Abstract
Routine laboratory procedures can be stressful for laboratory animals. We wanted to determine whether human handling of adult rabbits could induce a degree of habituation, reducing stress and facilitating research-related manipulation. To this end, adult New Zealand white rabbits were handled either frequently or minimally. After being handled over 3 wk, these rabbits were evaluated by novel personnel and compared with minimally handled controls. Evaluators subjectively scored the rabbits for their relative compliance or resistance to being scruffed and removed from their cages, being transported to a treatment room, and their behavior at all stages of the exercise. Upon evaluation, handled rabbits scored significantly more compliant than nontreated controls. During evaluation, behaviors that the rabbits displayed when they were approached in their cages and while being handled outside their cages were recorded and compared between study groups. Handled rabbits displayed behavior consistent with a reduction in human-directed fear. This study illustrates the potential for handling to improve compliance in laboratory procedures and reduce fear-related behavior in laboratory rabbits. Such handling could be used to improve rabbit welfare through the reduction of stress and exposure to novel stimuli.
Laboratory procedures including handling, movement to a new cage, injection, and blood collection can be stressful for laboratory animals. Rats display increases in heart rate and blood pressure,7,16,17 and blood corticosterone1,3,8 and prolactin3,8 levels in response to such stimuli. Rabbits have not been the subject of such study. Therefore, we sought to determine whether adult rabbits could be habituated to routine laboratory handling, and if such habituation would facilitate conducting research-related procedures and improve rabbit welfare by reducing stress associated with manipulation and exposure to novel stimuli.
Several studies have detailed the effects of human handling on rabbits during their first 3 wk of life.11,14,15,19 Rabbits that were handled briefly immediately before or after nursing were more likely to approach a human hand placed against their cage when they were 4 wk old (weaning age).14 On evaluation at 6 to 8 mo of age, rabbits handled as kits were more likely to approach a human and spend time in a space near that human. In another study, groups of kits were either handled by a human, exposed to a rabbit-friendly cat, or both. On weaning, the kits showed a significantly shorter latency to approach the species to which they had been exposed.15 Therefore, rabbits exposed to humans showed a short latency to approach humans and approached humans on more occasions but did not show a similar reduction in fear toward cats, indicating a specific reduction in human-directed fear.15
Brief handling of rabbits and exposure to novel environments during the first 3 wk of life has also been shown to affect fear after weaning in a manner not specific to humans.11,19 Rabbits handled throughout the first 3 wk of life were more likely to move over a larger area during an open-field test, approach a novel object, and approach another rabbit at weaning age.19 In a similar study, rabbits handled during days 10 to 20 of life displayed less fear-related behavior at 3 mo of age.11 These rabbits were more likely to show boldness rather than fear during this evaluation, where boldness was defined as moving forward (moving the forelimbs with the hindlimbs stationary), hopping, and rearing up on the hindlimbs while exploring a large part of the field, and where fear was defined as standing stretched (forelimbs forward with hindlimbs in place) and exhibiting little locomotion.13 These results indicate that human handling of neonates curtails general neophobia in addition to reducing human-directed fear.
Such effects persisted for at least 8 mo, although their total duration was not determined.14 However, daily handling of rats in their first 3 wk of life led to changes in the adrenocortical axis that persisted through 2 y of age.12 This effect was characterized by a lifelong increase in glucocorticoid receptors in the hippocampus, which play a vital role in negative feedback regulation, accompanied by decreased basal blood corticosterone levels and faster return to baseline corticosterone levels following stressful activity. The cited study suggests that handling can have a long-term physiologic effect on laboratory animals.
The success of neonatal handling studies in rabbits and the persistence of handling-related effects in other species suggest that handling adult rabbits—perhaps with greater duration, frequency, or both—with or without the presence of counter-conditioning (for example, the association of humans with pleasant events) might lead to similar outcomes. That rabbits are able to associate specific humans with positive stimuli, specifically food, suggests that these animals have the ability to interact with their handlers in a manner that the rabbits perceive to be pleasant.6 This behavior also suggests that rabbits use their prior experiences with humans to anticipate and respond to subsequent interactions. Therefore, handled rabbits might be more compliant during routine laboratory procedures, as seen in dogs socialized to their caretakers.2 We hypothesized that adult rabbits handled on a routine basis would be more compliant (lower scoring) when evaluated at a later date and would display observable changes in their behavior when compared with nonhandled controls. The hypothesis was tested by comparing rabbits handled each weekday for 5 min with those for which handling was minimized. After a 3-wk period of handling, all rabbits were evaluated by novel handlers to assess the value of this regimen as part of a rabbit socialization and enrichment program.
