Abstract
The identification of individuals at risk for Alzheimer's disease (AD) is essential for the timely administration of treatment approaches aimed at slowing the onset or progression of the disease. As amnestic forms of mild cognitive impairment (aMCI) may represent preclinical AD, the search for specific diagnostic biomarkers that characterize those with aMCI is a key research objective. Using surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDITOF-MS), we screened the cerebrospinal fluid (CSF) of Religious Orders Study participants with a clinical diagnosis of no cognitive impairment (NCI), aMCI, or mild/moderate AD for potential biomarkers. CSF was fractionated on immobilized metal affinity chromatography (IMAC) protein arrays preloaded with either gallium (IMAC-Ga), which binds phosphoproteins, or copper (IMAC-Cu) to isolate copper-binding proteins. SELDI TOF-MS analysis of the IMAC-Ga arrays revealed a phosphopeptide of 2490 Da that was selectively increased ~2-fold in aMCI and AD CSF compared to NCI. SELDI TOF-MS analysis of the IMAC-Cu arrays identified 2 proteins of 11.7 and 13.3 kDa that were both selectively increased ~1.5–1.6-fold in aMCI and AD CSF. Increasing levels of each protein were associated with poorer performance on the Mini Mental State Exam and higher Braak stage. Hence, increased CSF levels of these proteins may be potential biomarkers for preclinical AD and aid in the development of a CSF biomarker panel with high predictive value for identifying people who would most benefit from early therapeutic interventions to modify disease progression.
Keywords: Alzheimer's disease, mild cognitive impairment, cerebrospinal fluid, biomarker, proteomics
1. Introduction
Alzheimer's disease (AD) is currently defiined by its characteristic clinical manifestation of dementia. Since neuropathological examination of older people with a clinical diagnosis of no or mild cognitive impairment (MCI), a putative preclinical stage of AD [1], consistently reveal similar pathological signatures to those with frank AD [2–6], it is now suggested that the disease begins many years before the onset of clinical symptoms. Therefore, the ability to identify at-risk people prior to cognitive decline is a key objective for clinicians as well as for gauging the long-term therapeutic effects of AD modifying drugs. In this regard, several initiatives have been launched to understand whether a panel of diagnostic and prognostic biomarkers can be developed which characterize people at the earliest stages of the disease, particularly those with amnestic MCI (aMCI) who preferentially transition to AD [1]. Current studies have focused on detecting biomarkers associated with changes in amyloid-β (Aβ) peptide and tau protein levels in the cerebrospinal fluid (CSF) of people at varying stages of AD, since these markers may reflect underlying cerebral pathology [7]. In particular, studies have shown that the CSF of AD patients contains decreased levels of Aβ42 peptides, which form the nidus of amyloid plaques, and elevated levels of tau or phosphorylated tau, the principle constituent of neurofibrillary tangles (NFTs) [8–11]. In general, changes in the ratio of Aβ42 and tau or phospho-tau levels are predictive for longitudinal changes in cognitive measures [12–16]. However, the inherent variability and overlap of AD neuropathology in the aged control, MCI and diseased brain is also reflected by variation of these targets in the CSF, resulting in suboptimal specificity and sensitivity for identifying subjects in the prodromal stages of AD. These results underscore the need for the development of an unbiased CSF protein biomarker set to augment the predictive accuracy of CSF Aβ42 and tau ratios for the diagnosis of people with MCI and those in even earlier stages of the disease. The present study reports our inital CSF biomarker observations to differentiate people with aMCI from people with no cognitive impairment (NCI) using immobilized metal affinity chromatography (IMAC) with surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI TOF-MS) to screen postmortem ventricular CSF harvested from subjects enrolled in the Religious Orders Study who died with an antemortem clinical diagnosis of NCI, aMCI, or mild/moderate AD.
