Abstract
Methanoplanus petrolearius Ollivier et al. 1998 is the type strain of the genus Methanoplanus. The strain was originally isolated from an offshore oil field from the Gulf of Guinea. Members of the genus Methanoplanus are of interest because they play an important role in the carbon cycle and also because of their significant contribution to the global warming by methane emission in the atmosphere. Like other archaea of the family Methanomicrobiales, the members of the genus Methanoplanus are able to use CO2 and H2 as a source of carbon and energy; acetate is required for growth and probably also serves as carbon source. Here we describe the features of this organism, together with the complete genome sequence and annotation. This is the first complete genome sequence of a member of the family Methanomicrobiaceae and the sixth complete genome sequence from the order Methanomicrobiales. The 2,843,290 bp long genome with its 2,824 protein-coding and 57 RNA genes is a part of the Genomic Encyclopedia of Bacteria and Archaea project.
Keywords: obligately anaerobic, mesophilic, hydrogen, methane, Gram-negative, Methanomicrobiaceae, Euryarchaeota, GEBA
Introduction
Strain SEBR 4847T (= DSM 11571 = OCM 486) is the type strain of Methanoplanus petrolearius [1]. This strain was isolated from an offshore oil-producing well in the Gulf of Guinea, Africa [1]. Currently, the genus Methanoplanus contains three species: M. petrolearius, the type species M. limicola (isolated from an Italian swamp containing drilling waste near Baia in the Naples Area), and M. endosymbiosus (isolated from the marine ciliate Metopus contortus) [1]. The genus name derived from the Latin word “methanum”, and the adjective “planus”, meaning a flat plate, which refers to its flat cell morphology [1,2]. Methanoplanus therefore means “methane (-producing) plate”. The species epithet petrolearius derives from the Latin word “petra”, rock and the adjective “olearius”, which relates to vegetable oil [1]. “Petrolearius” means therefore related to mineral oil, referring to its origin of isolation [1]. No additional cultivated strains belonging to the species M. petrolearius have been described thus far. M. petrolearius SEBR 4847T is like other methanogens, strictly anaerobic. Here we present a summary classification and a set of features for M. petrolearius strain SEBR 4847T, together with the description of the complete genomic sequencing and annotation.
Classification and features
The type strains of the two other species in the genus Methanoplanus share an average of 93.5% 16S rRNA gene sequence identity with strain SEBR 4847T [1,2]. The 16S rRNA gene sequence of the strain SEBR 4847T shows 99% identity with an uncultured environmental 16S rRNA gene sequence of the clone KO-Eth-A (AB236050) obtained from the marine sediment [3]. The 16S rRNA gene sequences similarities of the strain SEBR 4847T to metagenomic libraries (env_nt) were all 83% or less, (status August 2010), indicating that members of the species, genus and even family are poorly represented in the habitats screened thus far.
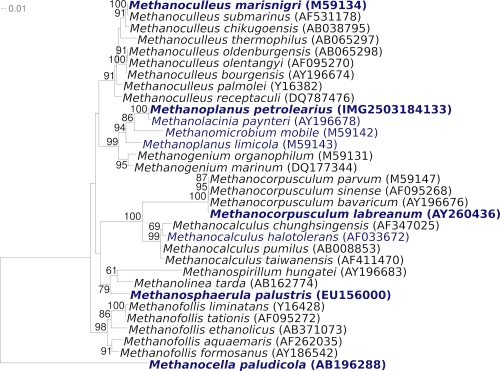
Figure 1 shows the phylogenetic neighborhood of M. petrolearius SEBR 4847T in a 16S rRNA based tree. The sequences of the two identical 16S rRNA gene copies in the genome do not differ from the previously published 16S rRNA sequence generated from DSM 11571 (U76631), which contained four ambiguous base calls.
Figure 1.
Phylogenetic tree highlighting the position of M. petrolearius SEBR 4847T relative to the other type strains within the order Methanomicrobiales. The tree was inferred from 1,275 aligned characters [4,5] of the 16S rRNA gene sequence under the maximum likelihood criterion [6] and rooted with Methanocellales [7]. The branches are scaled in terms of the expected number of substitutions per site. Numbers above branches are support values from 350 bootstrap replicates [8] if larger than 60%. Lineages with type strain genome sequencing projects registered in GOLD [9] are shown in blue, published genomes in bold [10,11] and GenBank accessions CP001338 (for Methanosphaera palustris E1-9c) and AP011532 (for Methanocella paludicola).
