Abstract
To many biophysical characterisation techniques, biological membranes appear as two-dimensional structures with details of their third dimension hidden within a 5 nm profile. Probing this structure requires methods able to discriminate multiple layers a few Ångströms thick. Given sufficient resolution, neutron methods can provide the required discrimination between different biochemical components, especially when selective deuteration is employed. We have used state-of-the-art neutron reflection methods, with resolution enhancement via magnetic contrast variation to study an oriented model membrane system. The model is based on the Escherichia coli outer membrane protein OmpF fixed to a gold surface via an engineered cysteine residue. Below the gold is buried a magnetic metal layer which, in a magnetic field, displays different scattering strengths to spin-up and spin-down neutrons. This provides two independent datasets from a single biological sample. Simultaneous fitting of the two datasets significantly refines the resulting model. A β-mercaptoethanol (βME) passivating surface, applied to the gold to prevent protein denaturation, is resolved for the first time as an 8.2 ± 0.6 Å thick layer, demonstrating the improved resolution and confirming that this layer remains after OmpF assembly. The thiolipid monolayer (35.3 ± 0.5 Å), assembled around the OmpF is determined and finally a fluid DMPC layer is added (total lipid thickness 58.7 ± 0.9 Å). The dimensions of trimeric OmpF in isolation (53.6 ± 2.5 Å), after assembly of lipid monolayer (57.5 ± 0.9 Å) and lipid bilayer (58.7 ± 0.9 Å), are precisely determined and show little variation.
Introduction
Currently, artificial planar layers of biological molecules on solid substrates are useful in two distinct research fields, the study of biological membranes1 and the formation of protein arrays.2 The biological membrane, a dynamic composition of lipid and protein which encloses most cells and organelles, is a structure still to yield its innermost secrets. The 35-year-old fluid mosaic model is the basis for our current thinking,3 but the detailed information on the distribution of phospho-, glyco- and sphin-golipids plus integral, surface and cytoskeletal proteins is still emerging.4 For example, lipid raft structures have recently revealed complex structure–function relationships within protein–lipid interactions.4 For many structural analysis tools, both the living cell and its established model, the lipid vesicle, do not provide sufficiently oriented samples. Lipid monolayers at the air–water interface are useful5,6 but flat substrates provide the possibility of assembling oriented bilayers.1 The substrate may be silicon, glass, gold, etc. and particularly for the latter, there may be a chemical link to create a tethered lipid bilayer.7,8 Detailed knowledge of immobilised biological layers also has biotechno-logical uses, since the need for the immobilisation of protein arrays on substrates for use in drug discovery and diagnostics is growing strongly.2,9–11 Furthermore, there is an increasing number of applications of nanoscale surfaces upon which cells will behave as though in complex tissues and thus provide a relevant in vitro model of cellular processes.12–15
Many physical methods are used to probe such layers. Microscopy, including electron, fluorescence16 and atomic force,17,18 can provide detailed data on the structure and/or dynamics of the 2D distribution of membrane components, whilst spectroscopic techniques such as ATR-FTIR19 can probe structural and dynamic aspects of molecular organisation. Surface plasmon resonance,20 quartz crystal microbalance,21 dual polarisation interferometry22 and ellipsometry23 are sensitive to effects based on mass or refractive index, but can also yield estimates of other parameters such as thickness and elasticity. Electrical impedance measurements can be used on conducting surfaces24 whilst solid-state NMR can use stacked planar bilayers on glass plates to determine the structure of membrane proteins, particularly the orientation of transmembrane helices.19
To determine the distribution of individual membrane components through the bilayer, methods that exploit the neutron’s ability to discriminate between hydrogen and deuterium are particularly powerful.25 Small-angle neutron scattering has been used to examine the molecular distribution of membrane proteins within lipid bilayers and detergent micelles,26,27 whilst neutron crystallography has revealed detergent28 and glycolipid distributions in membrane protein crystals.29 Specular neutron reflection (NR) can probe planar membranes and is the only method capable of fully defining the distribution of individual components throughout the profile (z-axis) of a single bilayer.30 We have recently determined the structure of a protein-reconstituted bilayer that contained the bacterial exotoxin, α-hemolysin, in high concentrations and were able to locate the protein within the bilayer with a precision of ~1 Å along the surface normal.31 These approaches are providing increasingly detailed pictures of membranes and may allow investigation of other structures such as bacterial peptidoglycan.
The system investigated in this paper was initially developed as a model of the Escherichia coli outer membrane for biological studies.32,33 Later it was realised that self-assembled monolayers of Gram-negative outer membrane proteins (Omp) on surfaces can also play a role in biotechnology.14 The protein used, OmpF from Escherichia coli, is one of a large group of β-barrel membrane proteins and this one in particular forms extremely stable 16-stranded β-barrels which are resistant to proteases, urea, guanidine hydrochloride, sodium dodecyl sulfate (SDS) and heat denaturation.32 The high-resolution crystal structure 2OMF34 shows it to have an asymmetric shape, resembling a cylinder with a length and diameter of ca. 60 and 30 Å, respectively. These monomers always associate into trimers with a flat base, on the periplasmic face, approximated by an equilateral triangle with a base length of 80 Å. The resilience of OmpF permits its immobilisation on surfaces and subsequent characterisation without detergent in aqueous buffer since exposure of the hydrophobic protein surface does not cause denaturation. This unusual stability of the protein enables the step-by-step assembly of a stable, electrically sealing bilayer membrane suitable for sensing applications.32,35 Such layers need to be electrically tight,36 and water content of the hydrophobic membrane centre is a good metric for successful fabrication because it correlates with membrane resistance to ion transfer. Neutrons are a prime tool to assess water content because of their isotopic sensitivity.
Here we use a modified OmpF containing a cysteine residue (OmpF-E183C) by which it can bind to a gold surface. To cover the high-energy gold surface entirely after surface ligation of the protein, we form contiguous membranes by backfilling the area between the immobilised protein with thiolipids that also attach through gold–sulfur bonds.32 Subsequently, the resulting OmpF/ self-assembled monolayer (SAM) system is terminated by precipitation of a terminal layer of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) using the method previously reported by McGillivray et al.8
The final architecture consists of a protein–lipid bilayer membrane that has its inner monolayer leaflet bonded to the gold film and is arranged around OmpF-E183C trimers also covalently bound to the gold surface (see Fig. 1). The self-assembly of the complex membrane is monitored sequentially through the deposition process, with the thickness and composition of the layers determined at each step. As we show, this general organisation of the membrane is confirmed by NR employing a combination of isotopic (2H vs. 1H) and magnetic contrast neutron reflection (MCNR). This uses polarised (up- or down-spin) neutrons to provide two independent data sets from a single membrane (see Experimental section for details). Compared to conventional reflectometry,37 this method results in significantly improved structural resolution and moreover enables determination of structures with a high degree of compositional complexity. To quantify these advantages, we have assessed the performance of the method by a Monte-Carlo-based resampling technique.15,38,39 The results demonstrate the application of MCNR for the determination of the molecular distribution across a complex biomembrane with Ångström resolution.
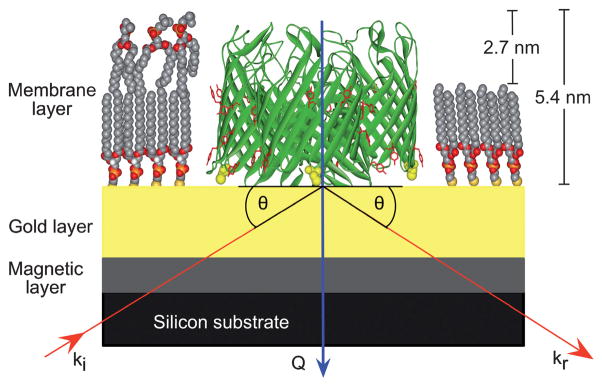
Fig. 1.