2. Experimental Procedures
2.1 Subjects
Postmortem ventricular CSF was available from participants in the Religious Orders Study, a longitudinal clinical-pathologic study of aging and AD in older Catholic nuns, priests and brothers [3, 17]. Subjects were clinically categorized as NCI (n = 24), aMCI (n = 16), or mild/moderate AD (n = 21). A summary of the study cohort is shown in Table 1. The Human Investigations Committee of Rush University Medical Center approved the study.
Table 1.
Case demographics
| Case demographics | NCI | aMCI | AD | P value | Pair-wise comparisons |
|---|---|---|---|---|---|
| N | 24 | 16 | 21 | ||
| Age, years (mean ± SD) (range) | 84.5 ± 4.5 (74–89) | 85.8 ±7.1 (74–98) | 86.5 ±4.1 (80–95) | 0.42a | |
|
| |||||
| Education, years (mean ±SD) (range) | 19.4 ±2.7 (15–23) | 18.2 ±4.2 (8–24) | 17.8 ±2.8 (12–25) | 0.34a | |
|
| |||||
| number (%) female | 12 (50%) | 7 (44%) | 11 (52%) | 0.89b | |
|
| |||||
| number (%) with apoE4 allele | 5 (21%) | 4 (25%) | 8 (38%) | 0.45b | |
|
| |||||
| PMI in hours (mean ± SD) (range) | 6.3 ±4.7 (2.2–12.4) | 6.7 ± 4.4 (2.8–16) | 4.7 ±1.0 (2.7–6) | 0.45a | |
|
| |||||
| MMSE score (mean ± SD) (range) | 28.1 ±1.5 (26–30) | 26.2 ±2.3 (22–29) | 17.8 ±7.2 (0–26) | 0.001c | (NCI,MCI)>AD |
|
| |||||
| Braak stage Distribution | 0.027c | AD > NCI | |||
| I/II | 8 | 3 | 3 | ||
| III/IV | 14 | 11 | 9 | ||
| V/VI | 2 | 2 | 9 | ||
|
| |||||
| Reagan Diagnosis (Likelihood of AD) | 0.003c | AD > (NCI,MCI) | |||
| NO | 1 | 0 | 0 | ||
| LOW | 10 | 9 | 4 | ||
| INTERMEDIATE | 12 | 6 | 9 | ||
| HIGH | 1 | 1 | 8 | ||
|
| |||||
| CERAD Diagnosis (Likelihood of AD) | 0.053c | ||||
| NO | 8 | 7 | 3 | ||
| LOW | 3 | 2 | 2 | ||
| INTERMEDIATE | 8 | 4 | 6 | ||
| HIGH | 4 | 3 | 10 | ||
One-WayANOVA
Fisher's Exact Test
Kruskall-Wallis with Dunn's multiple comparisons test
2.2 Clinical Evaluation
Details of the clinical assessment of the Religious Orders Study cohort was published previously [3, 17]. Briefly, an annual clinical evaluation including an assessment for stroke and parkinsonian signs was performed. Neuropsychology technicians carried out a battery of cognitive tests, including the MMSE, Boston Naming, Word List Memory, Word List Recall and Word List Recognition and Logical Memory (Story A) [3, 17]. Because neuropsychological tests do not measure cognition uniformly across different educational levels, cut-off points for rating impairment on each test were adjusted for education. A computer algorithm applied these cut-off points uniformly converting individual scores into impairment ratings in five cognitive domains (orientation, attention, memory, language and perception) [3, 17]. An impaired score for each domain required deficits on multiple tests within that domain. A board-certified neuropsychologist summarized impairment data in each of the five cognitive domains as not present, possible, or probable. The neuropsychologist was blind to age, gender, race, clinical data other than education, occupation, sensory or motor deficits, and effort. A board-certified neurologist with expertise in geriatrics made a clinical diagnosis after review of clinical data from that year and examination of all subjects. The diagnosis of dementia and AD followed the recommendations of the joint working group of the National Institute of Neurological and Communicative Disorders and the Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) [18]. Although there are no clear consensus criteria for the clinical classification of MCI, the criteria used in this study were similar to, or compatible with, those used by others in the field to describe persons who were not cognitively normal, but did not meet accepted criteria for dementia [1]. The MCI population is defined as those persons rated as impaired on neuropsychological testing by the neuropsychologist but who were not found to have dementia by the examining neurologist [3, 17]. Subjects in the MCI diagnostic group are subclassified as amnestic (aMCI) or non-amnestic based on the presence or absence of impairments in episodic memory, respectively. This differentiation is established to allow for studies focused on the aMCI subtype, which may be more representative of prodromal AD [1, 19]. As such, CSF from aMCI subjects was used for these studies. Postmortem interviews were carried out to determine medical conditions which occurred during the interval between the last clinical evaluation and death. A consensus conference of neurologists and neuropsychologists reviewed all available clinical data, and assigned a summary clinical diagnosis.
