Abstract
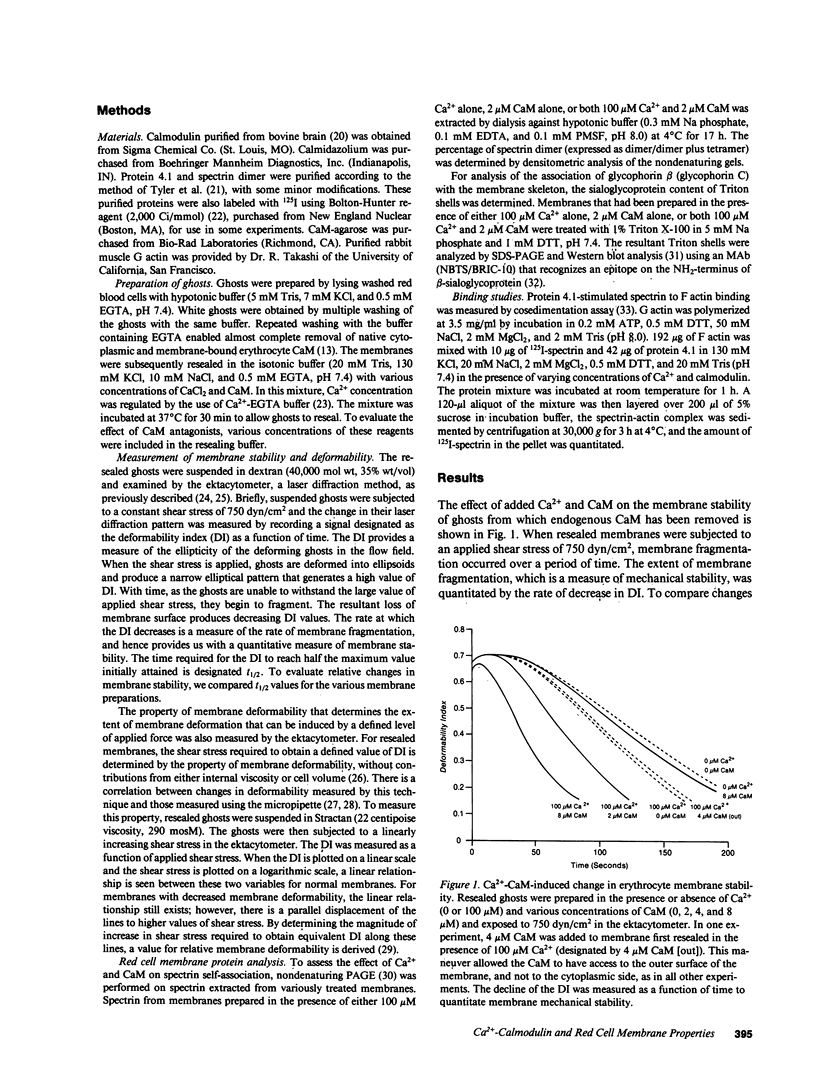
Skeletal proteins of the red blood cell apparently play an important role in regulating membrane material properties of deformability and stability. However, the role of various intracellular constituents in regulating membrane properties has not been clearly defined. To determine whether Ca2+ and calmodulin might play a role in this regulation, we measured the membrane stability and deformability of resealed ghosts prepared in the presence of varying concentrations of Ca2+ and calmodulin (CaM). For membranes resealed in the presence of Ca2+ and physiologic concentrations of CaM (2-8 microM), membrane stability decreased with increasing Ca2+ concentrations (greater than 1.0 microM). Moreover, Ca2+ and CaM-induced alterations in membrane stability were completely reversible. In the absence of CaM, an equivalent decrease in membrane stability was seen only when Ca2+ concentration was two orders of magnitude higher (greater than 100 microM). Calmodulin did not alter membrane stability in the absence of Ca2+. Compared with these changes in membrane stability, membrane deformability decreased only at Ca2+ concentrations greater than 100 microM, and calmodulin had no effect on Ca2+-induced decrease in membrane deformability. Examination of the effects of Ca2+ and CaM on various membrane interactions have enabled us to suggest that spectrin-protein 4.1-actin interaction may be one of the targets for the effect of Ca2+ and CaM. These results imply that Ca2+ and calmodulin can regulate membrane stability through modulation of skeletal protein interactions, and that these protein interactions are of a dynamic nature on intact membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Gardner K., Bennett V. Association between human erythrocyte calmodulin and the cytoplasmic surface of human erythrocyte membranes. J Biol Chem. 1983 May 25;258(10):6258–6265. [PubMed] [Google Scholar]