Schematic of the sample configuration for the neutron reflection studies. The substrate was exposed to a 1% (v/v) βME in ethanol solution.32 OmpF-E183C (300 μg ml−1 in buffer A) was incubated on the gold surface for at least 3 hours at room temperature. After incubation, the surface was washed, first with a 2% (w/v) SDS solution and then with distilled water, to remove any non-specifically bound protein. Thiolipid DPPTE (1,2-dipalmitoyl-sn-glyero-3-phosphothioethanol), (1.0 mg ml−11 in buffer A), was then deposited to infill around the OmpF trimers (right-hand side of figure). Finally DMPC (10 mg ml−1 in ethanol) was added to the assembled surface (left-hand side of figure) and incubated for 5 minutes. The DMPC solution was removed by washing the cell quickly with 50 ml of buffer B. The figure shows an OmpF trimer (PBD 2OMPF34) attached to the gold via cysteine residues surrounded by DPPTE (pdb file 870160.mol from Avanti Polar Lipids). DMPC molecules used to represent the upper layer are taken from a simulated DMPC bilayer structure.61 The cysteine residues are yellow and space-filled, and a belt of tyrosine residues (wire frame, red) delineates the bilayer interface in the bacterial membrane region. The incident neutron beam (red arrow labelled ki) was directed to reflect (kr) from the back of the complex interfacial structure. The blue arrow labelled Q shows the scattering vector.
Results
Resolving the β-mercaptoethanol layer
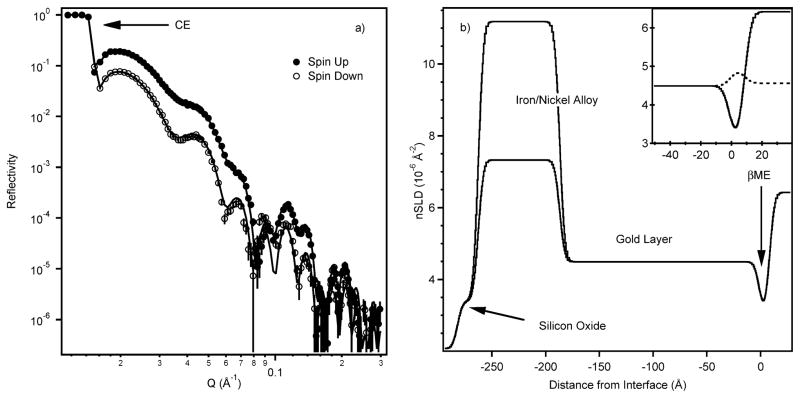
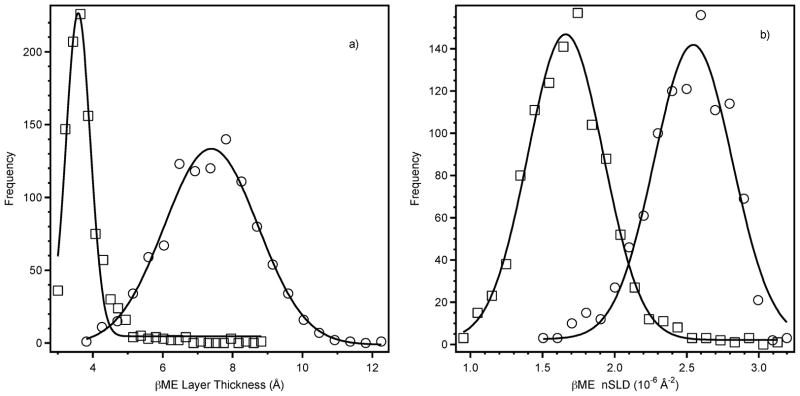
Gold pre-treatment with small hydrophilic thiol-containing molecules is a common method to passivate the high-energy surface1,40 and significantly reduces protein denaturation and non-specific binding to gold surfaces.32,41 Fig. 2a shows NR data for the ‘up’ and ‘down’ spin states after βME deposition onto a gold-coated substrate (thickness, d = 186.3 ± 0.3 Å) with an iron/nickel magnetic sub-layer (d = 75.6 ± 0.1 Å) in contact with D2O buffer. Because of the large difference of the neutron scattering length density (nSLD) values for the iron–nickel layer probed by the two spin states, the two NR spectra are different with the reflectivity for the spin ‘up’ state about an order of magnitude larger than that for the ‘down’ state. The derived nSLD profiles (Fig. 2b) provide excellent fits to the data and are the result of the simultaneous refinement of data from five different samples (three hydrogenous and two deuterated βME) each with two spin states. Data were collected on one of the hydrogenous βME samples with two different H2O/D2O contrast conditions, resulting in a total, using both spin states, of twelve different datasets. The data were fitted with the βME thickness constrained but with sub-layer (gold etc.) properties and βME surface coverage allowed to vary between different samples. A βME thickness of 8.2 × 0.6 Å with a nSLD of 3.07 ± 0.15 × 10 Å−2 was obtained for the sample shown in Fig. 2, corresponding to a βME surface coverage of 57%. Table 1 lists the volume fraction (Vf) of the βME in the layer calculated for various hydrogenous samples against a D2O buffer.
Fig. 2.
a) Reflectivity data (symbols) and fit (lines) after βME adsorption onto the surface, D2O buffer. Below the critical edge (CE) the neutrons are totally externally reflected and the reflectivity is unity. Q is the scattering vector defined in eqn (1). The separation in the data results from the different contrast of the magnetic layer to spin-up and spin-down neutrons. b) Real-space nSLD profile corresponding to the fit shown in a). The zero point has been set at the interface between the gold and the βME. The silicon substrate is on the left and D2O buffer on the right. The twp different nSLD values for the magnetic layer are clearly seen. The hydrogenous βME layer is clearly evident between the gold and the buffer. The inset shows the βME region of the sample expanded with the dashed line the fit obtained with d-βME next to a gold-matched water buffer. Note: Data presented in Figs. 2 and 4 are all from successive depositions on the same substrate.
Table 1.
Layer thicknesses and component volume fractions from constrained fits to multiple datasets. Numbers in brackets are the errors assessed via the Monte Carlo resampling method. Where data was collected for βME adsorption and subsequent OmpF adsorption, volume fractions are reported for both steps
| Sample number |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| βME thicknessa (Å) | 8.2 (0.6) | 8.2 (0.6) | 8.2 (0.6) | 8.2 (0.6) | 8.2 (0.6) |
| βME Vf | 0.54 (0.03) | 0.63 (0.05) | — | — | 0.83 (0.17) |
| OmpF thicknessb (Å) | — | 53.6 (2.5) | 53.6 (2.5) | 53.6 (2.5) | 53.6 (2.5) |
| βME Vf | — | 0.59 (0.02) | 0.35 (0.08) | 0.43 (0.01) | 0.31 (0.01) |
| OmpF Vf | — | 0.27 (0.01) | 0.39 (0.02) | 0.26 (0.01) | 0.34 (0.01) |
| Buffer Vf | — | 0.14 (0.02) | 0.26 (0.08) | 0.31 (0.02) | 0.35 (0.02) |
βME thickness from fits to βME adsorption step only.
Fitting from samples with βME + OmpF adsorbed where the βME thickness was constrained to that determined above.
