Abstract
Background and Purpose
The objective of this study was to determine the relationship between chronic kidney disease (CKD), race–ethnicity, and vascular outcomes.
Methods
A prospective, multiracial cohort of 3298 stroke-free subjects with 6.5 years of mean follow-up time for vascular outcomes (stroke, myocardial infarction, vascular death) was used. Kidney function was estimated using serum creatinine and Cockcroft-Gault formula. Cox proportional hazards models were fitted to evaluate the relationship between kidney function and vascular outcomes.
Results
In multivariate analysis, Cockcroft-Gault formula between 15 and 59 mL/min was associated with a significant 43% increased stroke risk in the overall cohort. Blacks with Cockcroft-Gault formula between 15 and 59 mL/min had significantly increased risk of both stroke (hazard ratio, 2.65; 95% CI, 1.47 to 4.77) and combined vascular outcomes (hazard ratio, 1.59; 95% CI, 1.10–2.92).
Conclusion
Chronic kidney disease is a significant risk factor for stroke and combined vascular events, especially in blacks.
Keywords: cardiac, chronic kidney disease, epidemiology, outcome, risk factors, stroke
Over the next decade, the worldwide epidemic of chronic kidney disease (CKD) will result in a continued increase in the number of individuals with decreased kidney function,1 a doubling in the number of patients with end-stage renal disease2 and a subsequent increase in morbidity and mortality related to CKD complications. Increased risk of cardiovascular disease (CVD) and CVD-related mortality is an important complication of CKD. As kidney function decreases, CVD risk increases exponentially; in the setting of other risk factors for CVD (ie, hypertension or diabetes), CKD confers a higher CVD risk than that in those patients without CKD.3 Although substantial investigations have been conducted on the relationship between CKD and CVD risk, it remains unclear if an independent association with stroke exists. Most investigations have included stroke as part of an aggregate cardiovascular end point, and studies that have evaluated stroke as a separate outcome have been conflicting.4,5
CVD epidemiology also differs by race–ethnicity;6,7 however, this relationship has not been completely elucidated in populations with CKD. In this regard, studies have noted that in patients with CKD, paradoxical relationships exist between CVD risk factors, race, and survival. Investigations of cardiovascular outcomes in Hispanics with CKD have demonstrated lower overall cardiovascular risk compared to non-Hispanics, although stroke risk was not independently investigated.8 Likewise, although blacks with CKD are known to have an increased risk of cardiovascular events compared to whites with CKD, the association for stroke is unclear.4,9 Therefore, it is particularly important to further investigate and identify race-specific risk and risk factors for stroke to promote more effective race-specific CVD risk reduction.
In this study, we used the multiethnic composition of the Northern Manhattan Study (NOMAS) to investigate the association between CKD and incident stroke and cardiovascular events, as well as the possible influences of race–ethnicity in modulating any such relationship.
Materials and Methods
Cohort
The NOMAS is a prospective population-based study designed to document the incidence of stroke, identify novel risk factors, and investigate stroke prognosis in a multiethnic urban community (63% Hispanic, 20% black, and 15% white). The methods of subject recruitment and enrollment into NOMAS have been described elsewhere.10 In brief, community participants were eligible if they: (1) never had stroke diagnosed; (2) were age 40 years or older; and (3) resided for at least 3 months in a household with a telephone in Northern Manhattan. Between 1993 and 2001, 3298 subjects were enrolled All subjects were followed-up annually by telephone starting in 1998 to gather information regarding incident cardiovascular events.10
Assessment of Kidney Function
Subjects were eligible for this investigation if they had laboratory data to assess kidney function, had a creatinine clearance (CCl) >15 mL/min, and were not using renal replacement therapy (dialysis or kidney transplant). Kidney function was determined by both serum creatinine (Scr) and CCl derived from the Cockcroft-Gault formula11:
Cockcroft-Gault CCl=(140–age)/(Scr)×(weight/72)×(0.85 for women).
CKD was defined as CCl between 15 and 59 mL/min.
Statistical Analyses
Statistical analyses were conducted on SAS 8.2 software (SAS Institute, Cary, NC). The Student t test or ANOVA were used to analyze continuous data, and χ2 test was used for categorical data. Univariate and multivariate Cox models were used to evaluate the association between kidney function and incident stroke (ischemic and hemorrhagic) and combined vascular events (stroke, MI, and vascular death). Confounding variables in multivariate analysis included age, gender, race–ethnicity, education, hypertension, serum LDL levels, diabetes, history of cardiac disease, cigarette smoking, and moderate alcohol consumption.
