Abstract
hTERT and NFAT were thought until recently to belong to separate metabolic compartments. The involvement of NFAT in the induction of hTERT transcription, suggested by hTERT expression variations during lymphocyte stimulation and immunosuppressive treatments, explains the link between hTERT expression and cell stimulation and offers new insights for therapeutic developments.
Key words: hTERT, NFAT, cell stimulation, tumorigenesis, immunosuppression
The integrity of cellular DNA can be compromised both by endogenous sources, such as oxidative by-products of cellular metabolism and stalled replication forks, and by environmental agents. Multiple DNA repair and protection systems have evolved to reduce the number of irreversible mutations. The ends of linear DNA could be particularly vulnerable because, at each round of replication, standard DNA polymerases are unable to complete DNA synthesis, leading to the “end replication problem”. Specific nucleoprotein structures capping the ends of chromosomes, named telomeres, prevent chromosome fusions and genomic instability. Telomerase, a nucleoprotein, was demonstrated to ensure de novo synthesis of telomeric repeats during DNA replication.1 Human telomeres are constituted by tandem TTAGGG DNA repeats of 5–15 kilobase pairs, proteins, such as shelterin proteins, and DNA repair proteins.2 Telomere maintenance is ensured by the telomerase complex, formed by the human telomerase RNA (hTR) template, containing the motif CUAAUCCCAAC (complementary to the telomere repeats), and the human telomerase reverse transcriptase (hTERT) catalytic subunit.3 Besides its major role in telomere elongation and cell life extension,4 hTERT is also implicated in metabolic processes independent of telomeres.5 Relationships between the expression and activity of hTERT and cell stimulation need to be known in order to understand consequences of anti-proliferative and anti-telomerase treatments.
hTERT Expression is Induced by Lymphocyte Activation
hTERT expression is restricted to a few normal human somatic cell types, such as germinal cells, several types of normally proliferative somatic cells, such as basal layer keratinocytes,6 or cells able to be activated, such as lymphocytes. Buchkovic and Greider7 demonstrated that peripheral blood lymphocytes are able to re-express telomerase activity when stimulated. eapamycin, which targets mammalian target of rapamycin (mTOR) and blocks cell cycle in G1 phase, was an inhibitor of telomerase activity, while aphidicolin and Hydroxyurea, inhibitors of S-phase progression, were not, suggesting that telomerase activity is regulated during the G1 phase of the cell cycle.7 However, relationships between hTERT expression and lymphocyte stimulation remained poorly understood. Telomerase activity appears 24 hours after lymphocyte stimulation, while hTERT mRNA is induced within the first 6 to 12 hours.8,9 Some discrepancies between telomerase activity and hTERT expression in lymphocytes at various stages of differentiation or maturation8 were related to post-translational modifications of hTERT, such as hTERT phosphorylation by Akt and protein kinase C (PKC), and protein interactions with heat shock protein-90, mTOR, S6-kinase, and hTR before nuclear translocation (Fig. 1).10 This may account for the delay between the beginning of hTERT mRNA expression and telomerase activity after lymphocyte activation. In both normal and malignant human cells, however, a positive correlation is consistently observed between telomerase activity and hTERT mRNA expression,11 thereby highlighting the importance of the transcriptional regulation of hTERT.
Figure 1.