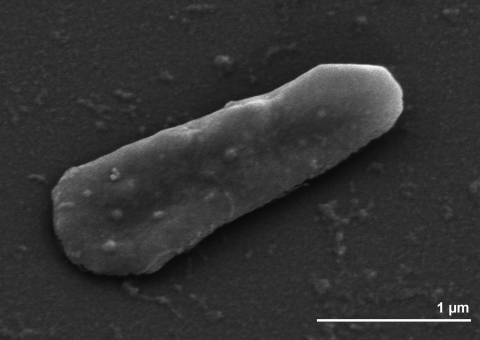
The cells of strain SEBR 4847T stain Gram-negative, but archaea do not have a Gram-negative type of cell wall with an outer envelope. Cells occur singly or in pairs and are irregularly disc-shaped of 1 to 3 µm size (Figure 2 and Table 1). A similar shape was found for two other strains of the genus Methanoplanus [1,2,24]. Strain SEBR 4847T was originally described as non-motile [1], however, in samples of this strain kept in the DSMZ culture collection motile cells were frequently detected in young cultures (H. Hippe, personal communication). The genome sequence of SEBR 4847T contains numerous genes encoding flagellins (Mpet_2052 - Mpet2054, Mpet_2057) and chemotaxis proteins (Mpet_2064 – Mpet_2069), which is in line with the observation of motility in this species. Round colonies of 1-2 mm are observed after three weeks of incubation on solid agar medium. The generation time of strain SEBR 4847T is about 10 hours under optimal conditions [1]. Strain SEBR 4847T grows optimally at 37°C, the temperature range for growth being 28-43°C. No growth was observed at 25°C or 45°C [1]. The optimum pH is 7.0; growth occurs from pH 5.3 to 8.4. The optimum NaCl concentration for growth is between 1 and 3% NaCl with growth occurring at NaCl concentrations ranging from 0 to 5% [1]. Substrates for growth of strain SEBR 4847T are H2 + CO2, formate and CO2 + 2-propanol [1]. Strain SEBR 4847T does not utilize methanol, trimethylamine, lactate, glucose, CO2 + 1-propanol, CO2 + 1-butanol and isobutyrate [1]. Acetate is required for growth as carbon source and yeast extract is stimulatory [1]. Addition of acetate reduces the lag time [25]. The addition of acetate slightly increases the amount of H2 available, theoretically [26,27]. When H2 is limiting and sulfate is in excess, sulfate reducers compete with methanogens and homoacetogens for the available H2 [27]. The sulfate reducers can out-compete hydrogenotrophic methanogens, due to a higher affinity [28] and higher activity of hydrogenase and the energetically more favorable reduction of sulfate [29]. Similar features were observed for M. limicola and M. endosymbiosus [1,2,24].
Figure 2.
Scanning electron micrograph of M. petrolearius SEBR 4847T
Table 1. Classification and general features of M. petrolearius SEBR 4847T according to the MIGS recommendations [12].
