Abstract
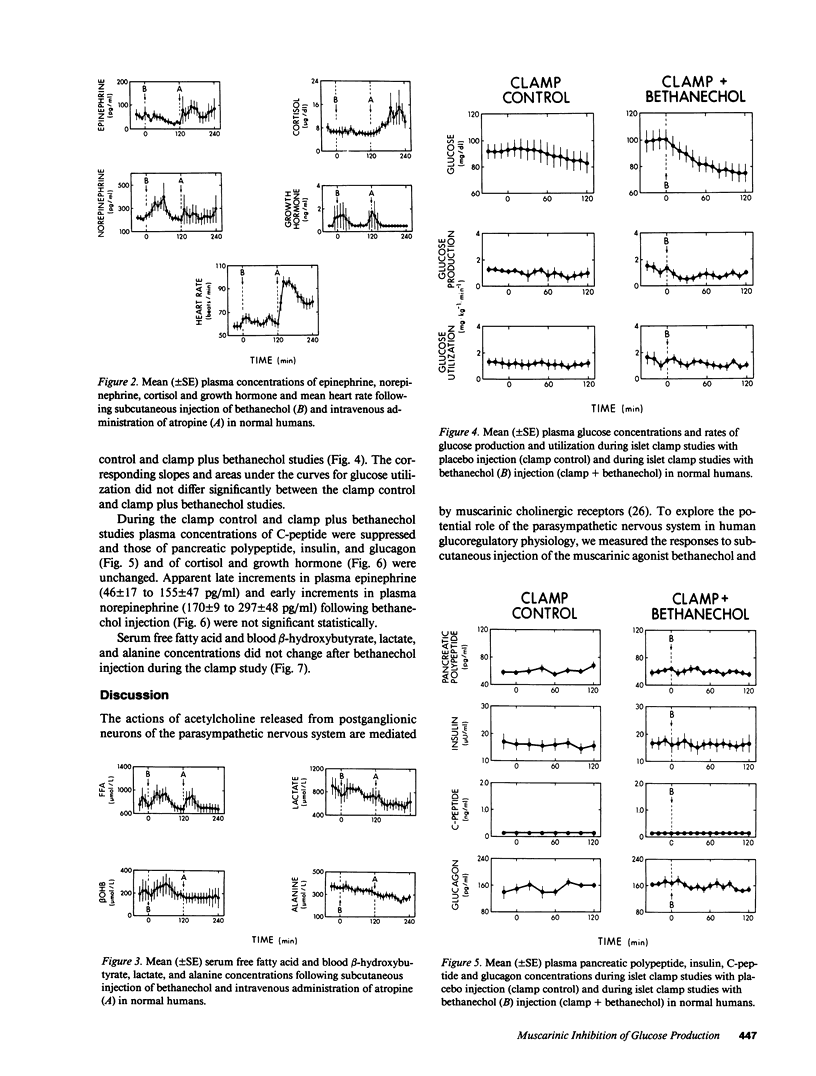
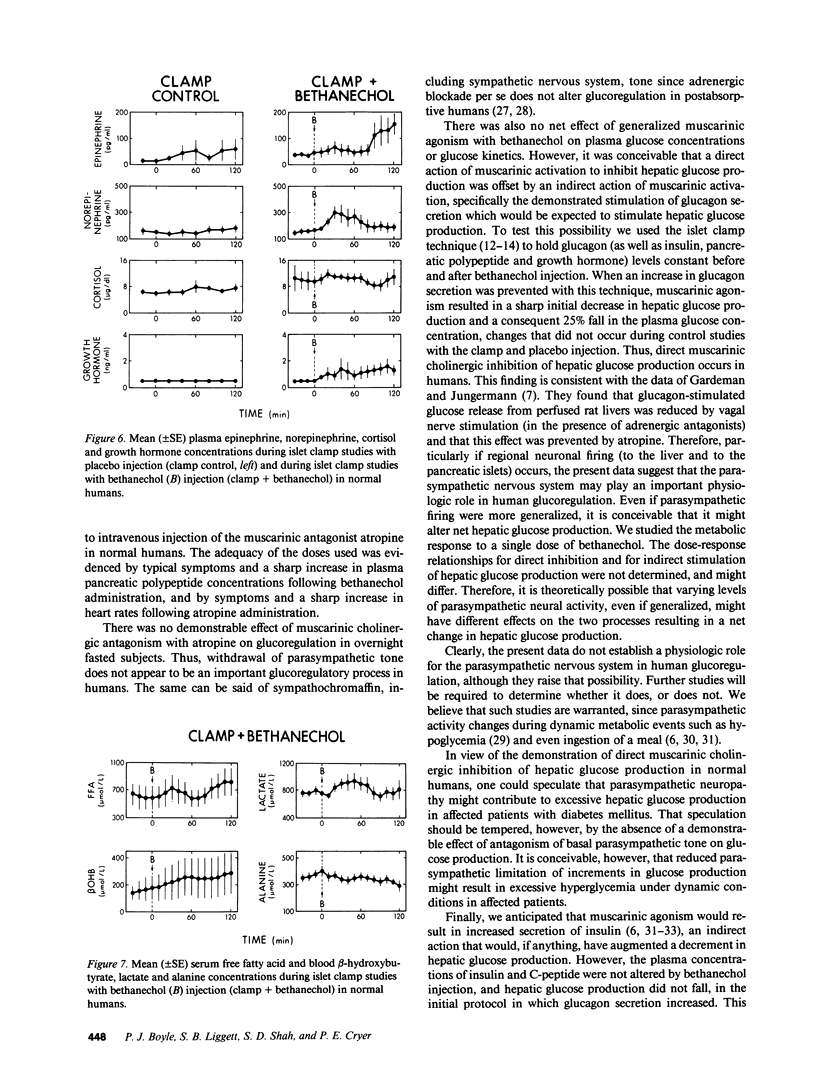
To explore the potential role of the parasympathetic nervous system in human glucoregulatory physiology, responses to the muscarinic cholinergic agonist bethanechol (5.0 mg s.c.) and antagonist atropine (1.0 mg i.v.) were measured in normal humans. There were no changes in the plasma glucose concentration or rates of glucose production or utilization following atropine administration. After bethanechol administration there were no changes in the plasma glucose concentration or fluxes despite increments in plasma glucagon (75 +/- 7 to 103 +/- 10 pg/ml, P less than 0.02). There were no changes in insulin or C-peptide levels. To test the hypothesis that direct muscarinic inhibition of glucose production was offset by an indirect action of the agonist, specifically increased glucagon secretion with consequent stimulation of glucose production, bethanechol was administered while glucagon levels were held constant with the islet clamp technique (somatostatin infusion with insulin, glucagon and growth hormone replacement at fixed rates). Under that condition the muscarinic agonist induced a 25% decrement in the plasma glucose concentration (101 +/- 8 to 75 +/- 8 mg/dl, P less than 0.05). When compared with separate clamp control studies (with placebo rather than bethanechol injection) both the rate of glucose production and the glucose concentration were reduced (P less than 0.05) following bethanechol injection; the rate of glucose utilization was unaltered. Thus, we conclude: Withdrawal of parasympathetic tone does not appear to be an important glucoregulatory process in humans. Direct muscarinic cholinergic inhibition of hepatic glucose production occurs in humans but during generalized muscarinic activation this is offset by an indirect muscarinic action, increased glucagon secretion with consequent stimulation of glucose production. Thus, particularly if regional neuronal firing occurs, the parasympathetic nervous system may play an important role in human glucoregulatory physiology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Taborsky G. J., Jr, Porte D., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986 Dec;29(12):827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Steele R., Rathgeb I., De Bodo R. C. Glucose metabolism and plasma insulin level during epinephrine infusion in the dog. Am J Physiol. 1967 Mar;212(3):677–682. doi: 10.1152/ajplegacy.1967.212.3.677. [DOI] [PubMed] [Google Scholar]