Results
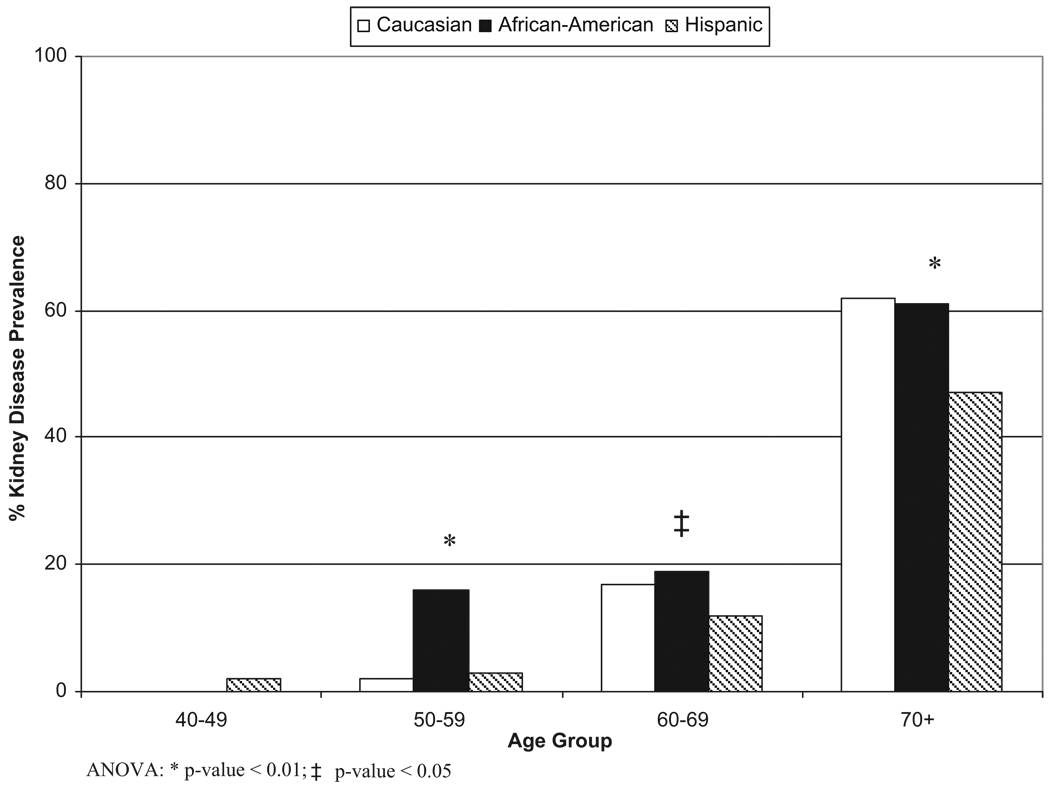
The NOMAS cohort included 3298 subjects. Baseline Scr levels were available for 3037 subjects; 23 subjects with a CCl <15 mL/min were excluded, bringing the cohort to 3014 subjects. Mean follow-up time was 6.5 years; mean age was 69 years; and most participants were women (63%). The group consisted of 52% Hispanics, 25% blacks, and 21% whites, and >32% had CKD. Table 1 describes the distribution of covariates by race–ethnicity. Hispanics had the lowest prevalence of kidney dysfunction. Hispanics and blacks had the highest prevalence of hypertension and diabetes, whereas whites had the highest prevalence of heart disease. The Figure shows the prevalence of CKD stratified by both race–ethnicity and age. Older Hispanics had significantly less CKD than age-stratified peers, and CKD prevalence in blacks notably exceeded that of other race–ethnic groups in the sixth decade.
Table 1.
