Abstract
Dopamine-producing neurons fire with both basal level tonic patterns and phasic bursts. Varying affinities of the five dopamine receptors have led to a hypothesis that higher affinity receptors are primarily activated by basal level tonic dopamine, while lower affinity receptors may be tuned to be sensitive to higher levels caused by phasic bursts. Genetically modified mice provide a method to begin to probe this hypothesis. Here we discuss three mouse models. Dopamine-deficient mice were used to determine which behaviors require dopamine. These behaviors were then analyzed in mice lacking D1 receptors and in mice with reduced phasic dopamine release. Comparison of the latter two mouse models revealed a similar failure to learn about and respond normally to cues that indicate either a positive or negative outcome, giving support to the hypothesis that phasic dopamine release and the D1 receptor act in the same pathway. However, the D1 receptor likely has additional roles beyond those of phasic dopamine detection, because D1 receptor knockout mice have deficits in addition to what has been observed in mice with reduced phasic dopamine release.
Introduction
Dopamine (DA)-producing neurons comprise a major modulatory system in the brain that is important for motor function, arousal, motivation, emotion, learning, and memory. Not surprisingly, alterations in the DA system are linked to numerous neurological and psychiatric disorders ranging from Parkinson’s disease to schizophrenia (Goto et al. 2007; Halliday and McCann 2010). Decades of research have gone into working out the pathways and mechanisms of DA action in order to better understand how alterations in DA signaling produce these disease phenotypes. Although there has been tremendous progress, our understanding remains incomplete.
DA neurons have been classified into nine distinct nuclei: A8–A16 (reviewed by Björklund and Dunnett 2007); the A9 and A10 groups, or substantia nigra (SNc) and ventral tegmental area (VTA), comprise the nigrostriatal and mesolimbic dopamine circuits, respectively. DA neurons of the nigrostriatal pathway innervate the caudate and putamen, or dorsal striatum. DA neurons of the mesolimbic pathway innervate the ventral striatum, as well as other cortical and limbic structures including the hippocampus, prefrontal cortex and amygdala. Because each of these brain regions simultaneously modulates distinct aspects of behavior and because they are innervated by subpopulations of DA neurons that exhibit a variety of electrophysiological characteristics, ascertaining the contribution of DA to behavior is often a complicated process.
The modulatory functions of DA are mediated by five distinct DA receptors that are differentially expressed in the brain and are classified into two major categories, the D1-like and D2-like receptor families. All five DA receptors are G-protein coupled and modulate various intracellular signaling cascades that act to either suppress or enhance neuronal activity. The D2-like family of receptors include D2, D3, and D4 and are coupled to heterotrimeric G-proteins that include the Gi/o subunit. Activation of the D2-like family of receptors typically results in the inhibition of adenylyl cyclase, activation of K+ channels, and the modulation of numerous other ion channels resulting in the dampening neuronal excitability and the facilitation of long-term depression of excitatory synapses. In contrast, the D1-like receptors, including D1 and D5, are coupled to heterotrimeric G-proteins that include the Gs/olf subunit. Activation of the D1-like family of receptors typically results in the increased activity of adenylyl cyclase, induction of immediate early gene expression, and the modulation of numerous ion channels that overall enhance neuronal excitability and facilitate longterm potentiation of excitatory synapses. Although these functions of D2- and D1-like receptor signaling generally apply, they can vary depending on the cell type and brain region in which they are expressed.
Members of the D2-like receptor family reportedly have a higher affinity for DA compared to the D1-like family (Richfield et al., 1989; Missale et al., 1998). Based on this higher affinity, it is hypothesized that D2-like receptors can detect the low levels of tonic DA release (Goto et al., 2007). The D1 receptor (D1R) has the lowest affinity for DA, which is up to 10-fold lower than even the D5 receptor (Sunahara et al., 1991; Tiberi et al., 1991). Consequently, the D1 receptor is hypothesized to be preferentially activated by transient, high concentrations of DA mediated by phasic bursts of DA neuron activity (Goto and Grace, 2005; Grace et al., 2007).
Most DA neurons in the SNc and VTA fire action potentials in a slow, irregular manner (~ 2 Hz) when recorded in either anesthetized or freely behaving animals and adopt a pacemaker-like firing pattern in slice preparations (Grace and Bunney, 1984; Kang and Kitai, 1993; Marinelli,et al., 2006; Overton and Clark, 1997). Low-frequency irregular firing of DA neurons is proposed to tonically generate a low basal level of extracellular DA (Grace et al., 2007). Tonic DA signaling is thought to preferentially activate high-affinity DA receptors in select cell types to dampen neuronal activity that would otherwise be saturated by phasic increases in DA. Release of this suppression of neural activity is facilitated by transient dips in DA mediated by pauses in DA neuron activity, such as those observed following omission of anticipated reward (Schultz, 1998, 2007). Superimposed on the low-frequency, irregular firing pattern are bursts of activity reaching 10–20 Hz that are composed of two or more spikes (mean 3–5) having initial inter-spike intervals of less than 80 ms (Grace and Bunney, 1984). These bursts are driven by excitatory (primarily glutamatergic) inputs in response to salient environmental events (Overton and Clark 1991; Schultz 2007) and lead to transient elevations of synaptic DA, referred to as phasic DA release that can be readily detected by fast scan cyclic voltammetry (Clark et al., 2010). These higher concentrations of phasic DA would recruit low-affinity DA receptors and result in the facilitated activation of select populations of neurons within the nigrostriatal and mesolimbic circuits. An illustration of the dichotomous function of DA receptor signaling can be found in the striatal direct and indirect, (or Go and No Go) pathways (Frank and O’Reilly, 2006). Within these striatal circuits, tonic DA would suppress activation of D2 receptor-expressing neurons of the No Go pathway. During periods of phasic DA release (superimposed on tonic DA levels) the striatal Go pathway is recruited which would facilitate the engagement of goal-directed behavior. In contrast, when tonic DA levels are reduced, neurons in the No Go pathway are relieved of tonic DA suppression leading to a disengagement, or inhibition of ongoing behavior.
Considerable electrophysiological, pharmacological, genetic, and modeling data support the hypothesis of distinct behavioral functions of tonic and phasic DA signaling. Recent advances in genetic techniques have also contributed to our understanding of DA action. In this review, we summarize behavioral data from genetic models of DA synthesis, release, and signaling in an attempt to draw parallels and contrasts in behavioral phenotypes between these various models as they relate to the role of D1 receptor selectivity for phasic DA release. Because of limited space, we deliberately avoid discussing the effects of genetic manipulaitons on most drug-related behaviors, which are interesting but complicated. For instances in which the behaviors regulated by D1 receptor signaling are absent from the literature, or the reported results are equivocal, we provide our primary data from D1 receptor knockout (D1R KO) mice to allow a more thorough comparison with other mouse models.
Genetic Mouse Models of DA Signaling
Progress towards understanding DA function began decades before the arrival of genetically-altered mouse models as research tools. Pharmacological and lesion methods were used to gain insight into DA actions during this time. Pharmacological studies continue to be necessary for temporal resolution of DA receptor activity at various time points during behavior. Genetic manipulations allow for reliable inactivation of gene function with cellular and anatomical specificity. The advent of genetically engineered mice has brought additional information that could not be gained from pharmacology alone. This review investigates what has been learned about DA signaling from genetically engineered mice.