As gold is readily contaminated upon exposure to air, it was necessary to confirm that the layer resulted from the βME treatment. This was achieved by repeating a set of experiments using deuterated βME (d-βME), inset Fig. 2b, where the dashed line represents the profile for d-βME when the buffer is contrast matched to gold. This clearly demonstrates that deuterated material is present on the surface. We note that the calculated nSLD for a filled layer of d-βME is 4.8 × 10−6 Å−2, very similar to that of gold (4.5 × 10−6 Å−2). This value is very different from that for a filled layer of hydrogenous βME (0.62 × 10−6 Å−2). Model calculations demonstrate that the disappearance of fine features observed in the h-βME NR datasets (details in the ESI†) is due to the replacement of hydrogenous material with deuterated material.
OmpF-E183C on surface-passivated gold
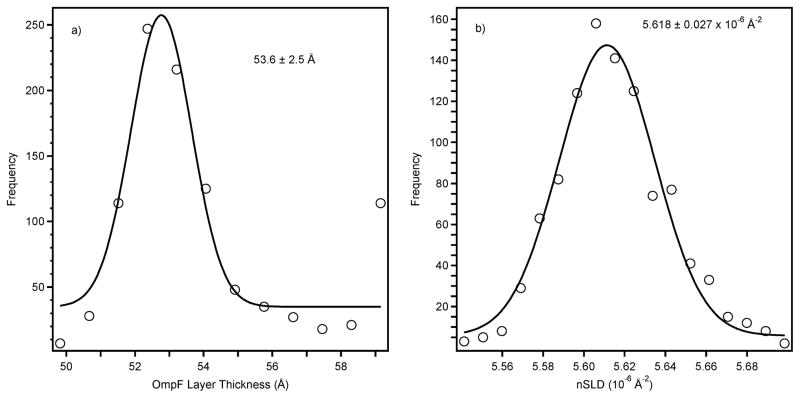
It is well-known that thiol–gold bonds are relatively weak. Even after the gold surface has been fully passivated, incubation with other thiolated species leads to spontaneous replacement of βME.42 However, the extent of this spontaneous exchange in the OmpF system was not known. There are therefore two approaches that can be taken to fit the OmpF data. One may either include a βME layer or assume that the βME has been completely displaced by the OmpF. Far superior fitting results were always obtained when a layer corresponding to βME was included in the fit. Fig. 3a shows the results of Monte-Carlo resampling39 applied to the constrained fit from four different samples, resulting in an OmpF best-fit thickness of 53.6 ± 2.5 Å. In comparison, a recent AFM study17 determined the protein thickness as 55 ± 13 Å from measurements of individual trimers. Here the thickness of the βME layer was fixed at 8 Å, and the nSLD of the βME and OmpF layers allowed to vary between samples to account for differences in surface coverage. Fig. 3b displays the resampling results for the protein nSLD for sample number 4 (Table 1) with the data and fit in shown in Fig. 4a (filled squares) and 4b (solid line) respectively.
Fig. 3.
Results from the Monte-Carlo resampling of magnetic contrast data for self-assembled OmpF layers where the line is a Gaussian fit intended to provide a guide to the eye. The frequency axis represents the number of times a particular result was obtained in the 1000 trial fits. A) OmpF layer thickness, and b) nSLD of the OmpF layer (see Fig. 4).
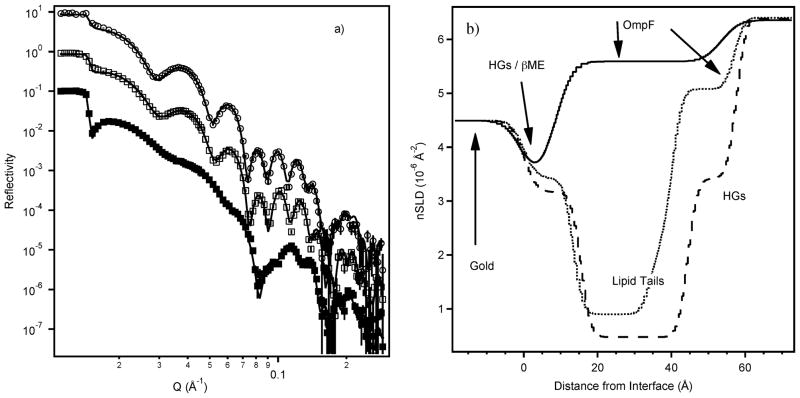
Fig. 4.
a) Data, ‘up polarisation’ state (symbols) and fit (lines) after OmpF adsorption to a βME-passivated gold surface (■), subsequent DPPTE adsorption (□) and subsequent precipitation of DMPC (○). The OmpF and DMPC datasets have been offset for clarity. b) The real-space nSLD profiles corresponding to the fits of all polarisation data. Solid line – OmpF adsorption; dots – DPPTE adsorption; dashes – DMPC adsorption. The figure has been labelled to illustrate the main constituent of each region; HGs indicate regions of lipid headgroup.
The Vf of βME, OmpF and D2O in the mixed layer near the gold surface was determined (Table 1). This calculation determined the OmpF Vf from the local nSLD measured within the two-component upper OmpF-D2O layer. The proportions of βME and D2O in the three-component layer were then determined from its nSLD under the assumption that the OmpF volume fraction is the same in both layers. This approach may underestimate the amount of βME in the three-component layer, because the base of the protein is not perfectly flat. This makes it likely that there is some reduced OmpF density immediately adjacent to the surface.
Thiolipid layer: Backfilling of the OmpF layer and termination with DMPC
In an early test experiment, NR data revealed that the deposition of the thiolipid 1,2-dipalmitoyl-sn-glycero-3-phosphothioetha-nol (DPPTE) was incomplete at room temperature, i.e. below its phase transition temperature (data not shown), producing layers with high water content and lipid tails that were not fully extended. This was overcome by undertaking the DPPTE deposition at 50 °C with the solution in contact with the surface for two hours. Longer exposure times ran the risk of the DPPTE displacing OmpF trimers.17 Fig. 4 displays exemplary data, i.e. the ‘up’ polarisation reflectivity of a sequence of sample preparation states, all measured under D2O-based buffer. The ‘down’ polarisation state and samples in contact with various contrast buffers have also been measured and were included in the constrained fit shown. The sample preparation steps involved sequential adsorptions of OmpF, DPPTE, and finally DMPC. The large change in signal between the OmpF and DPPTE datasets in Fig. 4a is due to the displacement of D2O (positive nSLD) from around the OmpF by hydrogenous thiolipid (negative nSLD). The extra fringes observed in the data are due to this hydrogenous layer. Subsequent addition of DMPC then produced a much smaller change, with the ‘new’ fringes shifted to slightly lower Q values, signifying a thickening of the hydrogenous lipid layer. While the adsorbed OmpF on the βME was fitted in a two-layer model and the final sample (OmpF/DPPTE/ DMPC) was well described by a three-layer model, a four-layer model was required to satisfactorily fit the data obtained from the intermediate step.
The interpretation of the models (Tables 1 and 2) is straightforward. Upon chemisorption, the protein partially displaces βME, and as a result, the surface film consists of a layer in which βME fills the area in-between surface-ligated protein, while at distances greater than ~8 Å from the interface solvent fills the corresponding volume, which results in a change in (average) nSLD. In the completed sample, the three subsequent layers contain OmpF and DPPTE headgroups (innermost layer), OmpF and DPPTE or DMPC acyl chains (central layer) and OmpF and DMPC headgroups (outermost layer). Interpretation of the interface film structure at the intermediate stage of the preparation (OmpF and DPPTE chemisorption) is more complex. We have not been able to realistically model the data with less than four layers. These layers are interpreted as the DPPTE head groups (plus OmpF), the aliphatic DPPTE chains (plus OmpF), a diffuse hydrocarbon layer (plus OmpF), and finally OmpF with some associated lipid or detergent protruding from these hydrocarbon layers (plus buffer). The inclusion of an intermediate layer, conceivably comprising detergent adsorbed from the washing solution to the hydrophobic DPPTE chains, resulted in sensible layer thicknesses and nSLDs. With such a layer included in the model, the overall OmpF thickness, 57.5 ± 0.9 Å, is in excellent agreement with AFM results.17 This protein layer thickness is in fact slightly larger than the thickness determined prior to DPPTE adsorption. Again, this is in excellent quantitative agreement with previous AFM data17 that suggested that surface-immobilised OmpF is stabilised by DPPTE back-filling which leads to a slightly longer projection of the protein’s crystallographic axis on the surface normal, resulting in a slightly larger layer thickness after backfilling. The result for the intermediate sample preparation suggests that detergent remains so tightly bound to the hydrophobically terminated DPPTE chains that it is resistant to copious rinsing with buffer.