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Current classification | Domain Archaea | TAS [13] | |
| Phylum Euryarchaeota | TAS [14,15] | ||
| Class Methanomicrobia | TAS [16] | ||
| Order Methanomicrobiales | TAS [17-19] | ||
| Family Methanomicrobiaceae | TAS [17,18] | ||
| Genus Methanoplanus | TAS [2,20] | ||
| Species Methanoplanus petrolearius | TAS [1,21] | ||
| Type strain SEBR 4847 | TAS [1] | ||
| Gram stain | negative | TAS [2] | |
| Cell shape | disc-shaped, irregular single or in pairs | TAS [1] | |
| Motility | motile | IDA | |
| Sporulation | not reported | NAS | |
| Temperature range | 28-43°C | TAS [1] | |
| Optimum temperature | 37°C | TAS [1] | |
| Salinity | 1-3% NaCl | TAS [1] | |
| MIGS-22 | Oxygen requirement | anaerobic obligate | TAS [1] |
| Carbon source | acetate, CO2, formate | TAS [1] | |
| Energy source | H2 + CO2, formate and CO2 + 2-propanol | TAS [1] | |
| MIGS-6 | Habitat | offshore oil field | TAS [1] |
| MIGS-15 | Biotic relationship | not reported | NAS |
| MIGS-14 | Pathogenicity | not reported | NAS |
| Biosafety level | 1 | TAS [22] | |
| Isolation | subsurface ecosystem | TAS [1] | |
| MIGS-4 | Geographic location | offshore oil field, Gulf of Guinea, West Africa | TAS [1] |
| MIGS-5 | Sample collection time | 1997 or before | TAS [1] |
| MIGS-4.1 MIGS-4.2 |
Latitude Longitude |
not reported | NAS |
| MIGS-4.3 | Depth | not reported | NAS |
| MIGS-4.4 | Altitude | not reported | NAS |
Evidence codes - IDA: Inferred from Direct Assay (first time in publication); TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from of the Gene Ontology project [23]. If the evidence code is IDA, then the property was directly observed by one of the authors or an expert mentioned in the acknowledgements
Chemotaxonomy
At the time of writing, no reports have been published describing the composition of the cell envelope of the strain SEBR 4847T. However, for the two other species in the genus Methanoplanus, M. limicola and M. endosymbiosus, several chemotaxonomic features have been reported [2,24]. Preparations of the cell envelope from M. limicola and M. endosymbiosius revealed the presence of a dominant band that appeared to be a glycoprotein when cells were disrupted in 2% SDS [2,24]. Methanoplanus spp. possesses a mixture of C20C20 and C40C40 core ethers [30]. For comparison, similar mixtures were also detected in other members of the family Methanomicrobiaceae: Methanogenium cariaci, Methanogenium marisnigri and Methanogenium thermophilicum, while C20C25 was absent in these species [30].
Genome sequencing and annotation
Genome project history
This organism was selected for sequencing on the basis of its phylogenetic position [31], and is part of the Genomic Encyclopedia of Bacteria and Archaea project [32]. The genome project is deposited in the Genome OnLine Database [9] and the complete genome sequence is deposited in GenBank. Sequencing, finishing and annotation were performed by the DOE Joint Genome Institute (JGI). A summary of the project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Tree genomic libraries: 454 pyrosequence standard library, paired end 454 library (9.5 kb insert size), Illumina GAii shotgun library |
| MIGS-29 | Sequencing platforms | 454 GS FLX Titanium, Illumina GAii |
| MIGS-31.2 | Sequencing coverage | 67.9 × pyrosequence, 52.2 × Illumina |
| MIGS-30 | Assemblers | Newbler version 2.3-PreRelease-09-14-2009, Velvet, phrap |
| MIGS-32 | Gene calling method | Prodigal 1.4, GenePRIMP |
| INSDC ID | CP002117 | |
| Genbank Date of Release | September 17, 2010 | |
| NCBI project ID | 40773 | |
| GOLD ID | Gc01372 | |
| Database: IMG-GEBA | 2503128011 | |
| MIGS-13 | Source material identifier | DSM 11571 |
| Project relevance | Tree of Life, GEBA |
Growth conditions and DNA isolation
M. petrolearius SEBR 4847T, DSM 11571, was grown anaerobically in DSMZ medium 141 (Methanogenium medium) [33] at 37°C. DNA was isolated from 0.2 g of cell paste using a phenol/chloroform extraction after cell lysis with a mixture of lysozyme and mutanolysin.
Genome sequencing and assembly
The genome was sequenced using a combination of Illumina and 454 sequencing platforms. All general aspects of library construction and sequencing can be found at the JGI website (http://www.jgi.doe.gov/). Pyrosequencing reads were assembled using the Newbler assembler Version 2.3 Pre-Release-09-14-2009 (Roche). The initial Newbler assembly consisted of 21 contigs in one scaffold that was converted into a phrap assembly by making fake reads from the consensus sequence. Illumina GAii sequencing data (148.5Mb) was assembled with Velvet [34] and the consensus sequences were shredded into 1.5 kb overlapped fake reads and assembled together with the 454 data. The draft assembly was based on 173.4 Mb of 454 data and all of the 454 paired end data. Newbler parameters are -consed -a 50 -l 350 -g -m -ml 20. The Phred/Phrap/Consed software package (www.phrap.com) was used for sequence assembly and quality assessment of the genome sequence. After the shotgun stage, reads were assembled with parallel phrap (High Performance Software, LLC). Possible mis-assemblies were corrected with gapResolution (http://www.jgi.doe.gov/), Dupfinisher, or sequencing cloned bridging PCR fragments with subcloning or transposon bombing (Epicentre Biotechnologies, Madison, WI) [35]. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR primer walks (J.-F.Chang, unpublished). A total of 139 additional reactions were necessary to close gaps and to raise the quality of the finished sequence. Illumina reads were also used to correct potential base errors and increase consensus quality using a software Polisher developed at JGI [36]. The error rate of the completed genome sequence is less than 1 in 100,000. Together, the combination of the Illumina and 454 sequencing platforms provided 120.1× coverage of the genome. The final assembly of the genoe contains 590,575 pyrosequences and 4,125,153 Illumina reads.