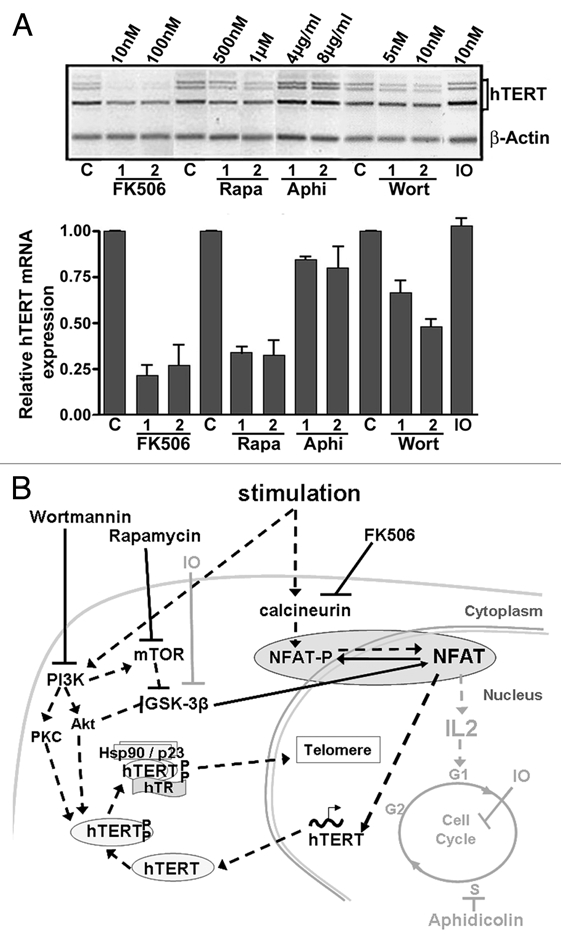
Modulation of hTERT expression during lymphocyte stimulation. (A) hTERT mRNA expression in PBL simultaneously stimulated (1 µg/µl PHA) and treated for 48 h with FK506, Rapamycin (Rapa), aphidicolin (Aphi), wortmannin (Wort) and indirubin-3′-monoxim (IO). hTERT mRNA expression is shown in a representative illustration after semi-quantitative RT-PCR and in a histogram summarizing the results from three independent experiments of real-time quantitative PCR (ratio of hTERT expression to control and normalized to actin expression). The strongest inhibitory effect was observed with FK506, while aphidicolin and IO did not clearly interfere with hTERT mRNA expression. (C: untreated control PBL). (B) Diagram representing lymphocyte stimulation and inhibition pathways. Lymphocyte stimulation activates the phosphatase calcineurin, which rapidly dephosphorylates NFAT, and also activates the PI3K/Akt/mTOR pathway, leading to the inhibition of the GSK-3β activity, a kinase for NFAT. Dephosphorylated NFAT is translocated into the nucleus, where it can exert its transcription factor role for hTERT and cytokines such as IL-2. The kinases Akt and PKC, both activated by PI3K, phosphorylate hTERT, which forms a complex with hTR and the chaperone proteins Hsp90 and p23. This complex is translocated into the nucleus where it can exert its function as a reverse transcriptase in telomere maintenance. FK506 induces the inhibition of calcineurin. Rapamycin and wortmannin inhibit mTOR and PI3K (and thus Akt) respectively, leading to GSK-3β activation. All of these compounds ensure NFAT phosphorylation and its subsequent inactivation. The actions of Aphidicolin and IO that do not interfere with NFAT activity and hTERT mRNA expression are marked in grey. The pathways implicated positively during lymphocyte stimulation are noted by black dashed arrows and those implicated negatively are noted by black solid arrows.
hTERT is located on chromosome 5p15.33 in a single copy, and sequence variants at the TERT-CLPTMIL locus associate with many cancer types.12 The hTERT promoter consists of three main regions described by their functional activity and localization relative to the +1 transcription initiation site.13 The first region, consisting of a sequence of 258 bp (from −203 to +55), corresponds to the core promoter and contains a fragment of 59 bp (−208 to −150) essential for maximal transcriptional activation of the hTERT gene. The second one, an activating region, is located between positions −1397 and −798. Finally, the third one, an inhibitory region, is located between positions −798 and −400. Globally, the hTERT promoter is GC rich and comprises mainly two CpG islands, a broad CpG island from −845 to exon 2 and a small one between −4245 and −4545 (reviewed in ref. 14).