- Anderson J. P., Morrow J. S. The interaction of calmodulin with human erythrocyte spectrin. Inhibition of protein 4.1-stimulated actin binding. J Biol Chem. 1987 May 5;262(13):6365–6372. [PubMed] [Google Scholar]
- Anstee D. J., Parsons S. F., Ridgwell K., Tanner M. J., Merry A. H., Thomson E. E., Judson P. A., Johnson P., Bates S., Fraser I. D. Two individuals with elliptocytic red cells apparently lack three minor erythrocyte membrane sialoglycoproteins. Biochem J. 1984 Mar 1;218(2):615–619. doi: 10.1042/bj2180615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster J. C., Welsh M. J., Brewer G. J., Maisel H. Identification of spectrin and protein 4.1-like proteins in mammalian lens. Biochem Biophys Res Commun. 1984 Mar 15;119(2):726–734. doi: 10.1016/s0006-291x(84)80311-3. [DOI] [PubMed] [Google Scholar]
- Baines A. J., Bennett V. Synapsin I is a spectrin-binding protein immunologically related to erythrocyte protein 4.1. 1985 May 30-Jun 5Nature. 315(6018):410–413. doi: 10.1038/315410a0. [DOI] [PubMed] [Google Scholar]
- Berglund A., Backman L., Shanbhag V. P. Calmodulin binding to human spectrin. FEBS Lett. 1984 Jun 25;172(1):109–113. doi: 10.1016/0014-5793(84)80884-4. [DOI] [PubMed] [Google Scholar]
- Boivin P., Galand C. Is spectrin a calmodulin-binding protein? Biochem Int. 1984 Feb;8(2):231–236. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burns N. R., Gratzer W. B. Interaction of calmodulin with the red cell and its membrane skeleton and with spectrin. Biochemistry. 1985 Jun 4;24(12):3070–3074. doi: 10.1021/bi00333a040. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N., Shohet S. B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985 Jun;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Korsgren C. Band 4.1 causes spectrin-actin gels to become thixiotropic. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1429–1435. doi: 10.1016/s0006-291x(80)80025-8. [DOI] [PubMed] [Google Scholar]