2.3 Pathological Evaluation
At autopsy, brains were processed as previously described [3, 20]. Brains were removed from the calvarium, cut into 1-cm thick slabs using a Plexiglas jig. One hemisphere was immersion-fixed in 4% paraformaldehyde and the opposite hemisphere was snap-frozen in liquid nitrogen and stored at −80°C. From the immersion fixed brain slabs, brain regions were dissected, paraffin embedded, cut at 8 μm, and stained with hematoxylin and eosin, Bielschowsky, thioflavin-S, and with an ubiquitin antibody. Neuropathologic designations of “normal” (with respect to AD or other dementing processes), “possible” or “probable AD”, and “definite AD” were based on the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) including a semi-quantitative estimation of neuritic plaque density, an age-adjusted plaque score, and presence or absence of dementia [21]. Each case also received a Braak score based upon NFT pathology [22] and a NIA-Reagan diagnostic likelihood-of-AD score [23]. Cases were excluded if they exhibited significant non-AD types of pathologic conditions (e.g., brain tumours, encephalitis, large strokes, multiple lacunar infarctions, Lewy body pathology, argyrophilic grains, tangle-only dementia).
2.4 CSF processing
Prior to brain removal at autopsy, CSF was carefully aspirated from the cerebral ventricles using a 30 ml syringe affixed with an 18g needle. The CSF sample was aliquotted into 1 ml volumes on ice and then snap-frozen in liquid nitrogen for storage at −80°C. For proteomic analysis, CSF was thawed on ice, spiked with a protease inhibitor cocktail (Sigma, St. Louis, MO), and screened for blood contamination using Hemastix reagent strips (Bayer, Elkhart, IN) to detect hemoglobin (Hb). Using serial dilutions of a control blood sample with a known Hb concentration, we estimated that the cut-off value for detectable Hb contamination was ~0.4 mg/dL or ~100 erythrocytes/μl. CSF samples were then microaliquotted and stored at −80°C. Samples were analyzed only if Hb concentration fell below the cut-off value.
2.5 CSF analysis on IMAC protein chip arrays
IMAC protein chip arrays (Bio-Rad, Hercules, CA) incorporate 8 nitrilotriacetic acid retention surfaces (spots) which bind cationic metals. IMAC chips were preloaded with either 50 mM gallium nitrate (IMAC-Ga) or 100 mM cupric sulfate (IMAC-Cu) to capture phosphorylated and copper-binding proteins, respectively. CSF was then diluted 1:10 in binding buffer (IMAC-Ga: 50 mM NaOAc/1M NaCl, pH 5.3; IMAC-Cu: 100mM NaPO4/0.5 M NaCl, pH 7.0) and incubated for 1 hour on the arrays. CSF was removed and the arrays were washed sequentially in binding buffer and water. α-Cyano-4-hydroxycinnamic acid (CHCA, in 50% v/v acetonitrile and 0.5% v/v trifluoracetic acid) was applied to each spot as the energy absorbing matrix (EAM) molecule. The arrays were then analyzed by SELDI TOF-MS in a ProteinChip reader system (PBScII, Bio-Rad). Arrays were analyzed at a setting optimized for low mass protein detection using the following parameters: laser intensity, 150 laser shots/spectra collected in the positive mode; sensitivity, 9; detector voltage, 2,800 V; mass range, 2–20,000 daltons (Da); focus mass, 6,000 Da; deflector mass, 1,500 Da.