Several transcription factors are implicated in hTERT expression: some are activators (e.g., c-Myc, Max, Sp1, Ets1 and 2, E2 and, in normal cells, E2F 1 to 5) and others are inhibitors (e.g., Mad1, WT1, p53, MZF-2 and, in neoplastic cells, E2F 1 to 3).14 c-Myc is considered to be a major regulatory factor of hTERT and can form heterodimers with Max proteins to directly activate the hTERT promoter through binding two E-boxes within the core promoter. c-Myc was shown to cooperate with the ubiquitous factor Sp1, which binds to five responsive elements located between the E-boxes. Furthermore, c-Myc may, in part, control the regulatory activity of the Ets1 and 2 proteins that act as activators or inhibitors depending on which responsive element they bind to. However, neither the known transcription factors nor variations in epigenetic regulation (such as methylation and acetylation) or post transcriptional regulation through alternative splicing of hTERT (reviewed in ref. 14) are sufficient to explain how early induction of hTERT mRNA expression during the activation of normal lymphocytes is controlled. Indeed, while c-myc deregulation may by-pass normal activation pathways in lymphoid tumor cells, it is not sufficient for the acquisition of competence to proliferate in normal lymphocytes.15
Metabolic Pathways Implicated in Lymphocyte Stimulation and in vitro Immunosuppressive Treatment Responses Lead to the Role of Nuclear Factor of Activated T cells (NFAT) in hTERT Regulation
In quiescent cells, NFAT proteins are phosphorylated, located in the cytoplasm, and inactive as transcription factors. NFAT dephosphorylation and nuclear translocation occur as a result of the transcription of genes implicated in lymphocyte proliferation and differentiation (reviewed in ref. 16). T lymphocyte stimulation results in calcium- and calmodulin-dependent activation of calcineurin, a serine/threonine phosphatase, which induces a rapid dephosphorylation of NFAT serine residues. On the other hand, stimulation induces the activation of phosphatidylinositol- 3-kinase (PI3K) and the inhibition of phosphatases, such as the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and SH2 domain-containing inositol 5-phosphatases 1 (SHIP-1) or 2 (SHIP-2), which favors the conversion of phosphatidylinositol 4,5-biphophate (PIP2) phosphorylation into phosphatidylinositol 3,4,5-biphophate (PIP3). PIP3 is recognized by 3-phosphoinositidedependent kinases 1 and 2 (PDK1/2) and the Akt kinase through their pleckstrin homology domain, allowing its recruitment to the plasma membrane.17 PDK1/2 induces the phosphorylation and activation of Akt, which in turn phosphorylates numerous substrates including the mTOR kinase. mTOR and Akt phosphorylate and inactivate Glycogen Synthase Kinase-3β (GSK-3β), a kinase for NFAT (Fig. 2). Dephosphorylation of NFAT triggers its translocation into the nucleus in about 5 to 15 min, where it can exert its transcription factor activity during several hours, as hTERT mRNA expression begins to be observed.9
Figure 2.
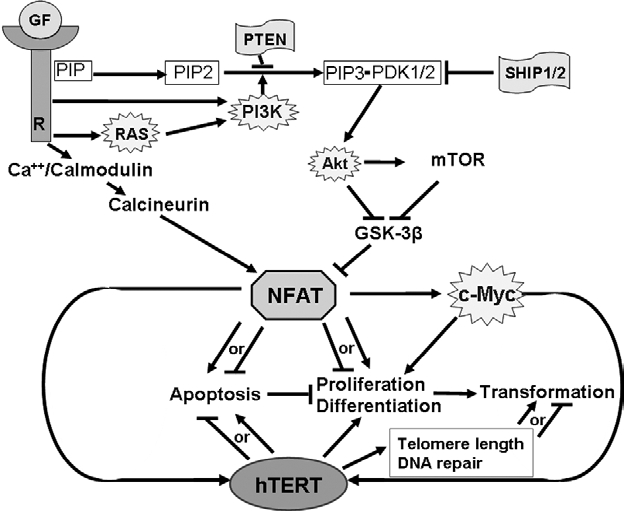
Putative relationships between NFAT and hTERT in cell proliferation, differentiation and malignant transformation. NFAT and hTERT are both positively and negatively implicated in apoptosis regulation and are also involved in cell proliferation, differentiation and malignant transformation. NFAT regulates hTERT transcription both directly and indirectly via c-Myc. In tumors, constitutive activation of NFAT can be due to an alteration of the upstream signaling pathways implicated in NFAT regulation, either by deletion of tumor suppressor genes (PTEN or SHIP1/2), overexpression of oncogenes (PI3K, Akt or RAS) or deregulation of the tumor microenvironment depending on autocrine/paracrine growth factors (GF) (R: receptor). NFAT overexpression will subsequently trigger an increase in hTERT activity amplifying its proliferative potential. (stars: oncogenes; flags: tumor suppressors; →: activation; ⊣: inhibition).