Genome annotation
Genes were identified using Prodigal [37] as part of the Oak Ridge National Laboratory genome annotation pipeline, followed by a round of manual curation using the JGI GenePRIMP pipeline [38]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) nonredundant database, UniProt, TIGRFam, Pfam, PRIAM, KEGG, COG, and InterPro databases. Additional gene prediction analysis and functional annotation was performed within the Integrated Microbial Genomes - Expert Review (IMG-ER) platform [39].
Genome properties
The genome consists of a 2,843,290 bp long chromosome with a 47.4% GC content (Table 3 and Figure 3). Of the 2,881 genes predicted, 2,825 were protein-coding genes, and 57 RNAs; thirty nine pseudogenes were also identified. The majority of the protein-coding genes (61.2%) were assigned a putative function while the remaining ones were annotated as hypothetical proteins. The distribution of genes into COGs functional categories is presented in Table 4.
Table 3. Genome Statistics.
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 2,843,290 | 100.00% |
| DNA coding region (bp) | 2,501,893 | 87.99% |
| DNA G+C content (bp) | 1,347,696 | 47.40% |
| Number of replicons | 1 | |
| Extrachromosomal elements | 0 | |
| Total genes | 2,881 | 100.00% |
| RNA genes | 57 | 1.98% |
| rRNA operons | 2 | |
| Protein-coding genes | 2,824 | 98.02% |
| Pseudo genes | 39 | 1.35% |
| Genes with function prediction | 1,793 | 62.24% |
| Genes in paralog clusters | 550 | 19.10% |
| Genes assigned to COGs | 1,939 | 67.30% |
| Genes assigned Pfam domains | 2,000 | 69.42% |
| Genes with signal peptides | 492 | 17.10% |
| Genes with transmembrane helices | 886 | 30.75% |
| CRISPR repeats | 0 |
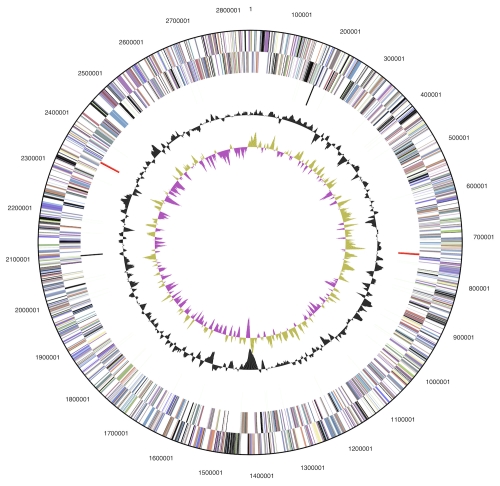
Figure 3.
Graphical circular map of the genome. From outside to the center: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, GC skew.
Table 4. Number of genes associated with the general COG functional categories.