Table 2.
Fitted thickness and layer compositions after thiolipid and then DMPC addition to sample 4 (Table 1)
| DPPTE addition | DMPC addition | |
|---|---|---|
| Inner head group thickness (Å) | 13.4 (0.1) | 14.4 (0.4) |
| Lipid tails thickness (Å) | 21.9 (0.5) | 31.6 (0.8) |
| Thickness of diffuse hydrocarbon layer (Å) | 4.6 (0.7) | — |
| Protruding OmpF thickness (Å) | 17.6 (0.4) | — |
| Outer head group thickness (Å) | — | 12.7 (0.3) |
| Layer total (Å) (dependent quantity) | 57.5 (0.9) | 58.7 (0.9) |
| OmpF Vf | 0.26 | 0.26 |
| Lipid tails Vf | 0.68 | 0.73 |
| Buffer Vf | 0.06 | 0.01 |
The final self-assembly step adds a single layer of DMPC to the substrate to complete the bilayer structure, resulting in a hydrophilic outer surface. The overall thickness of the acyl chain layer (31.6 × 0.8 Å) is as reasonably expected from the apposition of a palmitoyl and a myristoyl layer (combined length in the all-trans state: ~ 35 Å). Whether any detergent is retained in the final preparation and if so, how much, cannot be determined without performing dedicated experiments using deuterated detergent or DMPC. Splitting the lipid chains region into DPPTE and DMPC tails did not improve the fit. The quantitative interpretation of the surface structure at different solvent contrasts shows that the addition of DMPC displaces nearly all of the buffer that was resident in the DPPTE layer during the intermediate sample preparation stage (Vf = 0.06). In the final sample, the buffer Vf is ca, 0.01.
Discussion
The application of MCNR has enabled us to define the model membrane system with significantly improved accuracy compared to earlier work, and the Monte-Carlo parameter resampling enables a rigorous determination of the confidence limits. The improved resolution reveals that the OmpF protein only partially displaces the βME passivation layer. In distinction, the thiolipid back-fill displaces βME quantitatively and leads to the formation of a dense layer with little water penetration. The assembly of the fluid DMPC layer around the OmpF is shown to be complete. The OmpF height, which was difficult to measure by AFM in the absence of a rigid thiolipid layer,17 is shown to be remarkably constant in the different stages of membrane formation. The improvement of resolution that underlies this interpretation of the surface structure has been achieved without lipid or protein deuteration, the use of which will further extend the complexity of structures that can be determined.
Resolving the β-mercaptoethanol layer
MCNR enables the structural characterisation of the coherent layer formed by βME. Adsorption of d-βME leads to a slight increase in nSLD near the gold interface (inset in Fig. 2b). Contamination by adsorbed organic species would have produced a drop in nSLD near the gold surface similar to that seen for h-βME (Fig. 2b). That such a drop is not observed after d-βME is adsorbed indicates that the adsorbate layer is βME. Moreover, even after this passivation layer is penetrated and partially replaced by OmpF, the remainder of this thin βME film can be structurally quantified by neutrons, as any model without a βME layer leads to a significant reduction in the fit quality (for example, an increase in χ2 from 1.67 to 2.56 for the data shown in Fig. 2). The data further indicates that the βME layer is finally replaced during DPPTE deposition.
OmpF-E183C on surface-passivated gold
The production and analysis of OmpF layers is highly dependent upon the use of high-quality substrates. Compared with our previous low resolution NR study37 where about 10% of the surface was covered with protein, we are consistently achieving OmpF surface coverages of 30% or greater. To put this into context, the volume fraction of protein in a 2D OmpF crystal is about 0.50; so we are achieving 60% of this maximum packing. The precision obtained for the protein thickness and density is far in excess of that previously reported.37 In view of recent data on monomeric OmpG and OmpA porins in detergent micelles, the height of the protein array is an especially interesting parameter. As reported by NMR, the non-crystalline porins in micelles display shorter β strands and a lower overall height compared to their respective X-ray crystal structures.43,44 No NMR data exist yet for the larger trimeric OmpF, but AFM data17 on gold-immobilised OmpF showed that the proteins were too flexible to observe clearly before thiolipid addition. However, after backfilling the protein array with thiolipid, the trimers were clearly imaged by AFM but the average height was the same both cases. The results from NR reported here indicate the invariant dimensions of surface-bound OmpF, suggesting that the height of the trimeric OmpF may be less sensitive to the change from detergent-stabilised to tight packing than monomeric porins. The AFM work could not measure the absolute OmpF height in thiolipid and assumed a thiolipid thickness through which the OmpF penetrates. Neutrons on the other hand penetrate the whole layer, are non-destructive, and provide an average over the whole surface. Therefore, agreement between these very different but complementary techniques is strong evidence for the accuracy of the models.
Thiolipid addition
Deposition of the DPPTE below its phase transition was unsuccessful with poor surface packing and little extension of the lipid tails. In AFM studies17 of this system the thiolipid was deposited at 45 °C, above the phase transition temperature of DPPC. A similar protocol (at 50 °C) led to a dense, dry (6% water content) layer corresponding to the expected thickness of DPPTE (Fig. 4), confirming that assembly of a dense ordered DPPTE monolayer only occurs above the lipid phase transition temperature. To avoid this temperature requirement in future work with possibly less robust proteins, thiolipids using diphytanoyl chains with lower phase transition temperatures may be used.
The expected result at this stage of the assembly is a half bilayer facing the water, through which the proteins protrude. Due to the thinner cross-section of the E. coli outer membrane, compared to more usual fluid lipid bilayers, the outer membrane protein’s hydrophobic surface is largely covered by the single DPPTE layer.17 This leaves the thiolipid’s hydrophobic surface exposed to the aqueous phase. Such surfaces have a high free energy but, due to the covalent attachment of the thiolipid to the gold, are stable.45,46 In this work, however, the surfaces were rinsed between adsorption steps with SDS solutions of ca. 70× the nominal CMC. It also needs to be kept in mind that CMC is usually determined in pure buffer (i.e. in the absence of lipid aggregates) and that the effective CMC of a particular detergent may be affected by the presence of such aggregates. In our earlier AFM study, extensive washing was used and no evidence for an additional surface layer was obtained.17 MCNR reveals that there is a highly disordered hydrocarbon layer adsorbed onto the hydrophobic outer DPPTE surface. This layer is about 5 Å thick with a Vf of ca. 0.6, and most likely consists of detergent molecules. As with the AFM study, the samples were prepared ex situ, which allowed copious washing of the surfaces, so if the same layer was present in the AFM study17 it was either removed by the AFM tip or was incorporated into the measurement baseline. This residual layer corresponds to a coverage of ca. 100 pmol cm−2, compared to the total surface coverage of 280 pmol cm−2, determined by Sigal et al.47 prior to washing.
