Abstract
Identification and eradication of murine fur mite infestations are ongoing challenges faced by many research institutions. Infestations with Myobia musculi and Myocoptes musculinus can lead to animal health problems and may impose unwanted research variables by affecting the immune and physiologic functions of mice. The purpose of this study was to evaluate the utility and efficacy of soiled bedding sentinels in the detection of fur mite infestations in colony mice. Female young-adult CRL:CD1(ICR) mice (n = 140) were exposed over a 12-wk period to various volume percentages of soiled bedding (11%, 20%, 50%, and 100%) from fur-mite–infested animals. Mice were tested every 2 wk with the cellophane tape test to identify the presence of fur mite adults and eggs. At the end of 12 wk, all mice exposed to 11%, 20%, and 50% soiled bedding tested negative for fur mites. One of the 35 mice (3%) receiving 100% soiled bedding tested positive for fur mites at the end of the 12-wk follow-up period. These findings suggest that the use of soiled bedding sentinels for the detection of fur mite infestations in colony mice is unreliable.
Ectoparasite infestations present an ongoing threat to barrier facilities. Murine acariasis in laboratory mice frequently is caused by Myobia musculi, Myocoptes musculinus, and Radfordia affinis.1,13,17,40,41 These infestations can be challenging to identify and control and often lead to animal health problems and research complications. For this reason, many institutions strive to exclude these parasites from their barrier facilities.1,17,18,41 Infestations can further compromise ongoing research by disrupting collaboration with institutions affected by sporadic or endemic mite infestations in their facilities.18
Myocoptes musculinus is the most common fur mite identified among laboratory mice, although mixed infections with Myobia musculi are common.17 The life cycles of Myocoptes and Myobia are 14 and 23 d, respectively.2,17 Myobia mites most frequently are found to inhabit the head and neck of mice, whereas Myocoptes are reported to have a predilection for the back, ventral abdomen, and inguinal regions.2,17 Mite infestations in live animals are often diagnosed by using cellophane tape tests.5,14,25 A clear piece of cellophane tape is pressed against the fur of the mouse, affixed to a slide, and examined microscopically for the presence of eggs or adult mites. Pelage collection and examination and skin scraping are 2 other common diagnostic methods. These tests have been shown to have increased sensitivity when compared with the tape test, but they have the disadvantage of requiring an anesthetized or recently euthanized animal.2,5,17
Fur mites feed on the superficial skin tissues and secretions of the animals they infest.1,2,17 Mite infestations in mice have been associated with numerous health problems. Common clinical manifestations of acariasis include alopecia, pruritis, and scruffiness.1,2,10,15,17-20,22,26,31,42,44 Severe health problems including ulcerative dermatitis, hypersensitivity dermatitis, and pyoderma can develop also.1,2,10,17,41 Infested mice may also be prone to secondary infections, reduced life span, and decreased body weight.2,17,42 Several studies have analyzed the potential research complications associated with murine acariasis.10,15,18-20,22,26,31,42,44 Mite infestations have been shown to cause elevations in IgE, IgG, and IgA levels; mast cell degranulation; increased levels of inflammatory cytokines; and lymphocytopenia.18-20,22,26,31,44 The changes in the immunologic function of affected mice can persist even after mite eradication.18
Multiple chemical treatment modalities have been proposed for the eradication of fur mites in infested animals.2,3,5,8,12,14,17,25,29,30,32,36,43 Conflicting information exists regarding the success of many of these treatment regimes. In addition, several of the proposed treatments have been associated with toxicity, adverse health effects in mice, and alterations in the physiologic or immune function of the animals.2,3,5,8,12,14,17,25,29,30,32,36,43 The complications associated with identifying an effective treatment for murine acariasis while minimizing toxicity and the introduction of unknown research variables highlight the importance of rapid and effective detection of mite infestations in barrier facilities.
Many institutions rely on soiled bedding sentinels for their primary source of information on colony health status.9,21,33,35 Several studies have demonstrated the efficacy of soiled bedding sentinels to detect common murine pathogens such as mouse hepatitis virus, mouse norovirus, Helicobacter spp., and pinworms.4,7,24,28,37,38 However, not all pathogens are easily transmitted through soiled bedding exposure. Agents that are not routinely identified through soiled bedding sentinels include those that are shed in low numbers, are susceptible to environmental factors, or are not easily transmitted through the fecal–oral route.6,21,33 Examples of pathogens that are not easily transmitted or detected through soiled bedding exposure include mouse Sendai virus, Pasteurella pneumotropica, lymphocytic choriomeningitis virus, and cilia-associated respiratory bacillus.7,9,11,16,35 In addition, the sensitivity of soiled bedding sentinel programs varies with the number of animals affected within the colony.27,38
In 2008, our institution faced a fur-mite outbreak that affected more than 25 rooms in a single barrier facility. Animals positive for Myobia musculi, Myocoptes musculinus, or both were identified through either health check requests for itching and scratching animals and by testing of animals scheduled for export to other institutions. Despite the extent of this outbreak, the soiled bedding sentinels in all mite-positive rooms consistently tested negative on cellophane tape tests for fur mites.