Dopamine-deficient (DD) mice
The use of 6-hydroxy-dopamine (6-OHDA) has been used extensively to examine the consequence of ablating DA neurons (Beal, 2001; Deumens, et al., 2002; Blandini, et al., 2008; Salamone et al., 2007; Ungerstedt, 1971). Though this lesion method can be made specific to DA neurons, killing the neuron destroys all other normal signaling of that cell and therefore cannot isolate the function of DA alone. To address the behaviors that require DA synthesis, we generated a mouse model in which the gene encoding Tyrosine hydroxylase (Th), the rate-limiting enzyme in catecholamine synthesis, was inactivated. To overcome the developmental requirement for norepinephrine (Thomas et al., 1995; Zhou et al., 1995a), the Th gene was targeted to the Dopamine β-hydroxylase (Dbh) locus allowing Th to still be expressed in all noradrenergic and adrenergic neurons (Zhou and Palmiter, 1995b). Dopamine-deficient (DD) mice (Th−/−; DbhTh/+) are born at expected frequencies and grow normally for about 10 days, after which they become hypoactive, adipsic and aphagic and perish by ~3 weeks without intervention. However, these mice can be maintained by a daily injection of L-3,4-dihydroxyphenylalanine (L-Dopa), which is taken up by neurons and converted to DA by L-aromatic amino acid decarboxylase. After injection of L-Dopa, DD mice become active for ~10 hr during which time they are capable of performing most behaviors. Alternatively, some locomotion can be restored by caffeine, allowing for assay of select behaviors in the absence of DA. Although DD mice are unable to synthesize DA, the firing pattern of DA neurons is normal, indicating that these neurons are “firing blanks” during the DA-depleted state (Robinson et al., 2004). This DD mouse model is unique in that it allows one to train or test the mice in either a DA-depleted or DA-replete state. DA neurons and their post-synaptic cells develop normally in absence of DA; however, DA receptors are hypersensitive to DA even though receptor abundance appears to be normal (Kim et al., 2002). This hypersensitivity can be reversed by semi-chronic administration of L-Dopa for a day or two (Kim et al., 2006). More recently, we introduced another DD mouse model (fsDD) in which the Th gene is inactive due to insertion of a stop cassette into the first intron. This Th gene can be reactivated by Cre recombinase mediated excision of the stop cassette (Hnasko et al., 2006). Utilizing viral-mediated expression of Cre recombinase to reactivate DA signaling in selected brain regions allows for functional mapping of DA sufficiency for a variety of behaviors (Darvas and Palmiter, 2009, 2010; Fadok et al., 2009; Hnasko et al., 2006; Robinson et al., 2007).
D1 receptor knockout (D1R KO) mice
D1R KO mice were engineered by either deleting part (Drago et al., 1994) or most of the D1 receptor coding region (Xu et al., 1994) from the genome. Both methods result in complete inactivation of the D1 receptor demonstrated by autoradiography with a labeled D1 receptor antagonist (Drago et al.,1994; Xu et al., 1994). Because most D1 receptor agonists and antagonists affect both the D1 and D5 receptors, D1R KO mice have the advantage of allowing one to examine specific D1 receptor-dependent behaviors.
Genetic inactivation of other DA receptors
Although this review primarily describes D1R KO mice, genetic disruptions of each of the remaining four DA receptors have also been generated and their behavioral consequences have been examined (Table 1, see Drago et al. 1998 for review). The phenotype of D3R, D4R or D5R KO mice are relatively mild compared to that of either D1R or D2R. In some cases, mice lacking combinations of DA receptors have been produced. Whereas all of the single KO lines survive, mice lacking both D1R and D2R fail to thrive and their phenotype resembles that of untreated DD mice (Kobayashi et al., 2004), suggesting that signaling by these two DA receptors is most important for survival.
Table 1.
Overview of DA and DA receptor-mediated behaviors
| DD | DAT:NR1KO | D1R KO | D2R KO | D3R KO | D4R KO | D5R KO | |
|---|---|---|---|---|---|---|---|
| Genotype(s) | Th−/−;DbhTh/+ and Thfs/fs;DbhTh/+ | Slc6a3+/Cre; Grin1Δ/lox | Drd1a−/− | Drd2−/− | Drd2−/− | Drd4−/− | Drd5−/− |
| Growth | Growth stops at 17 days | Normal (Zweifel 2009) | 20–25% smaller (Smith 1998) | 15% smaller (Baik 1995) | Normal (Accili 1996) | Normal (Rubenstein 1997) | Normal (Holmes 2001) |
| Locomotion | Hypoactive (Zhou 1995) | Normal (Zweifel 2009) | Hyperactive/Hypoactive (Kobayashi 2004) | Hypoactive (Kobayashi 2004) Normal speed, hypoactive overall (Kelley 1998) | Normal (Karasinska 2000; Chourbaji 2008) Hyperactive (Accili 1996) | Reduced novel response (Dulawa 1999) Hypoactive (Rubenstein 1997) | |
| Rearing | Attenuated (Kobayashi 2004) | Attenuated (Kobayashi 2004) (Baik 1995) | Enhanced (Karasinska 2000) | Attenuated (Rubenstein 1997) | Decreased (Holmes 2001) | ||
| Motor Reflexes | Normal (Zhou 1995) | Normal (Zweifel 2009) | Normal (Drago et al. 1994) | Normal (Baik 1995) | Normal (Accili 1996) | Normal (Holmes 2001) | |
| Motor Coordination | Impaired (Zhou 1995) | Normal (Zweifel 2009) | Impaired (Kobayashi 2004) | Impaired (Kobayashi 2004) (Baik 1995) | Normal (Accili 1996) (Karasinska 2000) | Enhanced (Rubenstein 1997) | Normal (Holmes 2001) |
| Swim Speed | Impaired (Darvas 2009, 2010) | Normal (Karasinska 2000) | Normal (Karasinska 2000) | ||||
| Anxiety | Increased (Zweifel, submitted) | Increased (Zweifel, submitted) | Reduced (Steiner 1998; Karasinska 2000) Normal (Chourbaji 2008) | Increased (Falzone 2002) | Normal (Holmes 2001) | ||
| Morris Water Maze | Absent (Darvas 2009) | Impaired (Zweifel 2009) | Absent (El-Ghundi 1999, Granado 2008, Karasinska 2000, Smith 1998, Xing 2010) | Normal (Karasinska 2000) | Normal (Holmes 2001) | ||
| Working Memory | Normal (Zweifel 2009) | Impaired (Glickstein 2002) | Normal (Chourbaji 2008) Impaired (Glickstein 2002) | ||||
| Pavlovian conditioning | Normal (Parker, 2010) | Impaired (Parker, 2010; Ortiz 2010) | |||||
| Instrumental Responding | Absent (Darvas 2009) | Impaired (Zweifel 2009) | Impaired/Absent | Normal (Thanos 2010) | |||
| Cued aversive learning | Absent (Fadok 2009; Darvas 2009) | Impaired (Zweifel 2009) | Impaired (Fadok 2009; Oritz, 2010) | Normal (Falzone 2002) | Normal (Holmes 2001) | ||
| Contextual Aversive Learning | Absent (Fadok 2009) | Present/Altered (Zweifel 2009) | Normal (El-Ghundi 2001) Impaired, (Ortiz, 2010) | Impaired (Fadok 2009) | Normal (Falzone 2002) | Normal (Holmes 2001) |
Mice with reduced burst-firing (NR1:DAT KO mice)
Burst firing by DA neurons and subsequent phasic DA release are driven in part by glutamate signaling through the NMDA-type glutamate receptors (NMDAR) (Overton and Clark, 1997). To determine the contribution of DA neuron burst-firing to DA-dependent behaviors, a mouse model was generated in which the Grin1 gene encoding an essential NR1 subunit of the NMDAR was conditionally inactivated selectively in DA neurons (Engblom et al., 2008; Zweifel et al., 2008). This was achieved by crossing mice containing a major portion of the Grin1 gene flanked by loxP sites (Tsien et al.,1996) with mice expressing Cre-recombinase under the control of endogenous DA transporter (DAT) locus, Slc6a3 (Zhuang et al., 2005). These mice are referred to as NR1:DAT KO mice and have a 60–70% reduction in DA neuron burst firing (Zweifel et al. 2009). These mice also have a >50% reduction in phasic DA release in response to unexpected rewards or electrical stimulation of a major source of glutamatergic input, the pendunculopontine tegmental nucleus (Parker et al., 2010; Zweifel et al., 2009). In addition to phasic DA neuron activity, NMDAR signaling in DA neurons is also important for synaptic scaling of AMPA receptor currents (Zweifel et al., 2008; Engblom et al.,2008). Thus, alterations in behavior observed in DAT-NR1 KO mice could also be due to a lack of activity-dependent synaptic plasticity
General Characterization of Mice with Disruptions of DA Signaling
Feeding, growth and survival
Global DA depletion drastically affects growth, feeding and survival. Although the DA neurons and their post-synaptic targets in the striatum develop normally in DD mice and the gross anatomy in the DD brain is similar to controls, the overall size of the mutant brain and the number of DA neurons is reduced (Palmiter 2008, Zhou and Palmiter 1995b). Due to the aphagic phenotype of DD mice, they require a daily injection of L-Dopa to eat and drink enough to survive (Zhou and Palmiter, 1995b). As DA levels diminish due to metabolism, the mice become hypoactive, bradykinetic and fail to engage in most goal-directed behaviors including normal feeding (Palmiter 2008).