2.6 Statistical analysis
Demographic variables among the clinical groups were analyzed using one way analysis of variance (ANOVA), Fisher's Exact Test, or the Kruskall-Wallis test with Dunn's correction for multiple comparisons (Table 1). Each CSF sample was evaluated in triplicate in 3 separate analyses (n = 9 spectra profiles/sample). Protein profiles were normalized to total ion current. Peak clustering and quantitative analysis were performed using Biomarker Wizard software (Bio-Rad) with a first pass signal-to-noise ratio of 50 and peak clustering at 0.3% mass. Protein peak intensities were compared using ANOVA with post hoc Newman-Keuls testing. The Spearman rank test was used to correlate peak intensities with Mini Mental State Exam (MMSE, a measure of cognition), postmortem interval (PMI, time from death to autopsy), as well as neuropathologic variables (Braak scores, NIA Reagan, and CERAD diagnosis) for each subject. Statistical significance was set at p < 0.05.
3. Results
3.1 Case demographics
The three clinical groups were comparable in age, education, and PMI (Table 1). For all subjects, cognitive evaluations were performed within the last year of life. Subjects were clinically categorized as NCI (n = 24, mean ± SD for age = 84.5 ± 4.5 years, MMSE = 28.1 ± 1.5), aMCI insufficient to meet criteria for dementia (n = 16, age = 85.8 ±7.1 years, MMSE = 26.2 ± 2.3), or mild/moderate AD (n = 21, age = 86.5 ± 4.1 years, MMSE = 17.8 ± 7.2). MMSE scores were significantly higher in the NCI and MCI groups, whereas Braak and NIA-Reagan scores were significantly higher in the AD group (Table 1).
3.2 CSF blood screening
We could not screen the CSF samples for blood contamination by counting red blood cells due to hemolysis upon thawing of the samples. Therefore, we devised a Hemastix-based method to evaluate Hb content in the CSF (see Experimental Procedures). We verified that the cut-off values for Hb concentration used as exclusion criteria were appropriate by demonstrating that the CSF samples which passed the screening test exhibited no Hb peak in the SELDITOF-MS spectra regardless of the chromatographic platform (data not shown). Interestingly, we found that 82% of the NCI samples passed the screening test, whereas the pass rates for aMCI and AD samples were 58% and 60%, respectively. This suggests that there is breakdown of the ventricular vasculature allowing blood leakage into the CSF during the progression of the disease.