Membrane completion with DMPC
In the final step of the process, DMPC was used to complete the bilayer surrounding the OmpF. The fits to the experimental data (Fig. 4) indicate that this was successfully achieved. The OmpF thicknesses in the samples comprising (thiolipid + OmpF) and (thiolipid + DMPC + OmpF) were within 1.2 Å of each other (Table 2). The DMPC displaced both the disordered hydrocarbon and some of the water from the DPPTE during deposition. Both effects may have been aided by the fact that the rapid solvent exchange method is undertaken with the lipid dissolved in ethanol. The final bilayer with OmpF incorporated (Vf = 0.26) can obtain a maximum Vf of 0.74 for the phospholipid layer. Therefore the calculated Vf = 0.73 indicates the DMPC coverage of the available surface exceeds 98%, consistent with results with DMPC (and other lipids) on tethered bilayer lipid membranes.8 The versatility of the rapid solvent exchange method is demonstrated by the finding that the surface coverage in a layer formed around an existing protein protruding from a lipid monolayer was equal to or exceeded that obtained for lipids deposited onto a stable well-formed SAM.
Magnetic contrast
To quantify the benefit of MCNR, one can fit the data in an unconstrained manner, thereby examining each spin state in isolation. In this procedure, the fit often converges to a lower χ2 value as there are fewer boundary conditions to be satisfied. To demonstrate the benefit of the MCNR over conventional isotopic substitution, we analysed with the MC resampling approach the results of model fitting to magnetic contrast data at one isotopic composition (βME adsorbed to the gold surface under D2O) with model fitting to isotopic contrast brought about by three different buffer compositions (D2O, H2O, and a D2O/H2O mixture) without using magnetic contrast. Because there are three isotopic data sets, but only two magnetic contrasts, this should favour the isotopic approach. However, because there are sample manipulations (i.e. the exchange of buffer) involved that might slightly affect sample structure, one runs the risk in the isotopic contrast experiment of mistakenly assuming that the data sets derive from identical structures. If that is not strictly the case, one can easily arrive at a slightly distorted structural result, particularly if the real-space structure is near the length scale of resolution. In distinction, magnetic contrast variation is immune to such artifacts, because it involves no sample manipulation between the collection of the corresponding data sets. Fig. 5 displays the MC resampling analysis of the corresponding datasets. The total thickness of the entire interface structure is very similar, 220.6 Å versus 221.2 Å for the magnetic and subphase contrasts, respectively. There is a variation in both the βME thickness and nSLD values obtained. For the magnetic contrast datasets, the βME layer is thicker (7.6 ± 1.3 versus 3.7 ± 0.6 Å) with a lower Vf, 0.66 ± 0.07 versus 0.80 ± 0.14. The changes in βME parameters are compensated for by the gold layer being thicker for the subphase contrast fits. The βME layer appears more precisely determined in the isotopic contrast fits with a narrower distribution than for the magnetic contrast fits. This is not entirely surprising given that there are more data in those datasets. However, the result, an apparent layer thickness of ca. 3.5 Å, is unrealistically small, presumably due to slight differences in the real-space structures at different solvent compositions that were erroneously assumed to be isomorphic.
Fig. 5.
Monte-Carlo resampling analysis of the use of magnetic contrast upon the parameters for the βME layer. a) layer thickness and b) nSLD values where circles are from magnetic contrast fits with squares corresponding to fits relying solely on buffer contrast. The lines are simple Gaussian fits to guide the eye.
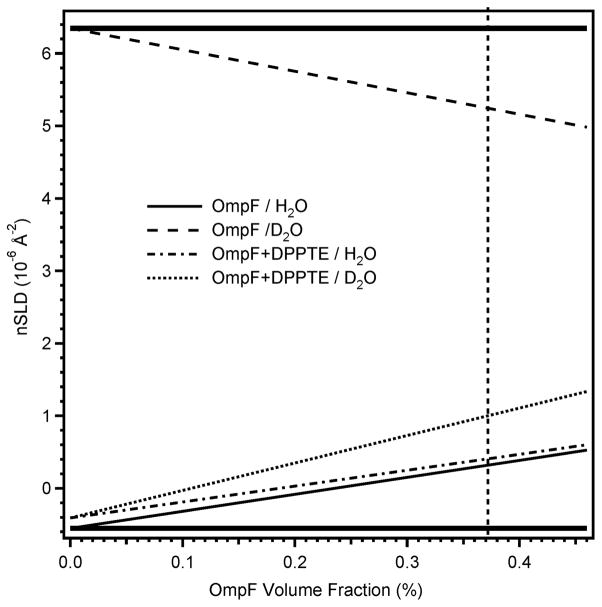
Available beamtime did not permit data collection on all samples with both D2O and H2O buffers. Therefore it was not possible to similarly demonstrate the impact of magnetic contrast for each assembly step. Nevertheless, when one analyses the nSLD profiles for samples to which the thiolipid had been added (Fig. 4) it is clear that H2O would have provided very poor contrast, especially since the thiolipid is only available in hydrogenous form. In fact, the calculated nSLD in H2O is almost indistinguishable before and after thiolipid adsorption (Fig. 6). Therefore magnetic contrast combined with a standard D2O buffer is the best approach available to readily access contrast variation in this system. Furthermore, the approach does not complicate the sample preparation, as a metallic bonding layer, such as titanium or chrome, is required between the gold and silicon substrate.
Fig. 6.
nSLD variation with OmpF surface coverage for D2O and H2O buffers before and after DPPTE adsorption. Pure H2O and D2O nSLD are shown as solid horizontal lines. The vertical dashed line represents maximal OmpF surface coverage observed in this work. The available contrast against the aqueous buffer is the difference between the nSLD of interest at the appropriate surface coverage and either the D2O or H2O lines.
Experimental
Magnetic scattering of polarised neutrons
The neutron is scattered from the atom’s nucleus and the application of neutron scattering techniques allows the spatial distribution of nuclear species to be determined. Biological systems are hydrogen-rich, and the two stable isotopes 1H (protium) and 2H (deuterium), although chemically very similar, interact very differently with thermal neutrons, as defined by their nuclear scattering lengths (b(1H) = −3.74 × 10−15 m, b(2H) = +6.67 × 10−15 m) related to a characteristic refractive index, analogous to that of a photon. The combination of b with the physico-chemical composition of a material (isotope density) defines its characteristic nSLD (eqn (2)) and regions of different nSLD provide the contrast variation needed to solve structures by neutron methods. Hydrogen contrast variation therefore provides a range of possibilities within a multi-component sample and has been extensively applied to studies of small molecules such as surfactants at interfaces; for a review see Lu et al.48 For example, the CH2 groups of cetyl trimethylammonium bromide have been selectively deuterated as a function of position along the surfactant tail, enabling high spatial resolution information on the self-assembled layer to be attained.49–51 As the molecules involved increase in size, selective deuteration becomes more labour-intensive and ultimately chemically intractable. Deuteration of proteins has been achieved in a limited number of systems52–54 and the use of aqueous phase deuteration is also somewhat limited. There is, therefore, a need to find alternative means of achieving increased contrast variation in a manner that neither complicates sample preparation nor alters the biological sample.