D1R KO mice have a 20–25% reduction in body weight compared to littermate controls (Smith et al., 1998) and a 22% reduction in striatal volume (Xu et al., 1994). D1R KO mice need moist food at weaning to survive, after which they require no further intervention to thrive. Removal of both D1 and D2 receptors produces the same wasting phenotypes as DD mice, indicating that these two receptors both contribute to normal growth and feeding (Kobayashi et al. 2004). NR1:DAT KO mice have normal appearance, body weight and food intake (Zweifel et al. 2009).
Locomotor activity and motor coordination
General locomotor activity is typically measured as total distance traveled (usually by infrared light beam breaks in an activity chamber) during one or several 24-hr periods. Locomotor activity during the first few hours reflects behavioral activation in response to a novel environment; after that one observes circadian activity which predominates at night. Other motor activities (rearing, resting/sleeping, climbing, hanging) can also be monitored from video recordings.
During their DA-depleted state, DD mice are ~10% as active as wild-type controls and they are very slow to initiate movements (Zhou and Palmiter 1995b). However, their righting reflex is intact and they will swim to avoid drowning, although their swim speed is reduced and they generally do not direct their swimming efforts towards a visible escape platform (Darvas and Palmiter, 2010; Denenberg et al., 2004; Szczypka et al., 1999). DD mice fail on simple tests involving coordinated movement; they can maintain balance on a stationary rod for at least 3 min, but will fall off as soon as the rod begins to rotate (Zhou and Palmiter, 1995b).
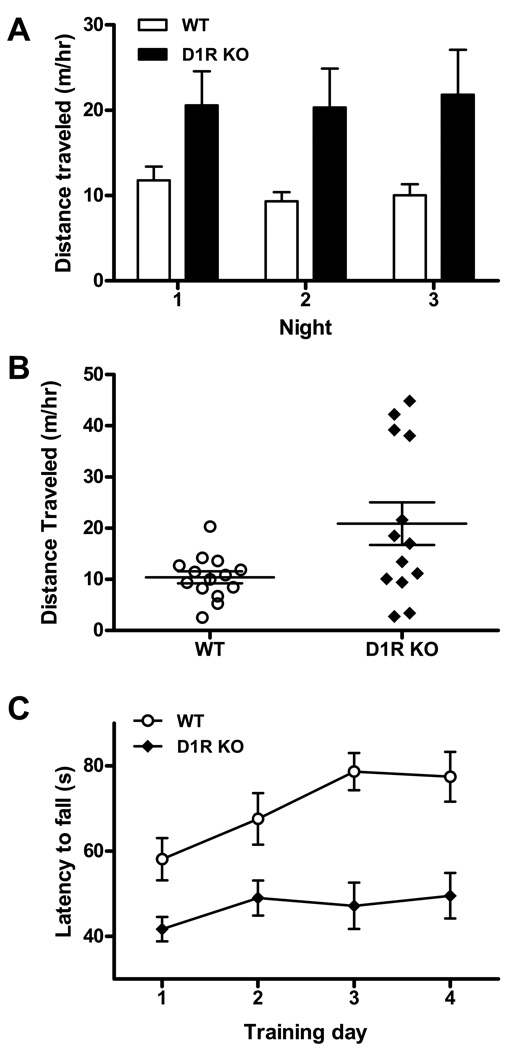
D1R KO mice have abnormal locomotor behavior and impaired motor coordination. Locomotor behavior ranges from hypoactive to hyperactive depending on the experimental conditions. Early experiments by Xu et al. (1994) showed that these mice were hyperactive in a novel environment and during the dark phase of the light-dark cycle. Granado et al. (2008) also reported hyperactivity in the same strain of D1R KO mice. Kobayashi et al. (2004) found that the strain of D1R KO mice generated by Drago et al. (1994) also had an enhanced locomotor response to novelty, but displayed reduced activity in a home cage. In contrast, Smith et al. (1998) noted an increased latency to move from the center of an open field, reflecting a hypoactive phenotype. To further explore the contribution of D1 receptors to novelty and activity during the light-dark cycle, we performed a 72-hr locomotion test. D1R KO mice (Drago et al. 1994 line; backcrossed onto C57/BL/6 > 10 generations) had an exaggerated locomotor response in the novel environment for the first 3 hr (p= 0.04), after which their activity closely resembled that of controls during each day (two-way repeated measures ANOVA; p= 0.147). However, during the dark phase, locomotion was significantly greater than that of control mice (Figure 1A, B). Analysis of night-time, locomotor data revealed that only a subset of D1R KO mice were responsible for this increase in activity. These high responders made up 31% of the total group, while the rest of D1R KO mice resembled wild-type mice. Given that the mice used in this study had undergone extensive backcrossing, the hyperactivity observed in this subset of mice is unlikely to be the result of genetic differences. Instead, this observation highlights the variability of locomotor responses by D1R KO mice that may underlie the prevalence of inconsistent reports on locomotor activity in these mice.
Figure 1. D1R KO mice have altered motor behavior.
(A) Locomotion for 13 D1R KO mice and 14 WT mice was monitored for 72 hr. A two-tailed t-test demonstrates that D1R KO mice were hyperactive each night (p=0.019), while displaying normal locomotion during each day (two-way repeated measures ANOVA F1,50 = 2.237; p=0.147). (B) Abnormal locomotion resulted from a subset of “high responders”. Night activity for 13 D1R KO mice and 14 WT mice was averaged over 3 nights. Four of the KO mice traveled ~4 times further than WT mice at night, while the rest were the same as controls (two-tailed t-test; p=0.502). (C) D1R KO mice (n = 11) and WT mice (n = 11) were tested in a 4-day accelerating rotarod paradigm (4–40 rpm over 120 sec; 3 trials/day). D1R KO mice had a generalized motor coordination deficit and attenuated motor learning. A Students t-test gave a significant difference between groups on the first day of training (p=0.009). Over days, two-way repeated measures ANOVA revealed significant effects of time (F3,80 = 3.189; p=0.0281) and genotype (F1,80 = 45.10; p < 0.0001). Further, one-way repeated-measures ANOVA shows that while WT performance improved during the experiment (F3,43 = 6.242; p=0.0020), D1R KO mice showed no improvement (F3,43 = 1.807; p=0.167).