3.3 SELDITOF-MS analysis ofIMAC-Ga CSFprotein arrays
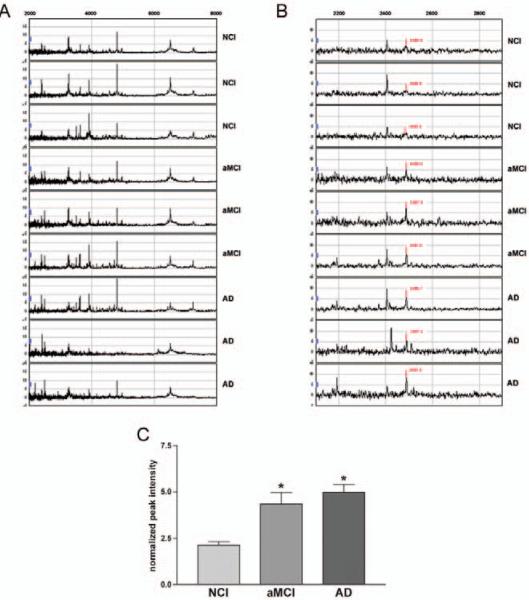
CSF samples were first incubated with the IMAC-Ga protein arrays to enrich for phosphoproteins which might be altered in aMCI and/or AD. SELDI TOF-MS spectra from these arrays demonstrated that most CSF phosphopeptide peaks were concentrated in the 2000 – 8000 Da range (Fig. 1 A). Focusing the spectra into the 2200–2800 Da range revealed an increase of a ~2490 Da phosphopeptide in the CSF of aMCI and AD subjects relative to NCI (Fig. 1B). Quantitative analysis indicated that the peak intensity of the CSF 2490 Da phosphopeptide was increased ~2-fold in aMCI and AD compared to NCI subjects (Fig. 1C). The increase in CSF levels of the 2490 Da peptide was correlated with poorer cognitive scores (Fig. 2A, Table 2). CSF levels of the 2490 Da peptide correlated with the Braak tangle score but not with NIA Reagan or CERAD criteria (Table 2). Significantly, there was no association between phosphopeptide levels and PMI (Fig. 2B, Table 2), indicating the protein level changes were unrelated to autolysis.
Fig. 1.
A 2490 Da phosphopeptide is increased in the CSF of aMCI and AD subjects. A) Representative SELDITOF spectra from IMAC-Ga arrays identify CSF peptides of differing mass (X-axis) and peak intensity (peptide concentration; Y-axis) in the 2000–8000 Da range. Individual spectra are normalized to total ion current. B) Inspection of the spectra show potential differences in peak intensity for a peptide with a predicted mass of 2485–2490 Da among NCI, aMCI and AD CSF samples C) Bar graph shows that peak intensities for the 2490 Da peptide in the CSF samples are increased ~2-fold in aMCI and AD relative to NCI. *,p< 0.001 via one-way ANOVA with post hoc Newman-Keuls test for multiple comparisons.
Fig. 2.
CSF levels of the 2490 Da peptide are inversely correlated with MMSE scores. A) Spearman rank correlations reveal a positive association between increasing levels of the 2490 Da phosphopeptide and lower MMSE scores. B) Spearman correlations show that 2490 kDa peptide levels are not associated with PMI.
Table 2.
Spearman correlations between CSF proteins and clinical pathologic variables
| CSF protein | MMSE | Braak | Reagan | CERAD | PMI |
|---|---|---|---|---|---|
| 2490 Da | r = −0.68 p < 0.0001 | r = 0.51 p < 0.0001 | r = 0.28 p = 0.08 | r = 0.02 p = 0.86 | r=−0.13 p = 0.33 |
| 11.7kDa | r = −0.57 p<0.01 | r = 0.45 p = 0.0003 | r = 0.20 p = 0.23 | r = 0.29 p = 0.18 | r=−0.16 p = 0.32 |
| 13.3 kDa | r = −0.52 p<0.01 | r = 0.40 p = 0.0018 | r = 0.18 p = 0.31 | r = 0.12 p = 0.59 | r=−0.26 p = 0.20 |
3.4 SELDI TOF-MS analysis CSF protein arrays
We also tested whether CSF samples could be enriched for differentially expressed Cu2+-binding proteins using the IMAC-Cu protein array. SELDI TOF-MS spectra from IMAC-Cu arrays showed a broad spectrum of protein peaks between 1500 and 15000 Da (Fig. 3A). Two protein peaks of ~11.7 kDa and ~13.3 kDa appeared increased in the CSF of aMCI and AD relative to NCI subjects (Fig. 3B). Quantitative analysis of the spectra indicated that both of the Cu2+-binding proteins were increased ~1.5–1.6-fold in the CSF of aMCI and AD cases compared to the NCI group (Fig. 3C). Similar to the phosphopeptide, higher CSF levels of the 11.7 and 13.3 kDa proteins were inversely correlated with lower MMSE cognitive scores and correlated with Braak but not NIA Reagan or CERAD neuropathologic scores. Protein levels were not associated with PMI (Table 2).