- Berk M. A., Clutter W. E., Skor D., Shah S. D., Gingerich R. P., Parvin C. A., Cryer P. E. Enhanced glycemic responsiveness to epinephrine in insulin-dependent diabetes mellitus is the result of the inability to secrete insulin. Augmented insulin secretion normally limits the glycemic, but not the lipolytic or ketogenic, response to epinephrine in humans. J Clin Invest. 1985 Jun;75(6):1842–1851. doi: 10.1172/JCI111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chap Z., Ishida T., Chou J., Lewis R., Hartley C., Entman M., Field J. B. Effects of atropine and gastric inhibitory polypeptide on hepatic glucose uptake and insulin extraction in conscious dogs. J Clin Invest. 1985 Sep;76(3):1174–1181. doi: 10.1172/JCI112073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutter W. E., Bier D. M., Shah S. D., Cryer P. E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980 Jul;66(1):94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutter W. E., Rizza R. A., Gerich J. E., Cryer P. E. Regulation of glucose metabolism by sympathochromaffin catecholamines. Diabetes Metab Rev. 1988 Feb;4(1):1–15. doi: 10.1002/dmr.5610040104. [DOI] [PubMed] [Google Scholar]
- Esterhuizen A. C., Spriggs T. L., Lever J. D. Nature of islet-cell innervation in the cat pancreas. Diabetes. 1968 Jan;17(1):33–36. doi: 10.2337/diab.17.1.33. [DOI] [PubMed] [Google Scholar]
- Farmer R. W., Pierce C. E. Plasma cortisol determination: radioimmunoassay and competitive protein binding compared. Clin Chem. 1974 Apr;20(4):411–414. [PubMed] [Google Scholar]
- Frier B. M., Corrall R. J., Ratcliffe J. G., Ashby J. P., McClemont E. J. Autonomic neural control mechanisms of substrate and hormonal responses to acute hypoglycaemia in man. Clin Endocrinol (Oxf) 1981 May;14(5):425–433. doi: 10.1111/j.1365-2265.1981.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Ezdinli E. Z., Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967 Jul;16(7):443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- Gardemann A., Jungermann K. Control of glucose balance in the perfused rat liver by the parasympathetic innervation. Biol Chem Hoppe Seyler. 1986 Jul;367(7):559–566. doi: 10.1515/bchm3.1986.367.2.559. [DOI] [PubMed] [Google Scholar]