Baseline Characteristics of Cohort by Race
| White (n=639) | Black (n=740) | Hispanic (n=1566) | P | |
|---|---|---|---|---|
| Events | ||||
| Stroke, N (%) | 43 (6.7) | 68 (9.2) | 89 (5.7) | <0.001 |
| Combined vascular, N | 142 (22.2) | 176 (23.8) | 191 (12.1) | <0.001 |
| Age, yr (SD) | 74 (10) | 72 (10) | 66 (9) | <0.001 |
| <0.001 | ||||
| Female, N (%) | 369 (58) | 500 (68) | 980 (63) | |
| Male, N (%) | 270 (42) | 240 (32) | 586 (37) | |
| Medical history | ||||
| Any heart disease, N (%) | 204 (32) | 168 (23) | 321 (21) | <0.001 |
| Hypertension, N (%) | 417 (65) | 586 (79) | 1167 (75) | <0.001 |
| Diabetes, N (%) | 93 (15) | 183 (25) | 371 (24) | <0.001 |
| Behavioral | ||||
| Former smoker, N (%) | 280 (44) | 269 (36) | 543 (35) | <0.001 |
| Current smoker, N (%) | 74 (12) | 165 (22) | 229 (15) | <0.001 |
| Moderate alcohol consumption, N (%) | 275 (43) | 234 (32) | 456 (29) | <0.001 |
| Completed high school, N (%) | 526 (82) | 469 (63) | 354 (23) | <0.001 |
| SBP, mm Hg, mean (SD) | 141 (20) | 147 (21) | 144 (21) | <0.001 |
| DBP, mm Hg, mean (SD) | 79 (11) | 84 (12) | 84 (11) | <0.001 |
| LDL, mg/dL, mean (SD) | 132 (35) | 126 (37) | 130 (36) | <0.023 |
| Kidney function parameters | ||||
| Prevalence of CCI 15–59 mL/min, N (%) | 291 (46) | 319 (43) | 335 (21) | <0.001 |
| Creatinine, mg/dL, mean (SD) | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | <0.001 |
| Creatinine clearance, mL/min, mean (SD) | 68 (30) | 69 (27) | 80 (27) | <0.001 |
Figure.
Prevalence of kidney disease in the NOMAS cohort stratified by race-ethnicity and age.
There were 177 ischemic strokes, 24 intracerebral hemorrhages, 179 MI, and 285 vascular deaths documented. Hazard ratios for the association between CKD and incident stroke and combined vascular outcomes are summarized in Table 2. For the combined cohort in multivariate hazard analysis, Scr had a significant relationship with combined outcomes, as did CCl (15 to 59 mL/min for stroke). Inclusion of confounders attenuated the association between CKD and all outcomes for Hispanics and whites. For blacks, Scr and CCl remained significant predictors of incident stroke and combined cardiovascular events after adjustment. We did not find a significant interaction between race–ethnicity and CKD for either outcome.
Table 2.
Hazard Ratio for Outcomes in Patients in the Both Univariate and Multivariate Models
| Univariate Models | ||||
|---|---|---|---|---|
| Hazard Ratio (95% Cl) Serum Creatinine (per mg/dL) |
P | Hazard Ratio (95% Cl) CCI (15–59 mL/min) |
P | |
| Stroke | ||||
| All | 1.87 (1.45–2.43) | <0.001 | 1.95 (1.48–2.57) | <0.001 |
| Caucasian | 2.17 (0.82–5.70) | <0.117 | 1.56 (0.85–2.85) | <0.148 |
| Hispanic | 1.78 (1.26–2.53) | <0.001 | 1.65 (1.05–2.60) | <0.030 |
| Black | 1.81 (1.06–3.11) | 0.031 | 2.55 (1.56–4.18) | <0.001 |
| All vascular events | ||||
| All | 1.93 (1.65–2.25) | <0.001 | 2.36 (1.99–2.81) | <0.001 |
| White | 2.84 (1.73–4.67) | <0.001 | 1.96 (1.40–2.75) | <0.001 |
| Hispanic | 1.75 (1.36–2.24) | <0.001 | 2.08 (1.54–2.79) | <0.001 |
| Black | 1.88 (1.35–2.61) | <0.001 | 2.35 (1.74–3.19) | <0.001 |
| Multivariate Models* | ||||
| Hazard Ratio (95% Cl) Serum Creatinine (per mg/dL) |
P | Hazard Ratio (95% Cl) CCI (15–59 mL/min) |
P | |
| Stroke | ||||
| All | 1.38 (0.94–2.02) | <0.098 | 1.43 (1.02–2.02) | <0.040 |
| White | 0.91 (0.24–3.40) | <0.888 | 1.08 (0.50–2.34) | <0.847 |
| Hispanic | 1.33 (0.81–2.19) | <0.264 | 0.93 (0.54–1.60) | <0.803 |
| Black | 1.58 (0.85–2.95) | <0.150 | 2.65 (1.47–4.77) | <0.001 |
| All vascular events | ||||
| All | 1.34 (1.06–1.76) | <0.015 | 1.20 (0.97–1.50) | <0.098 |
| White | 1.55 (0.80–3.01) | <0.195 | 1.12 (0.73–1.72) | <0.595 |
| Hispanic | 1.24 (0.85–1.82) | <0.269 | 0.93 (0.65–1.33) | <0.686 |
| Black | 1.51 (1.00–2.27) | <0.051 | 1.59 (1.10–2.92) | <0.014 |
Adjusted for age, gender, education, hypertension, LDL cholesterol, diabetes, prevalent cardiac disease, smoking, and alcohol consumption.