Materials and Methods
Animals.
Female New Zealand white rabbits (Oryctolagus cunniculus; n = 21) were obtained from a commercial vendor (Myrtles Rabbitry, Thompson's Station, TN). Rabbits were from stock free of Pasteurella multocida, Pasteurella pneumotropica, cillia-associated respiratory bacillus, Treponema cuniculi, Clostridium piliformis, oral papilloma virus, Psoroptes cuniculi, Cheyletiella parasitovorax, Listophorus gibbus, Passalurus ambiguous, Taenia pisiformis, Eimeria steidae, and intestinal coccidia. All rabbits were received in the same shipment when they were 4 mo old. On receipt, they weighed 3.2 to 3.6 kg and were allowed to acclimate to the animal facility for at least 3 wk prior to use in this study. Rabbits were housed individually in stainless steel cages (0.25 × 0.48 × 0.2 m) with plastic flooring containing 15-mm diameter round holes (Allentown, Allentown, PA) and a pan below the floor containing dust-free bedding (Teklad Pelleted Paper Bedding 7084, Harlan Laboratories, Indianapolis, IN). Rabbits were fed a commercial pelleted diet (Purina Lab Diet 5326 High Fiber Rabbit, Nestlé Purina, St Louis, MO) and given water ad libitum. In addition, they were given fresh spinach on 3 d each week. Personnel that had contact with these rabbits wore nitrile gloves and a clean white lab coat. All experiments were performed in an AAALAC-accredited facility with prior approval of the University of Georgia Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals.10
Rabbits were divided into 2 groups: a treatment group that was handled according to the protocol described (11 rabbits, handled group) and a control group (10 rabbits) that was minimally handled. Both handled and control rabbits experienced the minimal handling necessary for husbandry in addition to that prescribed by the study protocol. In addition, all rabbits in this study were used simultaneously for an unrelated immunization protocol and therefore underwent periodic subcutaneous injection and venipuncture while under sedation with acepromazine and ketamine. The 2 groups were composed of equal numbers of rabbits enrolled in each group of the immunization study.
For husbandry, immunization, and venipuncture, rabbits were picked up and transported using a plastic box (50 × 25 × 20 cm). Transportation was accomplished by turning the box on its long side, corralling the rabbit into the box using the cage wall, and then slowly turning the box to rest on its bottom. Facility personnel were informed about the nature of this study and were directed to refrain from touching or interacting with these rabbits unless necessary. During the study, 2 rabbits developed injection site abscesses, presumably due to administration of Freund adjuvant. These animals, both in the handled group, were removed from the study when these abscesses developed, and therefore are included in the first evaluation but not the second or third.
Handling protocol for treatment group rabbits.
Handling sessions took place in the afternoon Monday through Friday over 3 consecutive weeks. During this time, treatment group rabbits were each handled 14 times in total by the same person. During these sessions when rabbits were hand-carried, the scruff was held in the handler's dominant hand, while the nondominant arm was used to stabilize the body and the nondominant hand was used to control the hindlimbs.4 The head was tucked into the cavity formed by the nondominant elbow, thus covering the rabbit's eyes. Each rabbit in the treatment group was handled according to the following protocol. During stage 1 (30 s), each rabbit was removed from its cage by scruffing and was carried as described to a nearby procedure room. The rabbit was placed on a plastic cart covered with a no-slip mat. For stage 2 (2 min), the rabbit was stroked gently between the eyes and was palpated slowly and with moderate pressure over its entire body surface. Palpation began at the mouth, nose, and face and moved caudally to the front feet, back, chest, and hindfeet. The rabbit was stroked between the eyes once more. During stage 3 (1 min), the rabbit was scruffed and lifted into a plastic box (50 × 25 × 20 cm), where it was held still against the side of the box by the handler's dominant forearm. While the rabbit was held still, a gloved finger was placed midpinna on the central artery of one ear to simulate the tactile contact used in blood collection. If a rabbit was resistant, the ear was released but control of its body was maintained. In stage 4 (30 s), the rabbit was carried back to its cage as described. Food treats (one 0.5-cm3 piece each of apple, carrot, and collard green) were placed in the cage.