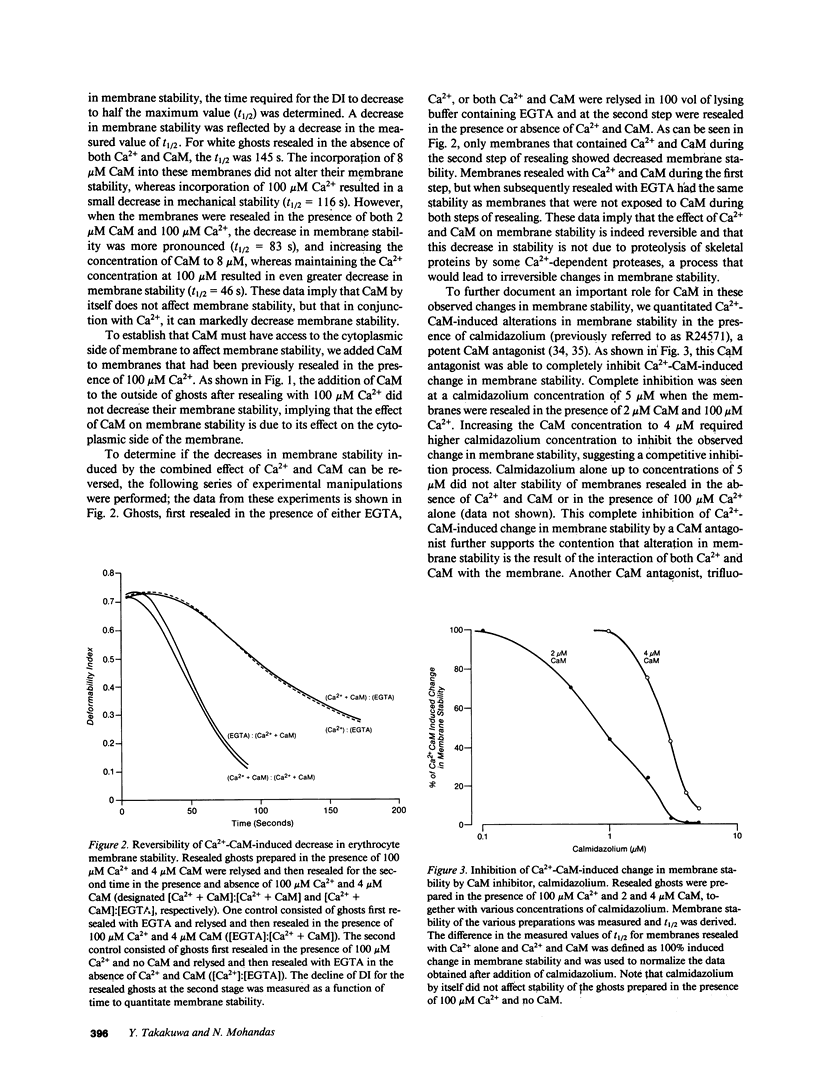
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
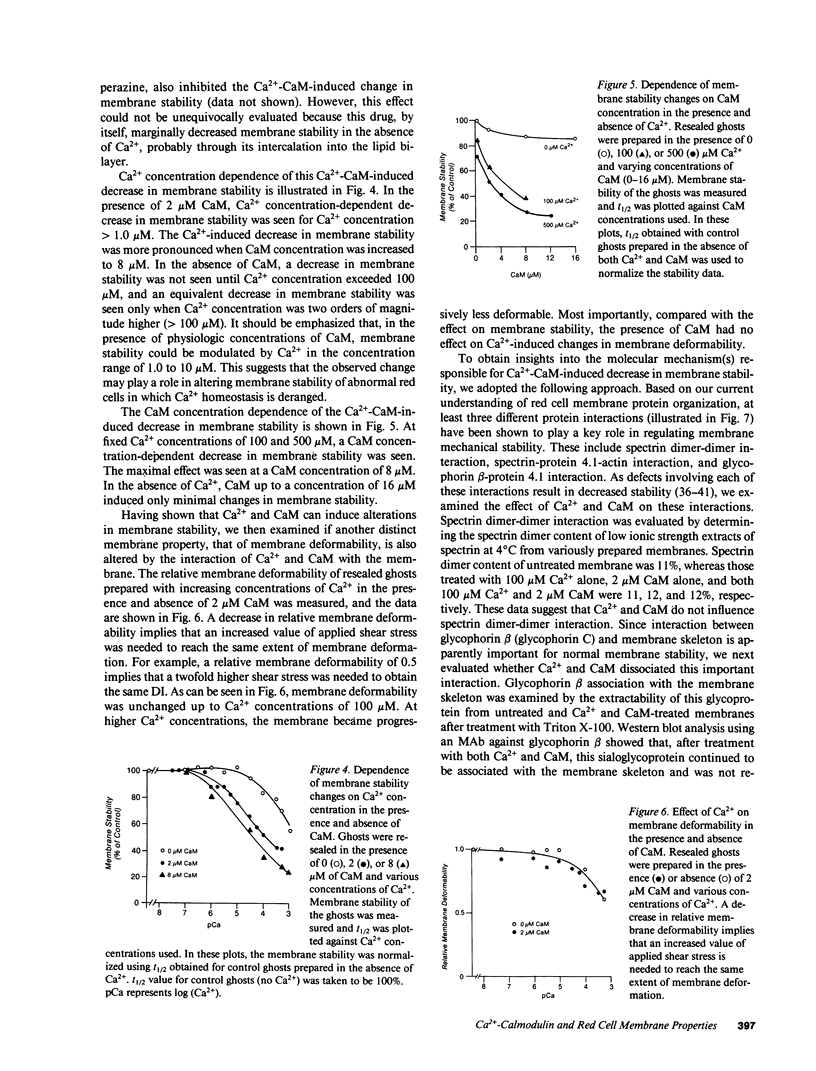
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K., Bennett V. A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. J Biol Chem. 1986 Jan 25;261(3):1339–1348. [PubMed] [Google Scholar]
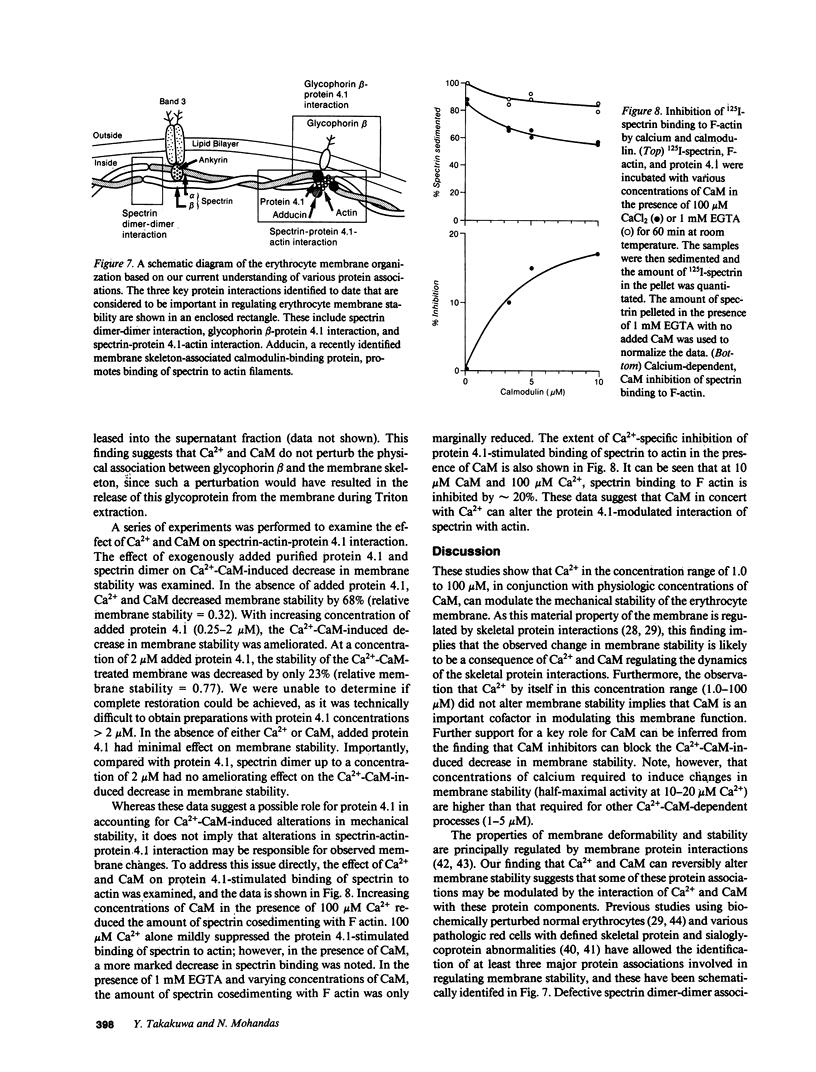
- Gardner K., Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987 Jul 23;328(6128):359–362. doi: 10.1038/328359a0. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Heath B. P., Mohandas N., Wyatt J. L., Shohet S. B. Deformability of isolated red blood cell membranes. Biochim Biophys Acta. 1982 Oct 7;691(2):211–219. doi: 10.1016/0005-2736(82)90409-6. [DOI] [PubMed] [Google Scholar]
- Husain A., Howlett G. J., Sawyer W. H. The interaction of calmodulin with erythrocyte membrane proteins. Biochem Int. 1985 Jan;10(1):1–12. [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen F. L., Vincenzi F. F. Calcium transport across the plasma membrane: stimulation by calmodulin. Science. 1979 Apr 20;204(4390):306–309. doi: 10.1126/science.155309. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Hockaday A., Sepulveda M. I., Somlyo A. P., Somlyo A. V., Ortiz O. E., Bookchin R. M. Compartmentalization of sickle-cell calcium in endocytic inside-out vesicles. Nature. 1985 Jun 13;315(6020):586–589. doi: 10.1038/315586a0. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Mentzer W. C., Turetsky T., Mohandas N., Schrier S., Wu C. S., Koenig H. Identification of the hereditary pyropoikilocytosis carrier state. Blood. 1984 Jun;63(6):1439–1446. [PubMed] [Google Scholar]