Fig. 3.
Two copper-binding proteins of 11.7 and 13.3 kDa are increased in the CSF of aMCI and AD subjects. A) Representative SELDI TOF spectra from IMAC-Cu arrays identify a wide array of CSF peptides and proteins in the 2000 Da to 15 kDa range. Individual spectra are normalized to total ion current. B) Inspection of the spectra show potential differences in the peak intensities of two proteins with predicted masses of 11.7 kDa and 13.3 kDa among NCI, aMCI and AD CSF samples C,D) Bar graph shows that levels of the 11.7 kDa protein (C) and the 13.3 kDa protein (D) are increased ~1.5–1.6-fold increase in the CSF of aMCI and AD subjects compared to NCI. *,p< 0.01, #, p < 0.05 via one-way ANOVA with post hoc Newman-Keuls test for multiple comparisons.
4. Discussion
In this report we demonstrate the utility of discovery-based expression proteomics for screening and identifying candidate CSF biomarkers for aMCI, a putative prodromal AD syndrome. The selective increase in SELDI TOFMS peak intensities of the 2490 Da, 11.7 kDa, and 13.3 kDa proteins in aMCI and AD cases among a vast array of resident CSF proteins suggest that these proteins may be potential biomarkers for the development of AD prior to a frank presentation of clinical symptoms. In this regard, increasing levels the candidate biomarker proteins were associated with poorer performance on the MMSE and a higher Braak score indicative of mounting neurofibrillary degeneration.
This study supports a growing list of candidate biomarkers for AD derived from CSF and other fluid compartments (e.g., plasma). With respect to CSF, alterations in the levels of proteins including brain-derived neurotrophic factor [24,25], apolipoprotein isoforms [25,26], prostaglandin-d-synthase:transthyretin dimers [27], and potentially synuclein isoforms [28] and ubiquitin [29, 30] may be useful for improving the diagnostic accuracy of AD. In addition, the SELDI TOF-MS platform has been used to identify several altered proteins in lumbar CSF sampled from living AD patients, including the 11.7 kDa protein β-2-microglobulin and the 13.3 kDa protein cystatin C [31, 32]. These latter findings suggest that the 11.7 kDa and 13.3 kDa CSF protein candidates discovered in the present study also may be β-2-microglobulin and cystatin C, respectively (Kaj Blennow, University of Goteburg, Sweden, personal communication). The fact that these CSF proteins display altered levels in people with a clinical diagnosis of aMCI supports their potential as novel biomarkers for the onset of preclinical AD. Furthermore, these data underscore the utility of using postmortem ventricular CSF from clinically well-characterized subjects as a screening tool for potential CSF biomarkers that can be validated in lumbar CSF samples when available.
β-2-microglobulin is a component of the class I major histocompatibility complex involved in the presentation of peptide antigens to the immune system. Increased levels of this protein occur in diseases characterized by elevated immune responses (e.g., multiple myeloma and lymphoma) [33], suggesting that brain inflammatory processes may be an early feature of aMCI. Cystatin C is a cysteine protease inhibitor which can also provide neuroprotection by binding Aβ to prevent oligomeric or fibrillar amyloid toxicity [34]. Hence, cystatin C levels in CSF may reflect aberrant turnover of Aβ peptides early in the disease process. A preferential association of cystatin C with small, diffusible oligomeric Aβ assemblies may also underlie the poor correlation between protein levels and CERAD or Reagan diagnostic criteria, which index insoluble amyloid plaques. The identity and function of the ~2490 Da phosphopeptide, which showed the strongest associations with MMSE and Braak stage, is currently under intensive investigation.