Wortmannin, an inhibitor of PI3K, and Rapamycin, an inhibitor of mTOR, induce the indirect activation of GSK-3β18 and thus favor NFAT phosphorylation. FK506 inhibits calcineurin and the subsequent dephosphorylation of NFAT.16 FK506, wortmannin and rapamycin, which inactivate NFAT as a transcription factor, caused inhibition of hTERT expression in activated lymphocytes.9 Conversely, aphidicolin, which inhibits DNA polymerase α, and indirubin-3′-monoxime (IO), an inhibitor of cyclin dependent-kinase (CDK) and GSK-3β, both inhibit cell cycle progression but did not inhibit hTERT expression (Fig. 1A).9 These observations suggest the induction of hTERT expression at an early stage of lymphocyte activation and propose a role for NFAT in its transcription (Fig. 1B).9 The NFAT family is comprised of four calcium-responsive transcription factors (NFAT1 through NFAT4). These proteins are highly homologous in structure and contain a DNA-binding domain (DBD) and an amino terminal domain called NFAT homology region (NHR). The DBD has sequence similarity with the Rel homology domain of the Rel family of transcription factors and permits NFAT binding to the DNA core sequence GGAAA. The NHR is unique to NFAT proteins and is comprised of multiple serine residues, a nuclear localization sequence, and a conserved calcineurin-binding sequence.16 DNA sequence analysis of the hTERT promoter reveals five potential GGAAA binding sites at positions −1575, −1225, −1200, −775 and −40 relative to hTERT transcription initiation site. In luciferase assays, NFAT1 (NFATp or NFATc2) overexpression induced an increase in hTERT promoter transcriptional activity. Subsequent 5′ deletions of the promoter sequence showed that NFAT1 could act mainly through a binding site located in the hTERT core promoter at position −40 and flanked by two Sp1 sites. Mutation of NFAT1 responsive elements inhibited hTERT promoter activity in vitro. Interestingly, simultaneous mutation of one or both Sp1 sites enhanced this effect, suggesting a possible relationship between these two factors. NFAT1 overexpression and inhibition correlated with variations in endogenous hTERT expression. In addition, chromatin immunoprecipitation assays demonstrated the direct binding of NFAT1 to the endogenous hTERT promoter sequence through the −40 and −775 sites. NFAT1 regulation of hTERT does not exclude a role for the other NFAT isoforms as NFAT1 silencing in stimulated Jurkat cells does not completely abolish endogenous hTERT expression.9
NFAT Implicated in Cell Death, Proliferation and Differentiation Regulation can be Constitutively Activated in Cancer Cells
NFAT proteins are expressed in multiple cell types both within and outside the immune system and their DNAbinding activity decline with age.19 NFAT1 and NFAT2 functions are the best known. NFAT1 implication in cell stimulation appears complex, since it is involved in the transcription induction of interleukins (IL)-2, IL-3, IL-4, IL-5, Granulocyte-macrophage colony stimulating factor, Interferon γ, TNFα, and cell cycle regulatory proteins such as p21Waf1, in cooperation with several transcriptional partners such as AP-1.20 However, in unstimulated lymphocytes, NFAT1 present in the nucleus at a basal level also negatively regulates cyclin A2 or downmodulates the expression of CDK4 as lymphocytes return to the quiescent state.21 NFAT1 was also shown to be implicated in apoptosis by enhancing activation-induced cell death in T-cells via the transcription of the FasL gene through early growth response proteins.22 NFAT2 acts as a positive regulator of cell proliferation and a repressor of cell death.23 Globally, in murine NIH 3T3 fibroblasts, it has been shown that NFAT1 acts as a tumor suppressor and NFAT2 as an oncogene, when constitutively activated.23
NFAT plays a role in hematological malignancies: constitutively active nuclear forms of NFAT1 and NFAT2 were found in a large panel of Non Hodgkin T-cell and B-cell lymphomas.24 Furthermore, persistent activation of the calcineurin/NFAT pathway in mouse models of human T-cell acute lymphoblastic leukemia (T-ALL) is pro-oncogenic in vivo by the constitutive activation of the JAK/STAT or NOTCH1 signaling pathways.24 In all these cases, the constitutive activation of NFAT proteins seems to be due to an alteration of the upstream signaling pathways implicated in NFAT regulation, either by deletions of tumor suppressor genes (e.g., PTEN or SHIP1/2), overexpression of oncogenes (e.g., Akt or RAS), or deregulation of tumor microenvironment depending on autocrine/paracrine signals as proposed by Meydiouf et al.24 (Fig. 2).