As in X-ray or neutron diffraction measurements, only the intensity of the scattered radiation is measured in an NR experiment. Without the corresponding phase information, a unique solution to the density profile that produced the scattering cannot be guaranteed, and ambiguities in the compositional depth profiles arise. This is especially the case when a single specular reflection dataset is considered, since there are usually multiple mathematically acceptable solutions. In certain cases, independent knowledge of parts of the nSLD profile can suffice to identify which of a number of model profiles, each an equally good mathematical solution to the reflectivity, corresponds to physical reality. Reference layers, incorporated into the sample, may be used to retrieve the missing phase information that is the cause of the inherent ambiguity in nSLD.55 X-ray protein crystallography employs an analogous approach where a heavy metal ion is incorporated into the protein crystal, providing a reference point that enables phase information to be retrieved from the data. Similarly, reflectivity curves from a sufficient number of samples, each consisting of an invariant unknown region plus a reference region with a known structure that bears distinct contrasts, would enable the determination of the complex reflection amplitude of the unknown.
One possible reference is a buried, saturated ferromagnetic layer that results in two different nSLD profiles, depending on the spin eigenstate of the neutrons in a polarised beam. By measuring two reflectivity datasets from a composite system, consisting of a ferromagnetic reference layer plus unknown, one with a beam of neutrons in the ‘up’ spin state and the other with spin ‘down’ neutrons, the complex reflection amplitude for the unknown segment alone can be exactly obtained and directly inverted to provide its corresponding scattering length density depth profile.55 In practice, however, statistical uncertainty and limited range of scattering vector can make an essential part of this calculation process – the selection of the physical root of a quadratic equation – problematic.56 A minimum of two magnetic contrast datasets were simultaneously refined with appropriate constraints to retrieve the unknown nSLD of the region of interest, an approach that implicitly takes into account the phase information distributed over the multiple datasets.
Without magnetic reference layers, contrast is much more tedious to achieve. Selective deuteration of components also enables contrast variation for the alkane and protein components of the model membrane. This might also provide independent datasets with different contrasts, but with the complication of inherent variability between independently prepared samples. Without magnetic contrast, the external reference approach to phase determination requires the assembly of two biological samples with identical thickness and surface coverage values upon different substrates with identical roughness and oxide layer thickness values. The application of the magnetic contrast approach to a single sample is far simpler than with the preparation of two or more independent samples with isotopic contrast. Also the self-assembly can be followed with the MCNR approach at each intermediate preparation step. Furthermore, this approach is entirely complementary to deuteration and has therefore the potential to greatly augment the information content of data if used in combination with deuterated samples. Moreover, future developments in data collection strategies may enable the direct inversion of MCNR data to obtain model-independent nSLD profiles.57
The metallic layers required a thickness homogeneity and surface roughness of better than 10 Å over the entire surface (area >4.5 cm2). To ensure appropriate smoothness, flatness and stability, a metallic binder layer was first deposited on the silicon, followed by the gold layer. Correct selection of the binder enabled a magnetic reference layer to be incorporated into the standard substrate. Application of an external magnetic field controlled the direction of the magnetisation in this binder layer. The incoming neutron beam was polarised so that the neutron spin was either parallel or anti-parallel to the direction of magnetisation in the layer, resulting in two datasets for the two distinct polarisation states. During data fitting all model parameters were kept exactly the same for the two datasets except for the nSLD values of the magnetic layer.
Materials
Unless otherwise stated all materials were obtained from Sigma and used without further purification. Thiolipid (1,2-dipalmi-toyl-sn-glycero-3-phosphothioethanol, DPPTE) and DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids. Sample preparation entailed two distinct procedures; firstly there was the deposition of the gold and magnetic layers in advance of the NR experiments. The second stage (Fig. 1) was solution-based with the protein and lipids sequentially self-assembled onto the gold surface. NR experiments were undertaken at the National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA, and at ISIS, Rutherford Appleton Laboratories, Oxfordshire, UK. The magnetic (40–80 Å) and gold (150–250 Å) layers were deposited sequentially in the same chamber. Three different magnetic layers were employed during the experiments: pure iron, permalloy (80% nickel, 20% iron) and mu metal (75% nickel, 15% iron, plus copper and molybdenum). Experiments at NIST were undertaken on silicon disks 75 mm diameter by 5 mm thick which were coated in a DC Magnetron Sputtering chamber (Auto A306; BOC Edwards, UK). Experiments at ISIS were carried out on silicon discs 100 mm diameter by 10 mm thick with the metal layers produced by INESC Microsystems & Nanotechnologies, Lisbon, Portugal, in a Nordiko 3000 Ion Beam system.
The self-assembly from solution and washing steps were carried out in buffer A (1% n-octyl-β-D-glucopyranoside, 20 mM Tris-HCl pH 7.5, 1 mM tris(2-carboxyethyl)phosphine) and B (5 mM Na2HPO4, 100 mM NaCl, pH 7.4). The OmpF was prepared and purified as has been previously described.17,32 The assembly of all components, except for the DMPC, was carried out ex situ.
Neutron reflection
The NIST Center for Neutron Research NG1 instrument is located on a reactor source and operates with a neutron beam of fixed wavelength incident on the sample whereas CRISP at ISIS is a time-of-flight reflectometer on a spallation source supplying a multiwavelength neutron beam. The different instrument configurations and neutron beam characteristics resulted in different data collection approaches for the two instruments. The reflectivity (ratio of the reflected to incident beam intensity) was measured as a function of Q, the scattering vector. Q is defined as follows;
| (1) |
where θ is the angle the incident beam makes with the interface and λ is the wavelength of the neutrons. It is therefore possible to vary Q by altering either the wavelength (CRISP) or the angle (NG1) during a measurement. Data were collected over Q values from 0.011 to 0.3 Å−1. Specular reflection occurs where the angle the incoming beam makes with the surface is equal to that of the reflected beam.
On NG1 data is collected with the neutron wavelength fixed at 4.75 Å and the incident beam angle scanned from 0.23 to 7.2° with increasing slit openings for each data point such that a fixed sample area is illuminated. At CRISP, a neutron beam, wavelength range 1.2–6.5 Å, is incident on the sample at three different incident angles also with a fixed illumination area producing an overall similar Q range on both instruments. The reflectometers were operated in polarised beam mode, achieved by the same method on both devices. The incoming neutron beam is first incident on a polarising supermirror, guide fields are then used to maintain the neutron polarisation and finally the beam passes through a spin flipper which sets the direction of the neutron beam polarisation onto the sample. The sample geometry is such that the polarisation is parallel to the silicon surface and the magnetisation of the buried layer is induced by the application of an external magnetic field. The spin flipper flips the direction of the neutron polarisation so that it is either parallel (‘up’ spin) or anti-parallel (‘down’ spin) to the direction of the reference layer magnetisation. It is possible to analyse the polarisation of the neutron beam downstream from the sample but this was not used because the biological layer did not contain any magnetic structure. Tests were performed on NG1 confirming the complete absence of spin-flip scattering (data not shown) which would have indicated non-uniform magnetic structure in the buried layer. As indicated in Fig. 1, the neutron beam is transmitted through the silicon substrate prior to reflecting from the interface.
Data fitting
The processing of the raw data to produce absolute reflectivity was different for each instrument. On NG1, the main correction to be applied is that related to the changing slit settings across the angular range. A slit scan with the beam transmitted through silicon was performed at the beginning of each experimental campaign to correct for this. On CRISP, corrections for silicon transmission and detector efficiency, which varies as a function of wavelength, were required. Nevertheless, once the corrections were applied and the data scaled there was no difference in the data-fitting programmes or routines used to treat the data. Fitting of the data was undertaken using the ga_refl programme.58 This approach is based upon the optical matrix method59 which assumes that the interface can be described as a series of slabs where there is a change in refractive index for neutrons at the interface of each slab. If protiated and deuterated material are mixed correctly it is possible to produce an interface which is contrast matched and becomes invisible to the neutron beam. There are three parameters that describe each slab or layer, the thickness (d, Å), a Gaussian interfacial roughness (σ, Å) and the nSLD (ρ, Å−2) of the layer. It was possible to simultaneously co-refine multiple datasets (from both NG1 and CRISP) with a range of constraints applied to the fit. For the simplest case, where two magnetic contrast datasets were co-refined, all parameters were set to refine to the same value except for the nSLD of the magnetic layer. If datasets distinct in magnetic contrast and buffer isotopic contrast were refined together then, all thickness and roughness values were equivalent between datasets with only the appropriate nSLDs (reference, buffer) allowed to vary.