The handling sessions were designed to fulfill multiple criteria. First, the handling had to be brief in duration, because it is likely that laboratories have insufficient personnel to carry out long handling sessions, and one of the goals was to identify a protocol that was both beneficial and practical. The protocol described was performed daily and required approximately 4 min per rabbit. A second criterion was to familiarize the rabbits with handling procedures that they would likely experience during handling for research, thus habituating them to these stimuli. This handling included both extensive nonpainful tactile contact between rabbit and handler, as well as brief restraint and touching of the central ear artery to simulate blood collection. A third criterion was to induce a degree of classical conditioning, so that the rabbits associated the human handlers with pleasant stimuli. This conditioning was accomplished through gentle stroking between the eyes before and after body palpation and the administration of treats at the conclusion of handling. Rabbits groom each other around the eyes, on the top of the nose, and on the top of the head, and touching in these areas is more likely to be perceived as pleasant.9
Evaluation protocol for all rabbits.
After conclusion of the 3-wk handling protocol, the behavior of all rabbits was evaluated. A preliminary evaluation was performed 3 d after the handling protocol was completed and was used to refine an evaluation rubric for use in all other data collection steps. Evaluations then were performed on all rabbits at 10, 14, and 21 d after completion of the handling protocol. Different evaluators, each of whom had extensive rabbit handling experience but no prior contact with these rabbits or knowledge of their previous performance or group assignment, performed each evaluation. Evaluations took place between 1300 and 1600 and were conducted in a random order determined by blindly drawing animal numbers from a bag. Each rabbit was evaluated as follows. During stage 1, a baseline behavior measurement was recorded for each animal 3 s after the evaluator approached within 3 ft of the cage. The rabbit's location (front, middle, or back of the cage), posture (standing, crouching, or lying down), head position (up, neutral or unable to tell, or down), and ear position (up or down) were recorded. The evaluator stood or crouched directly in front of the cage, and the same set of behavioral parameters was recorded after 3 s. The rabbit was offered a piece of collard green through the bars of the cage. The metal latch of the cage was opened and behavioral parameters were recorded after 3 s. Behaviors were recorded in an identical manner after the cage door was opened and after the evaluator's gloved hand was placed halfway into the cage at a level halfway between the cage floor and ceiling.
During stage 2, the evaluator scruffed, corralled, and removed the rabbit from its cage as described in the handling protocol.4 The rabbit was carried to a nearby treatment room. For stage 3, the rabbit was placed in a plastic box (50 × 25 × 20 cm), and baseline behavior was recorded after 3 s. Posture (standing, crouching, or lying down), head position (up, neutral or unable to tell, or down), and ear position (up or down) were recorded. The rabbit was stroked gently between its eyes, and its behavior was recorded after 5 s. In addition, the rabbit was observed to see whether a flinch could be detected in response to the handler's initial touch. Identical measurements were taken after the rabbit's central ear artery was held for 10 s by using a gloved finger, to simulate blood collection. Finally, the rabbit was carried back to its cage as described in the handling protocol.
The evaluator scored each rabbit's behavior on a scale of 1 to 5 (1, most compliant; 5, most resistant) when it was scruffed and removed from its cage and during transport to and from the treatment room. Evaluators also were asked to score each rabbit's overall behavior according to this scale, taking into account all parts of the evaluation.
Statistical analysis.
Behavior scores were compared between the handled and control groups in each evaluation using the Mann–Whitney U test (SPSS, Chicago, IL). Data on cage location, posture, head position, and ear position were compared between handled and control groups using a repeated-measures model (SAS version 9.2, SAS Institute, Cary, NC). The model was implemented using PROC GENMOD and included fixed factors of treatment and evaluator, a treatment × evaluator interaction effect, and a random factor of rabbit. A multinomial distribution with a cumulative logit link function was used for cage location, posture, and head position data, and a binomial distribution with a logit link function was used for ear position. An independent correlation structure was used. Cage location data was ordered back, middle, front; posture data was ordered crouching, standing, lying down; and head position data was ordered up, neutral, down. All hypothesis tests were 2-sided, and the significance level was α = 0.05.