D1R KO mice behaved normally in tests involving motor reflexes: righting reflex, placing and grasp reflexes as well as a simple coordination protocol involving walking along a wooden rod (Drago et al., 1994; El-Ghundi et al., 1999). However, they manifested deficits in complex coordinated behaviors (Kobayashi et al., 2004, Short et al., 2006), which appear to become more profound if the protocol included multiple training trials (Karasinska et al., 2000). To further investigate how training affects motor coordination in D1R KO mice, we monitored their performance on rotarod across 4 days. We found that D1R KO mice had a generalized motor coordination deficit on the first day of training (p= 0.009) and no improvement of performance with training (one-way repeated measures ANOVA, p= 0.167) (Figure 1C).
NR1:DAT KO mice had normal 24-hr locomotor activity and normal responses to novelty, drugs of abuse, and DA receptor-selective agonists (Zweifel et al., 2008). Motor coordination on an accelerating rotarod was also intact (Zweifel et al., 2009). Therefore, NMDAR-dependent phasic DA signaling, which contributes 60–70% of control phasic DA signaling, is not important for these motor activities.
Taken together, these locomotor experiments indicate that DA is essential for motor activity and coordination. However, genetic inactivation of any of the DA receptors individually does not result in greatly reduced locomotor activity (Table 1). Additionally, D1R KO mice are more active than control mice in response to a novel environment and during the dark cycle, when mice are normally active. Therefore, some combination of DA receptors, presumably both D1 and D2 receptors, is necessary to maintain normal locomotor activity. In contrast, general motor coordination during repeated training on an accelerating rotarod depends on D1R signaling but not NMDAR-dependent phasic DA release.
The Role of DA in Learning and Memory
As an animal explores its environment it acquires knowledge of important places and events that help to ensure its survival. This knowledge is thought to be stored as changes in synaptic efficacy in critical neuronal circuits. For example, long-term potentiation (LTP) allows subsequent excitations of the same synaptic pathway to elicit a greater post-synaptic response (reviewed by Bennett, 2000; Kauer and Malenka, 2007), whereas long-term depression (LTD) dampens post-synaptic responses. Studies in vitro and in vivo have demonstrated that D1 receptors are necessary for the induction of LTP and LTD and are sufficient for induction of LTP at some synapses (Centonze et al., 2001, 2003; Kerr and Wickens, 2001; Shen et al., 2008). Changes in dendritic spine morphology and density are strongly correlated with learning (reviewed by Leuner and Shors, 2004) and DA has been shown to influence these processes (Neely et al., 2007). While the contribution of DA learning has become widely recognized, the specific role of phasic DA signaling, the DA receptors that are involved, and the brain regions in which DA signaling is necessary for learning are just beginning to be deciphered. Genetic mouse models have proven an invaluable resource for unraveling these complex details.
T-Maze with food rewards
Because DD mice will not perform most tasks in a DA-depleted state, testing their ability to learn can be complicated. DD mice are particularly useful for investigating whether DA is necessary for the acquisition or performance of a reward-based learning task. For instance, training DD mice in a DA-depleted state and then monitoring their performance once DA is restored establishes whether DA is required for the acquisition phase of learning. In contrast, training DD mice in a DA-replete state and then testing them in the DA-depleted state will reveal the requirement of DA for the performance of a previously learned task. Robinson et al. (2005) employed such a strategy to ask whether DD mice could learn the location of a food reward in a simple T-maze task. The mice had to learn to turn into the right arm (always marked by horizontal stripes) to obtain a food reward. Thus, they could use either a cue- or response-based strategy to find the reward. DD mice were administered caffeine to stimulate movement and guided to the food reward if they did not find it at the end of each trial. After 100 trials (10 trials a day for 10 days) the DD mice showed no preference for the rewarded arm of the maze. In the second phase of the experiment these same mice were given L-Dopa just before testing for 10 additional days of training. On the first day of L-Dopa treatment they performed better than untrained DD mice that were given L-Dopa during their first training session. These observations indicate that the DD mice trained in the absence of L-Dopa must have learned the location of the reward when given only caffeine, even though they failed to express reward-directed behavior without DA. Although this experiment suggests that the DD-mice had learned the location of reward in the absence of L-dopa, it does not reveal how quickly they were able to form this association. The performance deficit of the DD mice in the absence of L-dopa cannot be explained by anhedonia since they have a normal preference for sweet (sugar or saccharin) solutions, even in the absence of DA (Cannon and Palmiter, 2003). Furthermore, all of the DD mice consumed all of the rewards they encountered, regardless of their DA state.
Mounting evidence specifically points to phasic DA signaling as being important for reward learning. The activity of DA neurons increases transiently in response to unexpected rewards, and this correlates with phasic DA release in the core of the nucleus accumbens (Schultz 2007). When reward delivery is repeatedly paired with a cue as during Pavlovian conditioning, the cue begins to elicit a phasic DA response (Schultz, 1998; 2007). The cue-elicited phasic DA response is thought to represent the learned incentive value of the predictive stimulus and is believed to be important for initiating behaviors for obtaining rewards (Berridge and Robinson 1998). The magnitude of the cue-elicited phasic DA response is influenced by the size of the reward, the time to reward delivery and the probability of receiving a reward (Fiorillo et al., 2003; 2008). In addition, the omission of an expected reward often results in an inhibition of DA neuron activity in well-trained animals. These observations suggest that phasic DA signaling is particularly important for using environmental cues to predict multiple aspects of reward availability.
The D1R KO mice had a profound deficit in the response-based, T-maze task. Analysis of correct trials by 2-way ANOVA revealed significant effects of genotype (p < 0.05), time (p <0.01) and time × genotype interaction (p < 0.01). Even after 100 trials, their performance was not equal to that of control mice (p < 0.05. Figure 2A). However, unlike the DD mouse model, it is not possible to dissociate acquisition and performance in D1R KO mice. Therefore, determining whether these mice are able to form the association is confounded by the known contribution of DA to the performance of goal-directed behaviors. Nonetheless, the deficit in D1R KO mice is unlikely due to decreased rewarding properties of the sucrose pellets because DA is not required to prefer sweet solutions and the D1R KO mice consumed all the sucrose rewards. Furthermore, D1R KO mice also had a normal preference for saccharin given a two-choice test (Figure 2B).
Figure 2. Appetitive learning is attenuated, while preference for sweet rewards remains intact.
(A) Learning in a T-maze for a food reward was attenuated in D1R KO mice. D1R KO mice (n = 9) and WT mice (n = 10) were food restricted to 85% body weight, then trained (10 trials/day) for 10 days to turn right to obtain a food reward. The correct arm was denoted by horizontal stripes (Zweifel et al. 2009). Two-way repeated measures ANOVA shows a significant effect of genotype (F1,153 = 6.32; p= 0.0223), time (F9,153 = 4.50; p <0.001) and time × genotype interaction (F9,153 = 2.62; p= 0.0076). One-way repeated-measures ANOVA with Bonferroni’s multiple comparison test shows that while WT mice learned the task by day 6 (p < 0.0001), D1R KO mice failed to acquire the task over 10 days (p=0.3341). (B) Saccharin preference was normal in D1R KO mice. D1R KO mice (n = 6) and WT mice (n = 7) were monitored for saccharin preference over 4 consecutive days. The first 2 days examined preference for 0.033% saccharin vs. H2O with no difference between genotypes (Students t-test; p=0.152). The final 2 days examined preference for 0.066% saccharin with no difference between genotypes (Students t-test; p=0.059). The locations of the saccharin and H2O bottles were alternated each day. (C) Instrumental conditioning is attenuated in D1R KO mice. D1R KO mice (n = 7) and WT mice (n = 8) were trained on a simple fixed-ratio schedule in which each lever press delivered a single food pellet up to a maximum of 50 (Zweifel et al., 2009). Two-way repeated- measures ANOVA shows an effect of both day (F6,91 = 6.284; p < 0.0001) and genotype (F1,91 = 498.1; p < 0.0001).