| Code | value | %age | Description |
|---|---|---|---|
| J | 150 | 7.1 | Translation, ribosomal structure and biogenesis |
| A | 0 | 0.0 | RNA processing and modification |
| K | 106 | 5.0 | Transcription |
| L | 80 | 3.8 | Replication, recombination and repair |
| B | 2 | 0.1 | Chromatin structure and dynamics |
| D | 18 | 0.9 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.0 | Nuclear structure |
| V | 28 | 1.3 | Defense mechanisms |
| T | 136 | 6.5 | Signal transduction mechanisms |
| M | 67 | 3.2 | Cell wall/membrane/envelope biogenesis |
| N | 54 | 2.6 | Cell motility |
| Z | 1 | 0.0 | Cytoskeleton |
| W | 0 | 0.0 | Extracellular structures |
| U | 32 | 1.5 | Intracellular trafficking and secretion, and vesicular transport |
| O | 80 | 3.8 | Posttranslational modification, protein turnover, chaperones |
| C | 185 | 8.8 | Energy production and conversion |
| G | 70 | 3.3 | Carbohydrate transport and metabolism |
| E | 155 | 7.4 | Amino acid transport and metabolism |
| F | 61 | 2.9 | Nucleotide transport and metabolism |
| H | 162 | 7.7 | Coenzyme transport and metabolism |
| I | 22 | 1.1 | Lipid transport and metabolism |
| P | 143 | 6.8 | Inorganic ion transport and metabolism |
| Q | 7 | 0.3 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 278 | 13.2 | General function prediction only |
| S | 267 | 12.7 | Function unknown |
| - | 942 | 32.7 | Not in COGs |
Acknowledgements
We would like to gratefully acknowledge the help of Maren Schröder (DSMZ) for growing cultures of M. petrolearius. This work was performed under the auspices of the US Department of Energy Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396, UT-Battelle and Oak Ridge National Laboratory under contract DE-AC05-00OR22725, as well as German Research Foundation (DFG) INST 599/1-2.
References
- 1.Ollivier B, Cayol JL, Patel BKC, Magot M, Fardeau ML, Garcia JL. Methanoplanus petrolearius sp. nov., a novel methanogenic bacterium from an oil-producing well. FEMS Microbiol Lett 1997; 147:51-56 10.1111/j.1574-6968.1997.tb10219.x [DOI] [PubMed] [Google Scholar]
- 2.Wildgruber G, Thomm M, König H, Ober K, Ricchiuto T, Stetter KO. Methanoplanus limicola, a plate-shaped methanogen representing a novel family, the Methanoplanaceae. Arch Microbiol 1982; 132:31-36 10.1007/BF00690813 [DOI] [Google Scholar]
- 3.Sakai S, Imachi H, Sekiguchi Y, Tseng IC, Ohashi A, Harada H, Kamagata Y. Cultivation of methanogens under low-hydrogen conditions by using the coculture method. Appl Environ Microbiol 2009; 75:4892-4896 10.1128/AEM.02835-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 2000; 17:540-552 [DOI] [PubMed] [Google Scholar]
- 5.Lee C, Grasso C, Sharlow MF. Multiple sequence alignment using partial order graphs. Bioinformatics 2002; 18:452-464 10.1093/bioinformatics/18.3.452 [DOI] [PubMed] [Google Scholar]
- 6.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web Servers. Syst Biol 2008; 57:758-771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 7.Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glöckner FO, Rosselló-Móra R. The all-species living tree project: A 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 2008; 31:241-250 10.1016/j.syapm.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A. How many bootstrap replicates are necessary? Lect Notes Comput Sci 2009; 5541:184-200 10.1007/978-3-642-02008-7_13 [DOI] [PubMed] [Google Scholar]
- 9.Liolios K, Mavromatis K, Tavernarakis N, Kyrpides NC. The genomes on line database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2008; 36:D475-D479 10.1093/nar/gkm884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson IJ, Sieprawska-Lupa M, Lapidus A, Nolan M, Copeland A, Glavina del Rio T, Tice H, Dalin E, Barry K, Saunders E, et al. Complete genome sequence of Methanoculleus marisnigri Romesser et al. 1981 type strain JR1. Stand Genomic Sci 2009; 1:189-196 10.4056/sigs.32535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson IJ, Sieprawska-Lupa M, Goltsman E, Lapidus A, Copeland A, Glavina del Rio T, Tice H, Dalin E, Barry K, Pitluck S, et al. Complete genome sequence of Methanocorpusculum labreanum type strain Z. Stand Genomic Sci 2009; 1:197-203 10.4056/sigs.35575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.List Editor Validation List no. 85. Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol 2002; 52:685-690 10.1099/ijs.0.02358-0 [DOI] [PubMed] [Google Scholar]
- 15.Garrity GM, Holt JG. The Road Map to the Manual. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 119-169. [Google Scholar]
- 16.Garrity GM, Bell JA, Lilburn T. Taxonomic Outline of the Procaryotes., Bergey's Manual of Systematic Bacteriology, Second Edition. Release 4.0, Fourth Edition, Springer-Verlag, New York, 2003. p. 1-39. [Google Scholar]
- 17.List 6. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol 1981; 31:215-218 10.1099/00207713-31-2-215 [DOI] [Google Scholar]
- 18.Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS. Methanogens: Reevaluation of a unique biological group. Microbiol Rev 1979; 43:260-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judicial Commission of the International Committee on Systematics of Prokaryotes The nomenclatural types of the orders Acholeplasmatales, Halanaerobiales, Halobacteriales, Methanobacteriales, Methanococcales, Methanomicrobiales, Planctomycetales, Prochlorales, Sulfolobales, Thermococcales, Thermoproteales and Verrucomicrobiales are the genera Acholeplasma, Halanaerobium, Halobacterium, Methanobacterium, Methanococcus, Methanomicrobium, Planctomyces, Prochloron, Sulfolobus, Thermococcus, Thermoproteus and Verrucomicrobium, respectively. Opinion 79. Int J Syst Evol Microbiol 2005; 55:517-518 10.1099/ijs.0.63548-0 [DOI] [PubMed] [Google Scholar]
- 20.List 14. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol 1984; 34:270-271 10.1099/00207713-34-2-270 [DOI] [Google Scholar]
- 21.List Editor Validation list 67. Validation of publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol 1998; 48:1083-1084 10.1099/00207713-48-4-1083 [DOI] [PubMed] [Google Scholar]
- 22.Classification of bacteria and archaea in risk groups. http://www.baua.de TRBA 466.