- Gingerich R. L., Lacy P. E., Chance R. E., Johnson M. G. Regional pancreatic concentration and in-vitro secretion of canine pancreatic polypeptide, insulin, and glucagon. Diabetes. 1978 Feb;27(2):96–101. doi: 10.2337/diab.27.2.96. [DOI] [PubMed] [Google Scholar]
- Hoelzer D. R., Dalsky G. P., Clutter W. E., Shah S. D., Holloszy J. O., Cryer P. E. Glucoregulation during exercise: hypoglycemia is prevented by redundant glucoregulatory systems, sympathochromaffin activation, and changes in islet hormone secretion. J Clin Invest. 1986 Jan;77(1):212–221. doi: 10.1172/JCI112279. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lautt W. W. Afferent and efferent neural roles in liver function. Prog Neurobiol. 1983;21(4):323–348. doi: 10.1016/0301-0082(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Lautt W. W. Hepatic nerves: a review of their functions and effects. Can J Physiol Pharmacol. 1980 Feb;58(2):105–123. doi: 10.1139/y80-019. [DOI] [PubMed] [Google Scholar]
- Leveston S. A., Shah S. D., Cryer P. E. Cholinergic stimulation of norepinephrine release in man. Evidence of a sympathetic postganglionic axonal lesion in diabetic adrenergic neuropathy. J Clin Invest. 1979 Aug;64(2):374–380. doi: 10.1172/JCI109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981 Fall;2(4):471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Palmer J. P., Werner P. L., Hollander P., Ensinck J. W. Evaluation of the control of glucagon secretion by the parasympathetic nervous system in man. Metabolism. 1979 May;28(5):549–552. doi: 10.1016/0026-0495(79)90196-3. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983 Dec;85(6):1411–1425. [PubMed] [Google Scholar]
- Shah S. D., Clutter W. E., Cryer P. E. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med. 1985 Dec;106(6):624–629. [PubMed] [Google Scholar]
- Sharp R., Culbert S., Cook J., Jennings A., Burr I. M. Cholinergic modification of glucose-induced biphasic insulin release in vitro. J Clin Invest. 1974 Mar;53(3):710–716. doi: 10.1172/JCI107609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubbe J. H. Effects of pancreas transplantation on insulin secretion in the rat during ingestion of varying glucose loads. Diabetologia. 1982 May;22(5):354–357. doi: 10.1007/BF00253581. [DOI] [PubMed] [Google Scholar]