- Mische S. M., Mooseker M. S., Morrow J. S. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987 Dec;105(6 Pt 1):2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A., Shohet S. B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983 Jul;20(3):225–242. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Reid M. E., Chasis J. A., Mohandas N. Identification of a functional role for human erythrocyte sialoglycoproteins beta and gamma. Blood. 1987 Apr;69(4):1068–1072. [PubMed] [Google Scholar]
- Shalev O., Mogilner S., Shinar E., Rachmilewitz E. A., Schrier S. L. Impaired erythrocyte calcium homeostasis in beta-thalassemia. Blood. 1984 Aug;64(2):564–566. [PubMed] [Google Scholar]
- Sheetz M. P., Casaly J. 2,3-Diphosphoglycerate and ATP dissociate erythrocyte membrane skeletons. J Biol Chem. 1980 Oct 25;255(20):9955–9960. [PubMed] [Google Scholar]
- Smith D. K., Palek J. Sulfhydryl reagents induce altered spectrin self-association, skeletal instability, and increased thermal sensitivity of red cells. Blood. 1983 Dec;62(6):1190–1196. [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Calmodulin-binding protein of erythrocyte cytoskeleton. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1063–1070. doi: 10.1016/0006-291x(81)91931-8. [DOI] [PubMed] [Google Scholar]
- Spiegel J. E., Beardsley D. S., Southwick F. S., Lux S. E. An analogue of the erythroid membrane skeletal protein 4.1 in nonerythroid cells. J Cell Biol. 1984 Sep;99(3):886–893. doi: 10.1083/jcb.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth J. A., Brewer G. J., Gnegy M. E., Treisman G., Near K. Calmodulin level in whole blood correlates with the percentage of reticulocytes. Am J Hematol. 1983 Sep;15(2):153–157. doi: 10.1002/ajh.2830150207. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]