Discussion
We found that in a multiethnic cohort, CKD maintained a robust association with CVD, even after adjustment for multiple risk factors causally related to both CKD and CVD. We also demonstrated that prevalence rates of CKD are not equivalent across different race-ethnic groups.
Differences in CVD-related morbidity and mortality among race–ethnic groups are of particular interest to this investigation. In multivariate analysis, blacks with CKD had a 2.65-fold increased risk of incident stroke and a 59% increased risk of incident vascular events, the former being a novel finding. The elevated burden of CVD in blacks compared to whites has been attributed to increased cardiovascular risk factor clustering in conjunction with their undertreatment.12 The addition of CVD risk factors to multivariate models did attenuate the association between CKD and combined CVD outcomes for blacks, suggesting a substantial burden of disease could be averted with better CVD risk factor control in this population. However, multivariate adjustment did not attenuate the relationship between CKD and stroke in blacks, suggesting a robust relationship with causal mechanisms that require further elucidation. It is also noteworthy that after multivariate adjustment, the increased association between CKD and risk of stroke and cardiovascular events for Hispanics was no longer significant. Other studies have demonstrated a lower risk of cardiovascular events compared to that of whites with CKD.8 However, those Hispanic populations were of different genetic admixture than predominantly Caribbean-Hispanics enrolled in our study.
This study has several limitations. NOMAS did not measure proteinuria, both a marker of CKD and a known risk factor for CVD, which may have resulted in an underestimation of the association between CKD and CVD. Furthermore, CKD classification was based on a single Scr measurement; thus, we were unable to assess the impact of changing kidney function on outcomes. Last, this study was not designed to measure the association between CKD and cardiovascular outcomes, and it may have been underpowered to determine the modulating effect of CKD on CVD risk across the three race–ethnic groups.
Despite these limitations, our data demonstrate the impact of CKD on CVD risk in an inner city population that is unique from other community-based cohorts with respect to race–ethnic composition, socioeconomic status, and access to medical care. This study is the first to our knowledge to measure CKD prevalence rates and to evaluate the association of CKD with CVD in the Caribbean-Hispanic community. Finally, our study clearly demonstrates that CKD is an independent risk for incident stroke and combined vascular events in blacks.
Conclusions
In a multiethnic, prospective, cohort study, we demonstrated that Caribbean-Hispanics had the lowest prevalence of CKD, that kidney dysfunction was associated with incident stroke and vascular events, and that CKD was an independent risk factor for incident stroke and vascular events in blacks. This has significant public health implications, especially in blacks, given the expected increase in CKD incidence and prevalence over the next decade.1,2 Further investigations are needed to determine the mechanisms by which CKD modulates stroke risk in this population. Until specific treatments are available that lower stroke risk in patients with CKD, aggressive control of traditional risk factors for stroke and CVD are recommended.
Acknowledgments
The authors thank the entire staff of the Northern Manhattan Study for their efforts, especially Janet DeRosa, Project Manager.
Sources of Funding
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (R01 NS 29993). Minesh Khatri was a research fellow supported by the Sarnoff Cardiovascular Research Foundation.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J AmSocNephrol. 2005;16:3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 3.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 5.Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67:224–228. doi: 10.1212/01.wnl.0000229099.62706.a3. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson RG, Watson RL, Davis CE, Barnes R, Brown S, Romm F, Spencer JM, Tyroler HA, Wu K. Racial differences in risk factors for atherosclerosis. The ARIC study. Atherosclerosis risk in communities. Angiology. 1997;48:279–290. doi: 10.1177/000331979704800401. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK. Risk factors for cardiovascular mortality in Mexican Americans and non-Hispanic whites. San Antonio heart study. Am J Epidemiol. 1990;131:423–433. doi: 10.1093/oxfordjournals.aje.a115517. [DOI] [PubMed] [Google Scholar]
- 8.Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17:2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 9.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a tri-ethnic cohort: The northern manhattan study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics CfDCaP, US Department of Health and Human Services. National health and nutrition examination survey (NHANES III) 1988–1994. 2006