NR1:DAT KO mice reveal that phasic DA signaling modulates the acquisition of both a response-based and cue-based T-maze tasks (Zweifel et al., 2009). The NR1:DAT KO mice were slower to acquire the simple, response-based T-maze task, although their performance was equivalent to controls after 100 trials. However, when the T-maze task required the NR1:DAT KO mice to use a cue presented in a pseudo-random order to find the food reward, they performed poorly and never reached the performance of control mice (Zweifel et al., 2009). This latter finding specifically implicates phasic DA in cue-based learning strategies.
Instrumental and Pavlovian conditioning for food rewards
Instrumental conditioning paradigms assess whether a mouse can learn to associate an action with a reward. A typical instrumental paradigm requires a mouse to press a lever for the delivery of a food reward. Instrumental conditioning not only requires the animal to form an association between the lever-press and the delivery of the food pellet, but it also requires the animal to be motivated to work to obtain the reward. More complex paradigms may require mice to discriminate between a correct and an incorrect lever and may impose delays for incorrect trials (during which the correct lever will no longer deliver a food reward).
Pavlovian conditioning measures the degree to which a mouse can form an association between a cue and a food reward. Unlike instrumental conditioning, the mouse is not required to perform any work to obtain the food reward. In our paradigm, learning is measured by the conditioned approach behavior of the mouse to the food delivery receptacle after training. Once an animal has learned to associate the previously neutral stimulus with the food reward, their head-entry rate to the food receptacle is selectively increased in the presence of the cue.
DD mice were unable to master a simple instrumental task in which they had to press either of two levers to obtain a food pellet (Robinson et al., 2007). However, given L-Dopa to restore DA everywhere, or after restoration of DA signaling in only the dorsal striatum by viral gene therapy they were capable of learning the task (Robinson et al., 2007; Darvas and Palmiter 2009, 2010). Although DD mice have not been tested in the Pavlovian task, it is unlikely that they would learn this task given that D1R KO mice fail to acquire Pavlovian-conditioned approach (see below).
Given the poor performance of D1R KO mice in a T-maze experiment, it is not surprising that D1R KO mice also performed poorly in a simple instrumental conditioning task, even after 7 days of training (Figure 2C). Other groups have examined D1R KO mice in slightly different instrumental paradigms. Olsen et al. (2009) reported that D1R KO mice could learn to discriminate between active and inactive levers normally; however, two groups reported that D1R KO mice had decreased preference for the active lever and reduced overall responses (Caine et al., 2007; El-Ghundi et al., 2003). In those experiments in which D1R KO mice displayed some learning, liquid food or sucrose was used as the reinforcer and longer training protocols were used than in our experiment. These distinctions may have promoted the higher degree of responding observed in those experiments. In addition to a deficit in instrumental conditioning, D1R KO mice were also unable to learn in a simple Pavlovian conditioning task (Parker et al. 2010) or a conditioned eye-blink task (Ortiz et al., 2010). The failure of D1R KO mice to learn to eye blink in response to a cue was associated with reduced hippocampal LTP (Ortiz et al., 2010). Our results support the conclusion that the D1 receptor is essential for both instrumental and Pavlovian conditioning.
NR1:DAT KO mice were slow to learn to lever-press for food rewards although they eventually performed equivalently to control mice (Zweifel et al., 2009). NR1:DAT KO mice also had similar motivation to work for food, as the maximum number of lever presses they would perform (their breakpoint) was the same as controls (Zweifel et al., 2009). Using the same Pavlovian task as in the D1R KO mouse experiment, NR1:DAT KO mice learned normally (Parker et al. 2010). Although phasic DA release in the nucleus accumbens core was greatly attenuated in the NR1:DAT KO mice during Pavlovian conditioning, they still developed enhanced DA release in response to the cue during training (Parker et al. 2010). In another Pavlovian task, conditioned place preference (CPP), NR1:DAT KO mice were slow to form a preference for a food-paired context (Zweifel et al., 2009). CPP for food is difficult to test for DD mice because they eat so little, and it has not been reported for D1R KO mice.
The results from the T-maze, instrumental and Pavlovian experiments are consistent with a role for DA in appetitive learning. DA signaling is not necessary to learn the very simplest T-maze task although DD mice will not manifest their learning in the absence of DA. D1 receptors are important for learning the simplest T-maze task. When D1R KO mice were required to make an instrumental response to obtain a food reward, they performed poorly in our task, despite being capable of discriminating active and inactive levers in similar tasks in which they were given more training. Still, they remained impaired in most reported instrumental paradigms. Because DA neurons are activated by unexpected food rewards and by cues that predict them, and because this activation depends upon signaling via NMDARs, we predicted that NR1:DAT KO mice would have difficulty learning to associate cues with reward delivery. This prediction was not borne out in our Pavlovian conditioning experiments, which suggests that residual phasic release of DA was sufficient to learn in this simple paradigm. However, in more difficult learning paradigms, NR1:DAT KO mice performed worse than controls. These mice were slower to master the response-based T-maze and instrumental tasks. Furthermore, they did not perform as well as controls in a cue-based, T-maze task, indicating that optimal phasic DA release facilitates learning in more difficult cue-based paradigms. Thus, we hypothesize that NMDAR-dependent phasic DA release and activation of D1 receptors are important for assigning value to cues during learning. In support of this hypothesis, a recent optogenetic experiment designed to precisely control the activity of DA neurons in vivo has shown that bursting of VTA DA neurons alone was sufficient to induce conditioned place preference in the absence of a reward (Tsai et al., 2009).
Water-based escape tasks
Although mice are able to swim, they will readily escape onto a visible platform, suggesting that swimming is a mildly aversive experience. Various water mazes have been used to address learning in the absence of reward. The simplest water escape task is a straight alley with a visible platform at one end. Such a task is used to assess an animal’s motivation and ability to swim and to escape from the water. The time it takes a mouse to swim to the platform (escape latency) gradually decreases with training, reflecting a learned behavioral response. DD mice in their depleted state will swim randomly and rarely approach the platform. At the end of each trial the DD mice are placed on the platform for 20 s. Despite not swimming to the platform the DD mice had learned its location as demonstrated by their more rapid escape latency when given L-Dopa compared to untrained DD mice given L-Dopa in their first session (Denenberg 2004). Thus, as in the appetitive T-maze task, the DD mice were able to learn the location of the platform, but could not manifest their learning in the absence of DA signaling.
A more complicated version of the straight alley swim task is a water-based, U-maze, which is essentially a T-maze with the two arms bent back so that the escape platform is not visible from the choice point. Mice can be trained with either response- or cue-based escape contingencies. Once the animal has learned to locate the escape platform, the contingency can be switched in order to assess their ability to change escape strategies. The behavioral flexibility needed to master a shift in response strategy is thought to rely on executive function and cortical inputs (Birrell and Brown 2000). DD mice were unable to learn the location of an escape platform in a cue-based paradigm, even if given L-Dopa after extensive training (Darvas and Palmiter, 2009, 2010).
The most popular water maze is the Morris water maze, which is used to assess spatial learning ability. In the Morris water maze, a platform is submerged in a circular pool, and the mouse has to use visual cues surrounding the pool to navigate to the platform. Spatial learning has been shown to depend upon the hippocampus, and during the exploration of a novel environment, DA levels increase in this brain region (Ihalainen et al., 1999). Because the hippocampus contains both D1R and D5R (Laurier et al., 1994; Tiberi et al., 1991), DA release in this brain structure could facilitate the formation of new spatial memories and require one or both of these DA receptors. DD mice failed to learn in a Morris water maze. Although supplementing the DD mice with caffeine increased their swim speed, they were still unable to learn the location of the platform, even if given L-Dopa after extensive training (Darvas and Palmiter, 2009, 2010).