Taken together, these and possibly other candidate proteins (see above) might be combined with Aβ42 and tau to derive a CSF biomarker panel with high predictive power for those who will convert from NCI to MCI or AD and perhaps even serve as a proxy for the efficacy of disease modifying therapies. Given the inherent heterogeneity of AD progression that confounds the predictive capacity of current biomarker sets, such a panel would provide a powerful tool to use in combination with clinical evaluations [35], structural MRI [36], and other diagnostic tools (e.g., plasma signaling proteins [37]) to identify people during the arc of disease progression when therapeutic intervention is most likely to be beneficial.
Acknowledgements
This work was supported by NIH grants, AG26032, AG14449 and AG10161. We thank Dr. Saleem Dar for expert technical assistance. We acknowledge the altruism and support of the Nuns, Priests and Brothers from the Religious Orders Study. A list of participating groups can be found at the website: http://www.rush.edu/rumc/page-R12394.html
References
- [1].Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- [3].Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- [4].Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [5].Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- [6].Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- [7].Mattsson N, Blennow K, Zetterberg H. CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann N Y Acad Sci. 2009;1180:28–35. doi: 10.1111/j.1749-6632.2009.04944.x. [DOI] [PubMed] [Google Scholar]
- [8].Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- [9].Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- [10].Nitsch RM, Rebeck GW, Deng M, Richardson UI, Tennis M, Schenk DB, et al. Cerebrospinal fluid levels of amyloid beta-protein in Alzheimer's disease: inverse correlation with severity of dementia and effect of apolipoprotein E genotype. Ann Neurol. 1995;37:512–518. doi: 10.1002/ana.410370414. [DOI] [PubMed] [Google Scholar]
- [11].Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ, et al. Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- [12].Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- [14].Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- [15].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- [17].Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- [18].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [19].Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer's disease. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- [21].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- [22].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [23].The National Institute on Aging Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- [24].Li G, Peskind ER, Millard SP, Chi P, Sokal I, Yu CE, et al. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. DOI:10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang J, Sokal I, Peskind ER, Quinn JF, Jankovic J, Kenney C, et al. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J Clin Pathol. 2008;129:526–529. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer's disease. Brain Res Mol Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- [27].Lovell MA, Lynn BC, Xiong S, Quinn JF, Kaye J, Markesbery WR. An aberrant protein complex in CSF as a biomarker of Alzheimer disease. Neurology. 2008;70:2212–2218. doi: 10.1212/01.wnl.0000312383.39973.88. [DOI] [PubMed] [Google Scholar]
- [28].Mukaetova-Ladinska EB, Milne J, Andras A, Abdel-All Z, Cerejeira J, Greally E, et al. Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement Geriatr Cogn Disord. 2008;26:32–42. doi: 10.1159/000141039. [DOI] [PubMed] [Google Scholar]
- [29].Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, et al. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- [30].Iqbal K, Grundke-Iqbal I. Elevated levels of tau and ubiquitin in brain and cerebrospinal fluid in Alzheimer's disease. Int Psychogeriatr. 1997;9(Suppl 1):289–96. doi: 10.1017/s1041610297005024. discussion 317–321. [DOI] [PubMed] [Google Scholar]
- [31].Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer's disease. Proteomics. 2003;3:1486–1494. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- [32].Simonsen AH, McGuire J, Podust VN, Davies H, Minthon L, Skoog I, et al. Identification of a novel panel of cerebrospinal fluid biomarkers for Alzheimer's disease. Neurobiol Aging. 2008;29:961–968. doi: 10.1016/j.neurobiolaging.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [33].Hoekman K, Van Nieuwkoop JA, Willemze R. The significance of beta-2 microglobulin in clinical medicine. Neth J Med. 1985;28:551–557. [PubMed] [Google Scholar]
- [34].Tizon B, Ribe EM, Mi W, Troy CM, Levy E. Cystatin C Protects Neuronal Cells from Amyloid-beta-induced Toxicity. J Alzheimers Dis. 2010;19:885–894. doi: 10.3233/JAD-2010-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer's disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- [37].Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]