NFAT proteins were found to mediate Ca2+ signals not only in immune cells but also in various mammalian cell types and tissues. They were also shown to play roles in human non-lymphoid cancers. Indeed, the ectopic activation of NFAT1 was shown to promote breast cancer cell invasion through the transcription of COX2 and the synthesis of prostaglandins.25 Furthermore, in pancreatic cancers, overexpression of NFAT2 increased cell proliferation and enhanced anchorageindependent growth by directly activating c-Myc expression.26
The Question about the Role of hTERT in NFAT-Controlled Cellular Metabolism Pathways must be Raised
After lymphocyte stimulation and in response to NFAT transcriptional activation, we can observe an early increase in hTERT mRNA expression and telomerase activity.9 Inversely, a decrease in hTERT expression and activity is observed during in vitro lymphocyte aging27 as NFAT DNA binding is also known to decrease.19 Moreover, the catalytic subunit of telomerase hTERT can physically bind telomeres and DNA repair proteins. Its ectopic expression in fibroblasts is accompanied by an increase in proteins involved in mismatch repair (MSH6, MLH1), non-homologous end joining (Ku80, XRCC4), and homologous recombination. hTERT favors genomic stability and DNA repair.28,29 It is worth noting that its declined expression during in vitro lymphocyte aging is accompanied by an increase in DNA damage.27 Thus, the direct transcriptional regulation of hTERT by NFAT offers an explanation for the close relationship between lymphocyte stimulation and the induction of hTERT expression allowing telomere maintenance during the cellular stimulation process.
Beyond its role in telomere maintenance, emergent extra-telomeric functions of hTERT have been reported: like NFAT, hTERT is involved in cell proliferation and transformation as well as in apoptosis regulation. Indeed, hTERT was able to induce cell immortalization and tumorigenesis when combined with two other oncogenes, H-Ras and large T-SV40.30 hTERT mutants unable to maintain telomere length also led, in combination with H-Ras, to the transformation of GM847 fibroblasts, highlighting a role of hTERT in tumorigenesis that is independent of its telomere lengthening ability.5
The ectopic expression of hTERT supports the proliferation of epithelial mammary cells and correlates with the induction of genes implicated in cell proliferation, in particular the epithelial growth factor receptor.31 Moreover, hTERT is involved in the proliferation of embryonic stem cells through its combination with specific transcription factors,31 and, likely, through the direct modulation of the Wnt/β-catenin signaling pathway, which has been implicated in stem cell proliferation and differentiation and in human tumorigenesis.32
Catalytically active hTERT also participates in apoptosis induction due to its localization in mitochondria, rendering cells more susceptible to oxidative stressinduced mitochondrial DNA damage.33 In contrast, when located in the nucleus, hTERT plays an anti-apoptotic role.28 hTERT may also counteract p53-induced apoptosis, as demonstrated in Burkitt lymphoma cells and colon carcinoma cells.34
NFAT may induce hTERT expression at two levels: directly, at the transcriptional level,9 and indirectly, by activating c-Myc.26 Thus, any aberrant activation of the NFAT upstream pathways, either through the calcineurin pathway or through the PI3K/Akt/mTOR pathway, as found in vivo in numerous lymphoid or non-lymphoid malignancies, could facilitate tumor growth in part via hTERT expression activation. The inhibition of NFAT activity obtained with immunosuppressors such as anti-calcineurin molecules or with mTOR inhibitors might counteract these tumoral processes. However, in normal proliferative cells, the decrease in hTERT expression and subsequent putative telomere shortening and uncapping induced by NFAT inactivation may favor DNA damage and the emergence of malignancies. The putative positive or negative consequences of the inhibition of hTERT expression related to the therapeutic inhibition of NFAT pathways remains largely to be studied.