The nSLD of a material is a function of the chemical composition (atomic number and density) and the nuclear scattering length as shown in eqn (2).
| (2) |
where NA is Avagadro’s number, pi the mass density, Ai the atomic weight and bi the nuclear scattering length of component i. From comparisons of the nSLD from a fitted layer with the theoretical value for a complete layer, it is straightforward to calculate the volume fraction or, if we assume that within the resolution of the experiment the nSLD profile of a component is homogeneous in all directions, we can calculate the surface coverage via projection of the volume fraction onto a planar surface.
The data fits were evaluated by the application of a Monte-Carlo resampling procedure using the best fit to the dataset as a starting point.39 At least N = 1000 synthetic datasets were produced by applying random Gaussian weighted deviations from the data based upon the counting statistics of each data point.60 These synthetic datasets were analysed in the same manner as real data, outputting N variations of each parameter. The fits to the synthetic data were analysed producing a frequency plot of fitted values. These parameter distributions were statistically analysed with the parameter value reported as the midpoint of the 95% confidence interval. Twice the standard deviation of the distribution is reported as the error. The frequency distribution obtained from this procedure is histogrammed (bin width = 3.49 × standard deviation × (N − 1/3)) for presentation.
Conclusions
Our previous study of this system by neutron reflection, while producing useful data about the buried proteins layers in situ and confirming OmpF orientation,37 did not demonstrate sufficient resolution to disentangle the scattering from all components. Structural and compositional resolution of this system has only been achieved through the production of high-quality samples and the application of magnetic contrast neutron reflectometry. The details of the βME layer have been observed in terms of precisely quantified structural parameters. Such layers are important in the formation of tethered lipid bilayers1,35,40,41 and this report reveals the structure of βME adsorbed to gold with unprecedented resolution. We have quantified the layer thickness resolution available from this method and showed that after OmpF adsorption the βME is still present on the surface between the OmpF trimers. It is not until the thiolipid is added to the surface that the βME is displaced. Through the use of magnetic contrast and some buffer contrast variation we have been able to accurately determine the relative proportions of water, lipid and protein in the biomolecular layer. The height of the OmpF has been measurable throughout with a small initial extension (3.9 Å) upon the assembly of the DPPTE. The final assessment of the structure indicates that the original aims of producing a stable robust protein scaffold, with a solution-accessible surface, embedded within a model membrane lipid bilayer have been achieved. The MCNR characterisation technique described here is especially suited to the study of biotechnical devices that employ gold substrates for electric field application. More broadly, it would be applicable to any layered soft matter system that incorporates a magnetic layer or substrate. This application is especially relevant when the selective deuteration of components is problematic.
Supplementary Material
Acknowledgments
Support by the National Institute of Standards and Technology (U.S. DOC) and ISIS (Science and Technologies Facility Council (STFC), UK) in providing the neutron research facilities used in this work is gratefully acknowledged. APLB is a BBSRC CASE student with support from STFC Centre for Structure and Dynamics and Orla Protein Technologies Ltd. JHL thanks BBSRC for funding and Helen Ridley for technical assistance. We thank Paul A. Kienzle (NCNR) for support in the NR data analysis and Tom Griffin (ISIS) for help in configuring computational resources. This work was supported by the NSF (CBET-0555201) and the NIH (1 RO1 RR14182). The identification of any commercial product or trade name does not imply endorsement or recommendation by the National Institute of Standards and Technology.
Abbreviations
- βME
β-Mercaptoethanol
- CE
Critical edge
- DMPC
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- DPPTE
1,2-Dipalmitoyl-sn-glyero-3-phosphothioethanol
- HGs
Head groups
- MCNR
Magnetic contrast neutron reflectometry
- NR
Neutron reflection
- nSLD
Neutron scattering length density
- OmpF
Outer membrane protein F
- SAM
Self-assembled monolayer
- Vf
Volume fraction
Footnotes
Electronic supplementary information (ESI) available: Data and model calculations demonstrating the sensitivity of the reflectivity data to βME surface coverage. See DOI: 10.1039/b822411k
Contributor Information
Stephen A. Holt, Email: Stephen.Holt@stfc.ac.uk.
Anton P. Le Brun, Email: Anton.Le-Brun@newcastle.ac.uk.
Charles F. Majkrzak, Email: charles.majkrzak@nist.gov.
Frank Heinrich, Email: frank.heinrich@nist.gov.
Mathias Lösche, Email: quench@andrew.cmu.edu.
Jeremy H. Lakey, Email: j.h.lakey@ncl.ac.uk.
References
- 1.Heyse S, Stora T, Schmid E, Lakey JH, Vogel H. Biochim Biophys Acta. 1998;1376:319–338. doi: 10.1016/s0304-4157(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 2.MacBeath G, Schreiber SL. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 3.Singer SJ, Nicolson GL. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Vaz WLC. Annu Rev Biophys Biomol Struct. 2004;33:269. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RK. Annu Rev Phys Chem. 2004;55:391. doi: 10.1146/annurev.physchem.54.011002.103830. [DOI] [PubMed] [Google Scholar]
- 6.Lösche M, Piepenstock M, Diederich A, Grünewald T, Kjaer K, Vaknin D. Biophys J. 1993;65:2160. doi: 10.1016/S0006-3495(93)81269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang H, Duschl C, Vogel H. Langmuir. 1994;10:197–210. [Google Scholar]
- 8.McGillivray DJ, Valincius G, Vanderah DJ, Febo-Ayala W, Woodward JT, Heinrich F, Kasianowicz JJ, Losche M. Biointerphases. 2007;2:21–33. doi: 10.1116/1.2709308. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Snyder M. Curr Opin Cell Biol. 2002;14:173–179. doi: 10.1016/s0955-0674(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 10.Hodneland CD, Lee YS, Min DH, Mrksich M. Proc Natl Acad Sci U S A. 2002;99:5048. doi: 10.1073/pnas.072685299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RB, Gordus A, Krall JA, MacBeath G. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 12.Mrksich M, Whitesides GM. Annu Rev Biophys Biomol Struct. 1996;25:55. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 13.Cooke MJ, Phillips SR, Shah DSH, Athey D, Lakey JH, Przyborski SA. Cytotechnology. 2008;56:71–79. doi: 10.1007/s10616-007-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah DS, Thomas MB, Phillips S, Cisneros DA, Le Brun AP, Holt SA, Lakey JH. Biochem Soc Trans. 2007;35:522. doi: 10.1042/BST0350522. [DOI] [PubMed] [Google Scholar]
