is how checkpoint recovery competence affects the outcome of genotoxic stress. The ability of a damaged cell to maintain or lose its competence to recover has clear consequences in the cellular response to damage. Loss of recovery competence could induce an irreversible cell cycle arrest or senescence. Alternatively, the cell could undergo apoptosis, or other forms of cell death. Importantly, a G2 cell that has lost its G2 state could also resume the cell cycle in G1, leading to endoreplication and polyploidy. Probably the type of damage and the cellular background are both determinants of such cell fate decision after loss of recovery competence. Finding the factors that determine the choice between all the potential cellular outcomes remains a challenge for future research.
Footnotes
Previously published online: http://www.landesbioscience.com/journals/transcription/article/12063
References
- 1.Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan DO. The Cell Cycle: Principles of Control. Primers in Biology. 2007 [Google Scholar]
- 3.Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. 1FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 5.Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterloh L, von Eyss B, Schmit F, Rein L, Hubner D, Samans B, et al. The human synMuv-like protein LIN-9 is required for transcription of G2/M genes and for entry into mitosis. EMBO J. 2007;26:144–157. doi: 10.1038/sj.emboj.7601478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katula KS, Wright KL, Paul H, Surman DR, Nuckolls FJ, Smith JW, et al. Cyclin-dependent kinase activation and S-phase induction of the cyclin B1 gene are linked through the CCAAT elements. Cell Growth Differ. 1997;8:811–820. [PubMed] [Google Scholar]
- 8.Saville MK, Watson RJ. The cell cycle regulated transcription factor B-Myb is phosphorylated by cyclin A/Cdk2 at sites that enhance its transactivation properties. Oncogene. 1998;17:2679–2689. doi: 10.1038/sj.onc.1202503. [DOI] [PubMed] [Google Scholar]
- 9.Chae HD, Yun J, Bang YJ, Shin DY. Cdk2-dependent phosphorylation of the NF-Y transcription factor is essential for the expression of the cell cycle-regulatory genes and cell cycle G1/S and G2/M transitions. Oncogene. 2004;23:4084–4088. doi: 10.1038/sj.onc.1207482. [DOI] [PubMed] [Google Scholar]
- 10.Laoukili J, Alvarez M, Meijer LA, Stahl M, Mohammed S, Kleij L, et al. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol Cell Biol. 2008;28:3076–3087. doi: 10.1128/MCB.01710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlando DA, Lin CY, Bernard A, Wang JY, Socolar JE, Iversen ES, et al. Global control of cell cycle transcription by coupled CDK and network oscillators. Nature. 2008;453:944–947. doi: 10.1038/nature06955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastan MB, Bartek J. Cell cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 13.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 15.Spurgers KB, Gold DL, Coombes KR, Bohnenstiehl NL, Mullins B, Meyn RE, et al. Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J Biol Chem. 2006;281:25134–25142. doi: 10.1074/jbc.M513901200. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Fernandez M, Halim VA, Krenning L, Aprelia M, Mohammed S, Heck AJ, et al. Recovery from a DNA damage-induced G2 arrest requires Cdk-dependent activation of FoxM1. EMBO R. 2010;11:452–458. doi: 10.1038/embor.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannefeld M, Klassen E, Gaubatz S. B-MYB is required for recovery from the DNA damage-induced G2 checkpoint in p53 mutant cells. Cancer Res. 2009;69:4073–4080. doi: 10.1158/0008-5472.CAN-08-4156. [DOI] [PubMed] [Google Scholar]
- 18.Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 19.Sudo T, Ota Y, Kotani S, Nakao M, Takami Y, Takeda S, et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001;20:6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindqvist A, de Bruijn M, Macurek L, Bras A, Mensinga A, Bruinsma W, et al. Wip1 confers G2 checkpoint recovery competence by counteracting p53-dependent transcriptional repression. EMBO J. 2009;28:3196–3206. doi: 10.1038/emboj.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Nguyen TA, Moon SH, Darlington Y, Sommer M, Donehower LA. The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008;27:123–135. doi: 10.1007/s10555-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci USA. 2000;97:11250–11255. doi: 10.1073/pnas.210171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]