D1R KO mice are capable of swimming and have normal or even elevated swim speeds in water tasks such as Morris water maze (El-Ghundi et al., 1999; Granado et al., 2008; Karasinska et al. 2000; Smith et al., 1998; Xing et al. 2010). When we tested D1R KO mice in a straight-alley swim task, they were slightly faster to find the platform than controls; however, in contrast to controls, D1R KO mice failed to improve their performance with training (data not shown). D1R KO mice were slower to learn a response-based strategy in a U-maze, they had a deficit in reversing from a turn-left strategy to a turn-right strategy, and they were slower to shift from a response-based to a cue-based strategy than control mice. Yet, given enough training, D1R KO mice could learn all U-maze tasks (Darvas et al., unpublished). D1R KO mice also have severely attenuated learning in the Morris water maze. In some cases, D1R KO mice were able to improve slightly during training trials (El-Ghundi et al., 1999; Granado et al., 2008), yet probe trials revealed no retention of this spatial memory (El-Ghundi et al., 1999; Granado et al., 2008; Karasinska et al. 2000; Smith et al., 1998; Xing et al. 2010). In some paradigms D1R KO mice learned a cued-version of the Morris water maze in which a flag denotes the location of the platform (El-Ghundi et al., 1999; Granado et al., 2008; Xing et al. 2010); however, others reported no learning in a similar version of the task (Karasinska et al., 2000; Smith et al., 1998). Our results with the straight alley swim task with a visible platform confirm this latter result. The deficits observed by D1R KO in these water-based escape tasks is also observed in dry Barnes maze, in which the animal learns to escape from open field into a tunnel, suggesting that the stress of being in the water is not a key factor (Ortiz et al., 2010). In support of D1 receptor mediation of spatial learning, recording from hippocampal place cells in the CA1 region showed that these cells had difficulty adjusting to changes in distal cues in D1R KO mice, an effect that was exacerbated in a novel environment (Tran et al., 2008). Thus, normal signaling through D1 receptors in the hippocampus appears to be modulate the encoding of spatial information.
NR1:DAT KO mice had no difficulty learning a simple straight-alley water escape task (Zweifel et al., 2009). They also learned a response-based, working-memory task in which the mouse is forced to choose one arm in a water-based U-maze, after which the mouse must choose the opposite arm in a free-choice trial to find a hidden platform (Zweifel et al., 2009). In contrast, when tested in a Morris water maze, these mice were slower to learn the location of the platform compared to control mice. However, once learned, they could recall the former location of the platform normally during a probe trial (Zweifel et al., 2009). The learning deficit in NR1:DAT KO mice in a Morris water maze indicates that NMDAR-dependent phasic DA release facilitates the acquisition of a spatial memory.
Results from DD mice indicate that DA is not only necessary for performance of a simple escape task, but it is also required for acquisition of cue-based and spatial-learning tasks. D1R KO mice had severe impairments in the acquisition of all response-based and cue-based water maze paradigms. However, their performance in some of these tasks improved with extensive training. Spatial learning tasks appear to be sensitive to both disruptions in D1 receptors and phasic DA signaling. As with reward learning, absence of D1 receptors produced a similar, but more pronounced learning deficit compared to NR1:DAT KO mice. Taken together, these data implicate DA signaling in the acquisition and performance of water-maze learning. Acquisition seems to be specifically dependent upon D1R signaling. Spatial learning, which uses diffuse predictive cues, appears to be particularly sensitive to the disruption of phasic DA.
Learning about aversive events
During fear conditioning, an animal learns to associate a context or cue with an aversive stimulus. Disruption of normal fear processing can manifest as phobias, post-traumatic stress and anxiety. DA is thought to play an important role in learning about aversive stimuli (Pezze et al., 2004 review). Altered DA neuron activity in response to aversive outcomes such as a shock, pinch, or an air puff directed at the face has been documented (Brischoux et al., 2009; Coizet et al., 2006; Joshua et al., 2008; Mantz et al., 1989; Matsumoto and Hikosaka 2009; Schultz and Romo, 1987; and Zweifel et al., submitted). When an aversive outcome is paired with a cue, behavioral and neuronal responses emerge in response to the cue as it becomes predictive of the aversive stimulus. It has been reported that a subset of DA neurons showed enhanced activity in response to a predictive cue as well as to the aversive outcome it predicted (Joshua et al., 2008; Matsumoto and Hikosaka, 2009). Additionally, DA has been demonstrated to facilitate long-term potentiation in key limbic brain areas important for fear conditioning (Brissière et al. 2003; Lemon and Manahan-Vaughan, 2006; Swant and Wagner, 2006). These observations provide strong evidence for the involvement of DA in Pavlovian fear conditioning.
Previous work on fear processing with primates and rats provides an extensive framework for a more detailed examination of the circuitry involved in fear conditioning. Here we review work using genetic mouse models. A commonly used paradigm used to assess Pavlovian fear conditioning is fear-potentiated startle. This paradigm is based on monitoring the animal’s natural acoustic startle response. After multiple pairings of a cue with a foot-shock, an animal will manifest an enhanced acoustic startle response in the presence as compared to the absence of the cue, a phenomenon referred to as fear-potentiated startle (FPS). The acoustic startle response is a reflex, and is independent of locomotion, making it an ideal paradigm for assessing aversive learning in the DD mouse.
DD mice in their depleted state are unable to learn FPS; however, they can learn it if L-Dopa is administered immediately after training (Fadok et al., 2009). There appears to be a stringent temporal requirement for DA, since administering L-Dopa >1hr later does not allow learning by DD mice. Once learned, DD mice can express FPS normally in the absence of DA signaling. These experiments suggest that DA is involved in the stabilization of fear memory, but is not required for the retrieval or expression of a learned fear response (Fadok et al., 2009). Subsequent experiments have indicated that simultaneous viral restoration of DA signaling to both the ventral striatum and the amygdala of fsDD mice is sufficient to restore normal FPS (Fadok et al., 2009, 2010).
Both D1R KO mice (Fadok et al., 2009) and NR1:DAT KO mice (Zweifel et al., 2009) fail to manifest FPS. Unlike DD mice, these two mouse models had elevated startle responses to both the cued and no-cued conditions. Further analysis of NR1:DAT KO mice has revealed that this enhanced potentiation of the acoustic startle reflex in the absence of the conditioned cue is context-independent (Zweifel et al., submitted) suggesting that attenuating NMDAR-dependent phasic DA results in a generalized fear phenotype.
In contextual fear conditioning, an animal learns to associate a particular environment with a shock, and learning is assessed by monitoring freezing behavior when the animal is returned to the training environment. D1R KO mice have normal contextual fear responses. In fact, D1R KO mice displayed a contextual fear response for several days longer than control mice (El-Ghundi et al., 2001), possibly indicating a role for D1 receptors in contextual fear extinction. In a passive-avoidance experiment, D1R KO mice also had a normal latency to enter a dark compartment where they had previously been shocked (El-Ghundi et al., 1999). However, more recent studies indicate that D1R KO mice have deficits in contextual and cued fear conditioning as well as in passive avoidance (Ortiz et al., 2010).