Aknowledgements
This work was supported in part by the “Ligues contre le Cancer du Rhône, et de Saône et Loire”, by the “Region Rhônes Alpes” (Contract 00 81 60 45), and by the “Cancéropôle Lyon Auvergne Rhône-Alpes”. We thank Dr. Nicolas Rachinel, Régine Catallo and Pr. Claire Pouteil-Noble for fruitful suggestions and discussions.
Footnotes
Previously published online: http://www.landesbioscience.com/journals/transcription/article/12062
References
- 1.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 3.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 5.Stewart SA, Hahn WC, O'Connor BF, Banner EN, Lundberg AS, Modha P, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA. 2002;99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolquist KA, Ellisen LW, Counter CM, Meyerson M, Tan LK, Weinberg RA, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19:182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- 7.Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, Weng NP. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci USA. 1999;96:5147–5152. doi: 10.1073/pnas.96.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chebel A, Rouault JP, Urbanowicz I, Baseggio L, Chien WW, Salles G, et al. Transcriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cells. J Biol Chem. 2009;284:35725–35734. doi: 10.1074/jbc.M109.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol. 2005;174:5261–5269. doi: 10.4049/jimmunol.174.9.5261. [DOI] [PubMed] [Google Scholar]
- 11.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 12.Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horikawa I, Cable PL, Afshari C, Barrett JC. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999;59:826–830. [PubMed] [Google Scholar]
- 14.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeland E, Godal T, Ruud E, Beiske K, Funderud S, Clark EA, et al. The specific induction of myc protooncogene expression in normal human B cells is not a sufficient event for acquisition of competence to proliferate. Proc Natl Acad Sci USA. 1985;82:6255–6259. doi: 10.1073/pnas.82.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 17.Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–167. [PubMed] [Google Scholar]
- 18.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahlavani MA, Harris MD, Richardson A. The age-related decline in the induction of IL-2 transcription is correlated to changes in the transcription factor NFAT. Cell Immunol. 1995;165:84–91. doi: 10.1006/cimm.1995.1190. [DOI] [PubMed] [Google Scholar]
- 20.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caetano MS, Vieira-de-Abreu A, Teixeira LK, Werneck MB, Barcinski MA, Viola JP. NFATC2 transcription factor regulates cell cycle progression during lymphocyte activation: evidence of its involvement in the control of cyclin gene expression. Faseb J. 2002;16:1940–1942. doi: 10.1096/fj.02-0282fje. [DOI] [PubMed] [Google Scholar]
- 22.Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD, et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- 23.Robbs BK, Cruz AL, Werneck MB, Mognol GP, Viola JP. Dual roles for NFAT transcription factor genes as oncogenes and tumor suppressors. Mol Cell Biol. 2008;28:7168–7181. doi: 10.1128/MCB.00256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medyouf H, Ghysdael J. The calcineurin/NFAT signaling pathway: A novel therapeutic target in leukemia and solid tumors. Cell Cycle. 2008;7:297–303. doi: 10.4161/cc.7.3.5357. [DOI] [PubMed] [Google Scholar]
- 25.Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J Biol Chem. 2006;281:12210–12217. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chebel A, Bauwens S, Gerland LM, Belleville A, Urbanowicz I, de Climens AR, et al. Telomere uncapping during in vitro T-lymphocyte senescence. Aging Cell. 2009;8:52–64. doi: 10.1111/j.1474-9726.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 28.Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem. 2002;277:38540–38549. doi: 10.1074/jbc.M202671200. [DOI] [PubMed] [Google Scholar]
- 29.Sharma GG, Gupta A, Wang H, Scherthan H, Dhar S, Gandhi V, et al. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene. 2003;22:131–146. doi: 10.1038/sj.onc.1206063. [DOI] [PubMed] [Google Scholar]
- 30.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 31.Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res. 2008;18:725–732. doi: 10.1038/cr.2008.74. [DOI] [PubMed] [Google Scholar]
- 32.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalenko OA, Caron MJ, Ulema P, Medrano C, Thomas AP, Kimura M, et al. A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cel. 2010 doi: 10.1111/j.1474-9726.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- 34.Rahman R, Latonen L, Wiman KG. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene. 2005;24:1320–1327. doi: 10.1038/sj.onc.1208232. [DOI] [PubMed] [Google Scholar]