- 15.Valincius G, Heinrich F, Budvytyte R, Vanderah DJ, McGillivray DJ, Sokolov Y, Hall JE, Losche M. Biophys J. 2008;95:4845–4861. doi: 10.1529/biophysj.108.130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez KL, Meyer BH, Hovius R, Lundstrom K, Vogel H. Langmuir. 2003;19:10925–10929. [Google Scholar]
- 17.Cisneros DA, Muller DJ, Daud SM, Lakey JH. Angew Chem, Int Ed. 2006;45:3252. doi: 10.1002/anie.200504506. [DOI] [PubMed] [Google Scholar]
- 18.Muller DJ, Engel A, Matthey U, Meier T, Dimroth P, Suda K. J Mol Biol. 2003;327:925. doi: 10.1016/s0022-2836(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 19.Aisenbrey C, Kinder R, Goormaghtigh E, Ruysschaert JM, Bechinger B. J Biol Chem. 2006;281:7708. doi: 10.1074/jbc.M513151200. [DOI] [PubMed] [Google Scholar]
- 20.Hong Q, Gutierrez-Aguirre I, Barlic A, Malovrh P, Kristan K, Podlesek V, Macek P, Gonzalez-Mañas JM, Lakey JH, Anderluh G. J Biol Chem. 2002;277:41916. doi: 10.1074/jbc.M204625200. [DOI] [PubMed] [Google Scholar]
- 21.Dorvel BR, Keizer HM, Fine D, Vuorinen J, Dodabalapur A, Duran RS. Langmuir. 2007;23:7344–7355. doi: 10.1021/la0610396. [DOI] [PubMed] [Google Scholar]
- 22.Terry CJ, Popplewell JF, Swann MJ, Freeman NJ, Fernig DG. Biosens Bioelectron. 2006;22:627. doi: 10.1016/j.bios.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Howland MC, Szmodis AW, Sanii B, Parikh AN. Biophys J. 2007;92:1306. doi: 10.1529/biophysj.106.097071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrettaz S, Stora T, Duschl C, Vogel H. Langmuir. 1993;9:1361–1369. [Google Scholar]
- 25.Büldt G, Gally HU, Seelig A, Seelig J. Nature. 1978;271:182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- 26.Jeanteur D, Pattus F, Timmins PA. J Mol Biol. 1994;235:898. doi: 10.1006/jmbi.1994.1047. [DOI] [PubMed] [Google Scholar]
- 27.Marone PA, Thiyagarajan P, Wagner AM, Tiede DM. J Cryst Growth. 1999;207:214. [Google Scholar]
- 28.Pebay-Peyroula E, Garavito RM, Rosenbusch JP, Zulauf M, Timmins PA. Structure. 1995;3:1051–1059. doi: 10.1016/s0969-2126(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 29.Weik M, Patzelt H, Zaccai G, Oesterhelt D. Mol Cell. 1998;1:411. doi: 10.1016/s1097-2765(00)80041-6. [DOI] [PubMed] [Google Scholar]
- 30.Krueger S, Meuse CW, Majkrzak CF, Dura JA, Berk NF, Tarek M, Plant AL. Langmuir. 2001;17:511–521. [Google Scholar]
- 31.McGillivray DJ, Valincius G, Heinrich F, Robertson JWF, Vanderah DJ, Febo-Ayala W, Ignatjev I, Lösche M, Kasianowicz JJ. Biophys J. 2009;96:1547–1553. doi: 10.1016/j.bpj.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrettaz S, Ulrich WP, Vogel H, Hong Q, Dover LG, Lakey JH. Protein Sci. 2002;11:1917. doi: 10.1110/ps.0206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baboolal TG, Conroy MJ, Gill K, Ridley H, Visudtiphole V, Bullough PA, Lakey JH. Structure. 2008;16:371–379. doi: 10.1016/j.str.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Paupit RA, Jansonius JN, Rosenbusch JP. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 35.Stora T, Lakey JH, Vogel H. Angew Chem, Int Ed. 1999;38:389. doi: 10.1002/(SICI)1521-3773(19990201)38:3<389::AID-ANIE389>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Terrettaz S, Mayer M, Vogel H. Langmuir. 2003;19:5567–5569. [Google Scholar]
- 37.Holt SA, Lakey JH, Daud SM, Keegan N. Aust J Chem. 2005;58:674. [Google Scholar]
- 38.Schalke M, Lösche M. Adv Colloid Interface Sci. 2000;88:243. doi: 10.1016/s0001-8686(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich F, Ng T, Vanderah DJ, Shekhar P, Mihailescu M, Nanda H, Lösche M. Langmuir. 2009;25:4219–4229. doi: 10.1021/la8033275. [DOI] [PubMed] [Google Scholar]
- 40.Cornell BA, Braach-Maksvytis VLB, King LG, Osman P, Raguse B, Wieczorek L, Pace RJ. Nature. 1997;387:580–583. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]
- 41.Keegan N, Wright NG, Lakey JH. Angew Chem, Int Ed. 2005;44:4801. doi: 10.1002/anie.200462977. [DOI] [PubMed] [Google Scholar]
- 42.Schlenoff JB, Li M, Ly H. J Am Chem Soc. 1995;117:12528. [Google Scholar]
- 43.Liang BY, Tamm LK. Proc Natl Acad Sci U SA. 2007;104:16140. doi: 10.1073/pnas.0705466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cierpicki T, Liang BY, Tamm LK, Bushweller JH. J Am Chem Soc. 2006;128:6947. doi: 10.1021/ja0608343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doshi DA, Watkins EB, Israelachvili JN, Majewski J. Proc Natl Acad Sci U S A. 2005;102:9458. doi: 10.1073/pnas.0504034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maccarini M, Steitz R, Himmelhaus M, Fick J, Tatur S, Wolff M, Grunze M, Janecek J, Netz RR. Langmuir. 2007;23:598–608. doi: 10.1021/la061943y. [DOI] [PubMed] [Google Scholar]
- 47.Sigal GB, Mrksich M, Whitesides GM. Langmuir. 1997;13:2749–2755. [Google Scholar]
- 48.Lu JR, Thomas RK, Penfold J. Adv Colloid Interface Sci. 2000;84:143. doi: 10.1016/s0001-8686(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 49.Lu JR, Li ZX, Smallwood J, Thomas RK, Penfold J. J Phys Chem. 1995;99:8233. [Google Scholar]
- 50.Lu JR, Hromadova M, Simister E, Thomas RK, Penfold J. Phys B. 1994;198:120. [Google Scholar]
- 51.Lu JR, Hromadova M, Simister EA, Thomas RK, Penfold J. J Phys Chem. 1994;98:11519. [Google Scholar]
- 52.Di Costanzo L, Moulin M, Haertlein M, Meilleur F, Christianson DW. Arch Biochem Biophys. 2007;465:82. doi: 10.1016/j.abb.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Etezady-Esfarjani T, Hiller S, Villalba C, Wuthrich K. J Biomol NMR. 2007;39:229. doi: 10.1007/s10858-007-9188-0. [DOI] [PubMed] [Google Scholar]
- 54.Meilleur F, Contzen J, Myles DAA, Jung C. Biochemistry. 2004;43:8744–8753. doi: 10.1021/bi049418q. [DOI] [PubMed] [Google Scholar]
- 55.Majkrzak CF, Berk NF, Perez-Salas UA. Langmuir. 2003;19:7796–7810. [Google Scholar]
- 56.Schreyer A, Majkrzak CF, Berk NF, Grull H, Han CC. J Phys Chem Solids. 1999;60:1045. [Google Scholar]
- 57.Majkrzak CF, Berk NF. Phys B. 1999;267–268:168. [Google Scholar]
- 58.Kienzle PA, Doucet M, McGillivray DJ, O’Donovan KV, Berk NF, Majkrzak CF. 2000–2009 http://www.ncnr.nist.gov/reflpak.
- 59.Penfold J. In: Neutron, X-Ray and Light Scattering: Introduction to an Investigative Tool for Colloidal and Polymeric Systems. Lindner P, Zemb T, editors. Elsevier; North Holland/Amsterdam/New York: 1991. pp. 223–236. [Google Scholar]
- 60.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes – The Art of Scientific Computing. 3. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- 61.Tieleman DP, Berendsen HJC. J Chem Phys. 1996;105:4871. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.