In 2-way active avoidance, a mouse has to learn to move from one chamber to another in response to a tone to avoid a shock. Thus, a cue-dependent motor response is necessary, which is even harder to learn. Not surprisingly, DD mice were unable to learn 2-way active avoidance even after 900 training trails. Although they could be trained to avoid the shock as well as control mice when given L-Dopa, they were unable to maintain their escape behavior in the absence of DA (Darvas, unpublished observations). Similar to DD mice, D1R KO mice were unable to use a tone to escape a footshock in a 2-way active avoidance task, even after 500 training trials (Ortiz et al., 2010; Darvas and Palmiter, unpublished); NR1:DAT KO mice demonstrated a significant delay in this task, but did perform at control levels after 200 training trials (Darvas and Palmiter, unpublished observations).
Together, these data show that DA in the ventral striatum and amygdala are necessary for forming a Pavlovian fear association. Furthermore, phasic DA and activation of D1 receptors are needed to encode information about cues that predict aversive stimuli. Unlike DD mice, NR1:DAT KO and D1R KO mice in FPS develop a cue- and context-independent FPS that is the result of fear generalization (Zweifel, Fadok, and Palmiter, unpublished observations); however, the nature of this behavioral manifestation remains unresolved. Together, these observations highlight the importance of dopamine, D1 receptors, and phasic DA signaling for fear processing.
Phasic dopamine release and D1R signaling facilitate learning about discrete cues
Table 2 summarizes the behavioral results obtained with the DD, D1R KO and NR1:DAT KO mice. DD mice in their DA-depleted state are unable to perform most of these behaviors or do so very slowly; hence these behaviors are relevant for further analysis of the underlying DA-dependent mechanisms. Virtually all of the behaviors of DD mice become normal after systemic restoration of DA signaling by administration of L-Dopa indicating that the behavioral deficits are due to lack of DA signaling rather than developmental abnormalities.
Table 2.
Comparison of specific behaviors in DD, D1R KO and DAT:NR1 KO mice. References are cited within the text.
| DD | D1R KO | DAT:NR1KO | |
|---|---|---|---|
| Feeding | Absent | Impaired at weaning | Normal |
| Sucrose/Saccharin preference | Normal | Normal | Normal |
| Locomotion | Hypoactive | No deficit - highly variable | Normal |
| Rotarod | Motor deficit | Motor coordination deficit | Normal |
| Straight Alley swim | Impaired | Slightly impaired | Normal |
| Appetitive T-maze | Impaired | Impaired | Impaired |
| Instrumental conditioning | Impaired | Impaired | Impaired |
| Morris water maze | Impaired | Impaired | Impaired |
| Fear-potentiated startle | Impaired | Impaired | Impaired |
| Active avoidance | Impaired | Impaired | Impaired |
| Contextual fear | Impaired | Normal/Impaired | Normal |
Work from these three mouse models has shown that DA signaling mediates a wide variety of behaviors, but that a subset of these behaviors are sensitive to both reductions in phasic release of DA as well as loss of D1 receptors. Both NR1:DAT KO and D1R KO mice are capable of performing several DA-dependent tasks such as seeking and consuming food, ambulatory behavior, water escape task and contextual aversive learning. However, in more complex tasks requiring mice to pay attention or respond to a discrete cue, both the NR1:DAT KO and the D1R KO mice are slower to learn. Learning deficits of the D1R KO mice were always more severe than those of the NR1:DAT KO mice, which could overcome most learning deficits with additional training. Attenuated learning in these two KO mouse models for the same types of tasks is consistent with the idea that burst-firing by DA neurons and phasic release of DA selectively activates D1 receptors in target brain regions (Goto and Grace, 2005). Phasic DA release and D1 receptors seem to be important for learning about both appetitive and aversive cues. There are several similarities between the D1R and NR1:DAT KO mice, but the phenotypes are not identical. One caveat to consider is that in addition to reduced phasic signaling, NMDARs are necessary for the induction of LTP (Bonci and Malenka, 1999). DA neurons in NR1:DAT KO mice have attenuated LTP and where also found to have chronically elevated AMPA receptor currents (Engblom et al., 2008; Zweifel et al., 2008). The role of LTP in DA neurons has been associated with learning and responses to psychostimulants in rats (Bowers et al., 2010; Stuber et al., 2008). Because NR1:DAT mice do not have any behavioral changes beyond those of the D1R KO mice, it is unlikely that loss of LTP in DA neurons exacerbates the phenotype associated with impaired phasic DA signaling. Rather, the D1R KO mice have a more severe phenotype, which could be due to several factors. First, it is possible that the growth reduction and reduced striatal volume in the D1R KO mouse could result in a generalized learning deficit that is not apparent in NR1:DAT KO mice. However, intact behaviors such as contextual fear conditioning in D1R KO mice argue that certain tasks can be learned normally. Another possibility is that D1Rs may receive and respond partially to tonic signaling in addition to greater activation by phasic bursts of DA release. This may explain why D1R KO mice perform poorly on the rotarod whereas NR1:DAT KO mice are normal. Coordinated movement is thought to depend primarily on tonic DA signaling to the dorsal striatum, an area rich in both D1 and D2 receptors. A pharmacological study comparing the contributions of these two receptors in coordinated movement found the D2R to be essential, whereas the D1 receptor was only important for acquiring more complex movement tasks (Yin et al., 2009). We observed that D1R KO mice had poor performance on a rotarod during the first trial and their performance did not improve with training, indicating that D1 receptor signaling is important for mastery of this complex motor task. Tonic input to the D1 receptor may also explain other D1 receptor-mediated deficits in innate behavior such as hypophagia and a decreased growth rate when compared to mice with reduced phasic signaling. Lastly, it is possible that the NR1:DAT KO mouse represents an intermediate phenotype. If phasic DA is acting primarily on the D1 receptor, then mice lacking this receptor would not be able to detect these bursts of DA release. However, the NR1:DAT KO still has ~30% of normal burst firing and phasic DA release. Therefore, with enough training, the residual phasic DA signaling may be sufficient to allow mice to learn the simplest tasks, e.g. Pavlovian association of a cue with reward availability and rotarod. Similarly, given enough training NR1:DAT mice can learn some more complex tasks, although they are slower to do so than control mice, e.g. response-based T-maze, instrumental conditioning and Morris water maze. Phasic DA may facilitate the acquisition of a task rather than the overall degree to which a mouse is able to master that task with extensive training. Being slower to learn, but capable of performing certain tasks in the DAT:NR1 KO model may explain some of the discrepancies in the literature. For instance, in our cocaine conditioned place preference paradigm, which included three cocaine-pairing sessions alternated with test days, NR1:DAT KO mice failed to form a preference for the cocaine-paired side (Zweifel et al., 2008). Whereas in a more extensive training protocol, with four cocaine-pairing sessions spread over eight days an no intermittent testing, NR1:DAT mice formed a preference that was indistinguishable from that of controls (Engblom et al., 2008).
Continuing research is needed to complete our understanding of the specific role of phasic DA in dopamine-dependent behavior. Though the research cited in this review provides a framework for behaviors that are similarly affected by reduction of phasic bursts and loss of D1 receptor detection, it is difficult to determine in what stage of learning (acquisition, consolidation, retrieval, or expression) DA signaling is important. Techniques that allow one to manipulate the activity of DA neurons, to either enhance or suppress their firing in a manner that resembles what occurs physiologically, would be a valuable step in this direction. In most cases it is not clear where in the brain DA signaling is important for mediating a specific behavior. Experiments aimed at restoration or inactivation of DA synthesis, DA receptors, and ion channels within DA neurons responsible for burst firing will provide a greater understanding of DA-dependent modulation of behavior. Although we have emphasized the role of D1R in this review, the other DA receptors play important roles as well. Furthermore, DA receptors may serve different functions depending on where they are located in the cell relative to active synapses. We anticipate that genetically modified mice, in conjunction with other classical and emerging techniques, will promote a deeper understanding of how DA signaling affects behavior.