Results
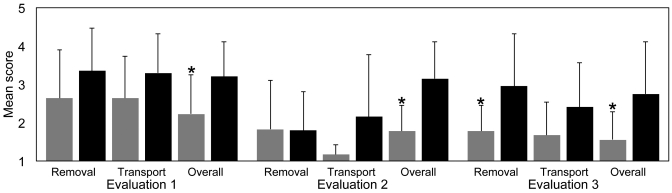
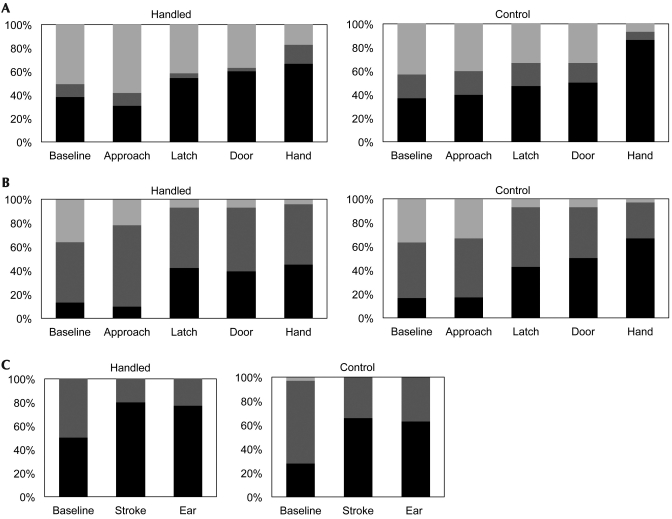
At all 3 evaluations, handled rabbits had lower overall scores than did controls (evaluation 1, P = 0.05; evaluation 2, P = 0.01; and evaluation 3, P = 0.04; Figure 1). No significant differences in removal and transport scores were noted between handled and control rabbits, except for the removal phase of evaluation 3 (P = 0.05). Given the significant differences in overall behavior scores between these groups, we wanted to determine whether specific behavioral differences between handled and control rabbits could be identified. To facilitate detailed behavioral analysis, the location, posture, head position, and ear position of each rabbit was recorded when the evaluator approached its cage (evaluation stage 1; Figure 2). The posture, head position, and ear position was recorded similarly when each rabbit was placed in a plastic box on a treatment table (evaluation stage 3, Figure 2). These data were analyzed statistically by comparing the behavior distributions of handled and control rabbits. No significant differences were detected between the 2 groups of rabbits or between evaluators.
Figure 1.
Rabbits were scored on a scale of 1 (most compliant) to 5 (most resistant) when scruffed and removed from their cages (removal), when transported to and from the treatment room (transport), and for overall behavior (overall). Bars indicate the mean scores of handled (gray bars) and control (black bars) group rabbits for each evaluation; error bars indicate the value of 1 SD about each mean. *, Value significantly (at the 95% level, Mann–Whitney U test) different from their corresponding controls.
Figure 2.
(A, B) Cage location and posture. After recording of baseline behavior (baseline), measurements were taken 3 s after the evaluator approached the rabbit's cage (approach), opened the cage latch (latch), opened the cage door (door), and inserted his or her hand into the middle of the cage (hand). (A) Bars indicate the percentages of handled and control rabbits in the front (light gray), middle (dark gray), and back (black) of the cage. (B) Bars indicate the percentages of handled and control rabbits lying down (light gray), standing (dark gray), and crouching (black). (C) Posture outside of cage. After recording of baseline behavior (baseline), measurements were taken 3 s after the evaluator stroked the rabbit between its eyes (stroke) and held the ear to simulate blood collection (ear). Bars indicate the percentages of handled and control rabbits lying down (light gray), standing (dark gray), and crouching (black).