- 23.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruggen JJA, Zwart KB, Hermans JGF, VanHove EM, Stumm CK, Vogels GD. Isolation and characterization of Methanoplanus endosymbiosus sp. nov., an endosymbiont of the marine sapropelic ciliate Metopus contortus Quennerstedt. Arch Microbiol 1986; 144:367-374 10.1007/BF00409886 [DOI] [Google Scholar]
- 25.Wu SY, Chen SC, Lai MC. Methanofollis formosanus sp. nov., isolated from a fish pond. Int J Syst Evol Microbiol 2005; 55:837-842 10.1099/ijs.0.63475-0 [DOI] [PubMed] [Google Scholar]
- 26.He J, Sung Y, Dollhopf ME, Fatherpure BZ, Tiedje JM, Löffler FE. Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ Sci Technol 2002; 36:3945-3952 10.1021/es025528d [DOI] [PubMed] [Google Scholar]
- 27.Weijma J, Gubbels F, Hulshoff LW, Stams AJM, Lens P, Lettinga G. Competition for H2 between sulfate reducers, methanogens and homoacetogens in a gas-lift reactor. Water Sci Technol 2002; 45:75-80 [PubMed] [Google Scholar]
- 28.Boone DR, Bryant MP. Proprionate-degrading bacterium, Syntrophobacter wolinii sp.nov. gen.nov., from methanogenic ecosystems. Appl Environ Microbiol 1980; 40:626-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupton FS, Zeikus JG. Physiological basis for sulfate-dependent hydrogen competition between sulfidogens and methanogens. Curr Microbiol 1984; 11:7-11 10.1007/BF01567568 [DOI] [Google Scholar]
- 30.Grant WD, Pinch G, Harris JE, Rosa MD, Gambacorta A. Polar lipids in methanogen taxonomy. J Gen Microbiol 1985; 131:3277-3286 [Google Scholar]
- 31.Klenk HP, Göker M. En route to a genome-based classification of Archaea and Bacteria? Syst Appl Microbiol 2010; 33:175-182 10.1016/j.syapm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 2009; 462:1056-1060 10.1038/nature08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.List of growth media used at DSMZ: http://www.dsmz.de/microorganisms/media_list.php
- 34.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims D, Brettin T, Detter J, Han C, Lapidus A, Copeland A, Glavina Del Rio T, Nolan M, Chen F, Lucas S, et al. Complete genome sequence of Kytococcus sedentarius type strain (541T). Stand Genomic Sci 2009; 1:12-20 10.4056/sigs.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapidus A, LaButti K, Foster B, Lowry S, Trong S, Goltsman E. POLISHER: An effective tool for using ultra short reads in microbial genome assembly and finishing. AGBT, Marco Island, FL, 2008. [Google Scholar]
- 37.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal Prokaryotic Dynamic Programming Genefinding Algorithm. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pati A, Ivanova N, Mikhailova N, Ovchinikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: A gene prediction improvement pipeline for microbial genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]
- 39.Markowitz VM, Ivanova NN, Chen IMA, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271-2278 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]