Acknowledgements
This investigation was supported in part by the Pacific Northwest Udall Center NS062684 (M.D.), by PHS NRSA 2T32 GM007270 from NIGMS (J.P.F.) and by National Institute on Drug Abuse Grants DA07278-13 (J.G.P.), F32 DA022829 (L.S.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl. Acad. Sci. USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Muer A, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Beal MF. Experimental models of Parkinson's disease. Nat. Rev. Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- Bennett MR. The concept of long term potentiation of transmission at synapses. Prog. Neuro. 2000;60:109–137. doi: 10.1016/s0301-0082(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat. Disord. 2008;14 Suppl 2:S124–S129. doi: 10.1016/j.parkreldis.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierly DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J. Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without dopamine. J. Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur. J. Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martín AB, Gubellini P, Pavón N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J. Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Mueller R, Drescher KU, Gross G, Gass P. Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharm. Res. 2008;58:302–307. doi: 10.1016/j.phrs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PEM. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurons are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc. Natl. Acad. Sci. USA. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J. Neurosci. 2010;30:1158–1165. doi: 10.1523/JNEUROSCI.4576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Kim DS, Palmiter RD. The role of dopamine in learning, memory and performance of a water escape task. Behav. Brain Res. 2004;148:73–78. doi: 10.1016/s0166-4328(03)00183-9. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, Bartlett PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl. Acad. Sci. USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Padungchaichot P, Accili D, Fuchs S. Dopamine receptors and dopamine transporter in brain function and addiction behaviors: Insights from targeted mouse mutants. Dev. Neurosci. 1998;20:188–203. doi: 10.1159/000017313. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. Spatial learning deficit in dopamine D1 receptor knockout mice. Eur. J. Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dawd BF, George SR. Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Res. 2001;892:86–93. doi: 10.1016/s0006-8993(00)03234-0. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur. J. Neurosci. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Luhán R, Halbout B, Mameli M, Parlato R, Sprengel R, Lüscher C, Schütz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TMK, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J. Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Darvas M, Dickerson TMK, Palmiter RD. Long-Term Memory for Pavlovian Fear Conditioning Requires Dopamine in the Nucleus Accumbens and Basolateral Amygdala. PLoS ONE. 2010;5(9):e12751. doi: 10.1371/journal.pone.0012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. Eur. J. Neurosci. 2002;15:158–164. doi: 10.1046/j.0953-816x.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat. Neurosci. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav. Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J. Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The yin and yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: Burst firing. J. Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Granado N, Ortiz O, Suárez LM, Martín ED, Ceña V, Solís JM, Moratalla R. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-induced arc and zif268 expression in the hippocampus. Cereb. Cortex. 2008;18:1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann. N Y Acad. Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc. Natl. Acad. Sci. USA. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav. Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Jr, Freenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using micro dialysis. Neurosci. Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons record differences between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Kitai ST. A whole cell patch-clamp study on the pacemaker potential in dopaminergic neurons of the rat substantia nigra pars compacta. Neurosci. Res. 1993;18:209–221. doi: 10.1016/0168-0102(93)90056-v. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, El-Ghundi M, Fletcher PJ, O’Dowd BF. Modification of dopamine D1 receptor knockout phenotype in mice lacking both dopamine D1 and D3 receptors. Eur. J. Pharmacol. 2000;399:171–181. doi: 10.1016/s0014-2999(00)00347-2. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 receptor-deficient mice is determined by gene dosage, genetic background. and developmental adaptations. J. Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JND, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kim DS, Froelick GJ, Palmiter RD. Dopamine-dependent desensitization of dopaminergic signaling in the developing mouse striatum. J. Neurosci. 2002;22:9841–9849. doi: 10.1523/JNEUROSCI.22-22-09841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD, Cummins A, Gerfen CR. Reversal of supersensitive striatal dopamine D1 receptor signaling and extracellular signal-regulated kinase activity in dopamine-deficient mice. Neuroscience. 2006;137:1381–1388. doi: 10.1016/j.neuroscience.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrellio E. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc. Natl. Acad. Sci. USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurier LG, O’Dowd BF, George SR. Heterogeneous tissue-specific transcription of dopamine receptor subtype messenger RNA in rat brain. Brain Res. Mol. Brain Res. 1994;25:344–350. doi: 10.1016/0169-328x(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition on novel information through hippocampus long-term potentiation and long-term depression. J. Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol. Neurobiol. 2004;29:117–130. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selectiveactivation of the mesocortical system. Brain Res. 1989;476:377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol. Disord. Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz O, Delgado-Garcia JM, Espadas I, Bahi A, Trullas R, Dreyer J-L, Gruart A, Moratalla R. Associative laearning and CA3-Ca1 synaptic plasticity are impaired in D1R null, Drd1a−/− mice and hippocampal siRNA silenced Drd1a mice. J. Neurosci. 2010;30:12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P, Clark D. N-methyl-D-aspartate increases the excitability of nigrostriatal dopamine terminals. Eur J. Pharmacol. 1991;201:117–120. doi: 10.1016/0014-2999(91)90332-k. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res. Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.1007827107. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog. Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neurosci. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Robinson S, Smith DM, Mizumori SJ, Palmiter RD. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc. Natl. Acad. Sci. USA. 2004;101:13329–13334. doi: 10.1073/pnas.0405084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting and/or learning about rewards. Behav. Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Robinson S, Rainwater AJ, Hnasko TS, Palmiter RD. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology (Berl.) 2007;191:567–578. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- Rubenstein M, Phillips TJ, Bunzow JR, Falzone TL, Dzeiwczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine and methanphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Pharmacology (Berl.) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high intensity somatosensory stimulation in the anesthetized monkey. J. Neurophysiol. 1987;57:201–217. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopamine control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JL, Ledent C, Drago J, Lawrence AJ. Receptor crosstalk: characterization of mice deficient in dopamine D1 and adenosine A2A receptors. Neuropsychopharmacology. 2006;31:525–534. doi: 10.1038/sj.npp.1300852. [DOI] [PubMed] [Google Scholar]
- Smith DR, Striplin CD, Geller AM, Mailman RB, Drago J, Lawler CP, Gallagher M. Behavioral assessment of mice lacking D1A dopamine receptors. Neuroscience. 1998;86:135–146. doi: 10.1016/s0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- Steiner H, Fuchs S, Accili D. D3 Dopamine receptor deficient mouse: evidence for reduced anxiety. Physiol. Behav. 1998;63:137–141. doi: 10.1016/s0031-9384(97)00430-7. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HHM, Hyman B, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Swant J, Wagner JJ. Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor. Learn. Mem. 2006;13:161–167. doi: 10.1101/lm.63806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, Palmiter RD. Feeding behavior in dopamine-deficient mice. Proc. Natl. Acad. Sci. USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Habibi R, Michaelides M, Patel UB, Suchland K, Anderson BJ, Robinson JK, Wang G, Grandy DK, Volkow ND. Dopamine D4 receptor (D4R) deletion in mice does not affect operant responding for food or cocaine. Behav. Brain Res. 2010;207:508–511. doi: 10.1016/j.bbr.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT, Jr, Caron MG. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc. Natl. Acad. Sci. USA. 1991;88:7491–7495. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AH, Uwano T, Kimura T, Hori E, Katsuki M, Nishijo H, Ono T. Dopamine D1 receptor modulates hippocampal representation plasticity to spatial novelty. J. Neurosci. 2008;28:13390–13400. doi: 10.1523/JNEUROSCI.2680-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptordependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand.Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Xing B, Kong H, Meng X, Wei SG, Xu M, Li SB. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169:1511–1519. doi: 10.1016/j.neuroscience.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995a;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995b;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J. Neurosci. Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–486. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]