Discussion
Rabbits in the group that was handled intensively received significantly lower overall behavior scores, indicating that they were more compliant when evaluated by novel personnel. In addition, the handled group had lower mean scores than did controls when rabbits were scruffed and removed from their cage during evaluation 3. When removed from their cages during evaluation 2, rabbits from both groups displayed nearly identical mean scores. When measured from the base of the neck to the wrist, evaluator 2 had a shirt sleeve length of 68 cm compared with 86 to 89 cm for evaluators 1 and 3. This difference might have made rabbit removal more difficult and thus reduced her scoring sensitivity at this evaluation phase, although other causes may exist. The overall behavior scores (Figure 1) suggest that rabbits that are handled regularly, even in brief sessions for a short time, show decreased resistance to handling in the subsequent 3 wk after regular handling is discontinued. This reduction in resistance may be a function of many different protocol aspects that promote classical conditioning in response to stimuli, such as food, contact that mimics grooming, and habituation to routine laboratory handling procedures. Statistically significant differences between our groups of rabbits were not found for specific behaviors exhibited during handling. However, because blinded experienced evaluators assigned handled rabbits significantly lower overall behavior scores, differences in the behaviors of these groups likely existed.
Throughout evaluation, rabbits in both groups shifted to a crouching position when they were stroked and when their ears were touched (Figure 2). Head and ear position followed a similar pattern, with a greater percentage of rabbits holding their heads and ears down as evaluation progressed. Interestingly, rabbits from both groups reacted to the cage latch opening with location and postural changes. The noise generated by the latch itself may have startled rabbits from both groups, although the presence of a human nearby may also have triggered an avoidance response in this context. The cages used in this study had stainless steel latch mechanisms, and steel is associated with louder noise levels in rat caging.18 Handled rabbits were also less likely to flinch in response to the evaluator's initial touch than controls (10% versus 60%, data not shown). This response may indicate a reduction in human-specific fear, laboratory procedure-related neophobia, or both.11,14,19 Additional studies incorporating larger group sizes and more intensive behavioral measurement could be used to determine the precise behavioral consequences of human handling in rabbits.
Overall behavior scores indicate that handled rabbits experienced a human-specific reduction in fear and experienced less stress when exposed to research-related manipulation. Novel handlers were used for each of the 3 evaluation trials, so person-specific reductions in fear are not responsible for the changes observed.6 The effects produced in this study were achieved with 4 min of intensive individual handling each weekday for 3 wk, whereas those seen in neonatal handling studies were achieved with only seconds of direct handling each day.9,13,14,17 Therefore, adult rabbits may require more handling than do neonates to achieve significant behavioral changes, although the exact amount of handling required is not known. Our study design does not allow for determination of which components of our handling regime are responsible for the changes. In addition, we used only female rabbits; sex-related differences in the response to handling are possible. The persistence of handling-related behavioral changes has yet to be studied in adult rabbits, so whether these changes persist as they do in neonates is unknown.8,13 Furthermore, whether the permanent physiologic changes seen in handled neonatal rats, such as modulation of the adrenocortical axis,11 can be achieved after studies such as the current one is unknown. Such modulation, even if temporary, could provide rabbits with a reduction in stress levels that they might otherwise experience in the laboratory.
Socialization to humans has been used in laboratory dogs to reduce stress, improve compliance with laboratory procedures, expose them to novel stimuli, and promote pleasant interaction between the dogs and their caretakers.2 The present study shows that similar programs can be applied successfully to rabbits. Regular handling during an initial acclimatization period could be used to habituate rabbits to human contact, reducing fear, improving laboratory procedural compliance, and facilitating routine care. More compliant rabbits might be less likely to experience injury, particularly lumbar vertebral fracture, due to kicking while being carried or restrained. Therefore, a handling protocol such as the one in this study could be used to improve rabbit welfare in a laboratory setting by reducing injury, reducing stress, and creating a pleasant interaction between rabbits and their caretakers. Because this protocol is time- and resource-efficient, a similar program could be continued for an extended period of time, or handling could be reduced in frequency as the animals habituate and experimentation begins. Such handling programs have been performed as part of prior rabbit behavior studies.5 Handling at a low frequency likely would be necessary to maintain the desirable behavioral effects. Additional research to determine the minimal amount of handling necessary for long-term efficacy would be helpful in refining such programs.
Acknowledgments
We thank Dr Elliot Altman (Department of Biological and Agricultural Engineering, University of Georgia) and Ms Liz Stich (University Research Animal Resources, University of Georgia) for use of the rabbits, Dr Deborah Keys for performing aspects of the statistical analysis, and Dr Kelly Pate (Department of Molecular and Comparative Pathobiology, Johns Hopkins University) for critically reading the manuscript.
References
- 1.Armario A, Montero JL, Balasch J. 1986. Sensitivity of corticosterone and some metabolic variables to graded levels of low-intensity stresses in male rats. Physiol Behav 37:559–561 [DOI] [PubMed] [Google Scholar]
- 2.Bayne KAL. 2003. Environmental enrichment of nonhuman primates, dogs, and rabbits used in toxicology studies. Toxicol Pathol 31:132–137 [DOI] [PubMed] [Google Scholar]
- 3.Brown GM, Martin JB. 1974. Corticosterone, prolactin, and growth hormone responses to handling and new environment in the rat. Psychosom Med 36:241–247 [DOI] [PubMed] [Google Scholar]
- 4.Cheeke PR, Patton NM, Templeton GS. 1982. Rabbit production. Danville (IL): Interstate Printers and Publishers [Google Scholar]
- 5.Chu L, Garner JP, Mench JA. 2004. A behavioral comparison of New Zealand white rabbits (Oryctolagus cuniculus) housed individually or in pairs in conventional laboratory cages. Appl Anim Behav Sci 85:121–139 [Google Scholar]
- 6.Davis H, Gibson JA. 2000. Can rabbits tell humans apart? Discrimination of individual humans and its implications for animal research. Comp Med 50:483–485 [PubMed] [Google Scholar]
- 7.Duke JL, Zammit TG, Lawson DM. 2001. The effects of routine cage-changing on cardiovascular and behavioral parameters in male Sprague–Dawley rats. Contemp Top Lab Anim Sci 40:17–20 [PubMed] [Google Scholar]
- 8.Gärtner K, Büttner D, Döhler K, Friedel R, Lindena J, Trautschold I. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14:267–274 [DOI] [PubMed] [Google Scholar]
- 9.Harriman M. 1995. How to live with an urban rabbit. Alameda (CA): Drollery Press [Google Scholar]
- 10.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 11.Kersten AMP, Meijsser FM, Metz JHM. 1989. Effects of early handling on later open-field behaviour in rabbits. Appl Anim Behav Sci 24:157–167 [Google Scholar]
- 12.Meaney MJ, Aitken DH, Van Berkel C, Bhatnagar S, Sapolsky RM. 1988. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239:766–768 [DOI] [PubMed] [Google Scholar]
- 13.Meijsser FM, Kersten AMP, Wiepkema PR, Metz JHM. 1989. An analysis of the open-field performance of subadult rabbits. Appl Anim Behav Sci 24:147–155 [Google Scholar]
- 14.Pongrácz P, Altbäcker V. 1999. The effect of early handling is dependent on the state of the rabbit (Oryctolagus cuniculus) pups around nursing. Dev Psychobiol 35:241–251 [DOI] [PubMed] [Google Scholar]
- 15.Pongrácz P, Altbäcker V, Fenes D. 2001. Human handling might interfere with conspecific recognition in the European rabbit (Oryctolagus cuniculus). Dev Psychobiol 39:53–62 [DOI] [PubMed] [Google Scholar]
- 16.Sharp JL, Zammit TG, Azar TA, Lawson DM. 2002. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp Top Lab Anim Sci 41:8–14 [PubMed] [Google Scholar]
- 17.Sharp JL, Zammit T, Azar T, Lawson D. 2003. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci 42:9–18 [PubMed] [Google Scholar]
- 18.Voipio HM, Nevalainen T, Halonen P, Hakumäki M, Björk E. 2006. Role of cage material, working style, and hearing sensitivity in perception of animal care noise. Lab Anim 40:400–409 [DOI] [PubMed] [Google Scholar]
- 19.Wyly MV, Denenberg VH, De Santis D, Burns JK, Zarrow MX. 1975. Handling rabbits in infancy: in search of a critical period. Dev Psychobiol 8:179–186 [DOI] [PubMed] [Google Scholar]