To our knowledge, only one study has specifically examined the efficacy of soiled bedding sentinels in the detection of fur mites in mice.34 A separate study, examining the transmission of mouse hepatitis virus to soiled bedding sentinels,38 demonstrated that 75% of cages (3 of 4) exposed to soiled bedding from colony animals tested positive for fur mites after 19 wk of exposure. That previous study used 8 cages of 12 mice each; 4 cages received soiled bedding from colony animals, whereas the other 4 cages received clean nonsoiled bedding. In that study,38 56.3% of colony mice were known to be mite-positive. Other literature suggests that spread of mites to naïve animals requires direct contact and that soiled bedding does not serve as an effective mechanism for transmission.1,17,23,39 However, we were unable to identify any research or experiments that substantiated these conclusions.
The purpose of the present study was to evaluate whether CRL:CD1(ICR) mice housed in static microisolation caging on soiled bedding from mice with Myobia and Myocoptes infestations can be used as sentinels for the detection of fur mites and to determine how the efficacy of these soiled bedding sentinels for fur-mite detection varies with the prevalence of fur-mite infestation among colony animals.
Materials and Methods
Subjects.
History of fur-mite–infested colony.
Mite-infested mice of several strains from multiple research groups were collected and housed in static microisolation caging in a conventional housing room. To expand the pool of infested animals, 6- to 8-wk-old CRL:CD1(ICR) female mice (Charles River Laboratories, Hollister, CA) were cohoused with fur-mite–infested mice. Breeding of mite-infested and CRL:CD1(ICR) mice continued over a 3-mo period to establish the infested colony. Male mice were removed from the infested colony prior to the start of the experiment.
Fur-mite–infested colony.
The infested mouse colony contained approximately 250 female mice of mixed strains and ages and was housed on a 2-sided conventional rack in static microisolation caging in the same housing room as the experimental colony. Colony mice were housed in groups of 3 to 4 per cage, 70 cages in all. One mouse per cage was screened for fur mites by using the cellophane tape test within 7 d of the onset of the experiment (week 0). All slides were read by 2 experienced independent readers. The number of eggs identified per slide among the mite-infested animals was classified into 5 ordinal groups: 1 to 2, 3 to 5, 6 to 10, 11 to 20, and greater than 20. The number of each mite species identified per slide was documented also. At week 0, all samples from the fur-mite–infested colony tested positive for fur-mite adults, eggs, or both.
Experimental colonies.
We obtained 280 female 6- to 8-wk-old CRL:CD1(ICR) mice from Charles River Laboratories for use in 4 experimental colonies. The vendor reported that the originating colonies were seronegative for mouse hepatitis virus, pneumonia virus of mice, mouse parvovirus, minute virus of mice, epizootic diarrhea of infant mice, Theiler murine encephalomyelitis virus, and ectromelia virus and were free of ectoparasites and endoparasites. Mice were individually housed on 4 double-sided conventional racks with 70 cages per rack. All 280 mice were screened twice on site for fur mites by using the cellophane tape test within 7 d prior to the onset of the experiment and were confirmed to be negative for ectoparasites by 2 experienced slide readers. In addition, 10% of the slides collected from this group were sent to an outside comparative pathology laboratory at the University of California–Davis for confirmatory testing. All slides submitted to the laboratory were confirmed to be negative for fur-mite adults and eggs.
Facilities.
The Laboratory Animal Resource Center at the University of California–San Francisco is an AAALAC-accredited animal care and use program. All animal care and experimental procedures were in accordance with federal policies and guidelines governing the use of animals and were approved by the University of California–San Francisco Institutional Animal Care and Use Committee. All animals in both groups were kept on paper chip bedding (Shepard Specialty Papers, Watertown, TN) with continuous access to food (Purina PicoLab 5053 irradiated, Purina Mills, St Louis, MO) and tap water. The housing room was maintained at 66 to 74 °F (18.9 to 23.3 °C) and with an average humidity between 33% and 52% under a 12:12-h light:dark cycle. All racks were maintained in a quarantined room in an isolated facility and were kept separate from any investigators’ animals to prevent the possible transmission of mites to other research animals.
Experimental design.
Cage changing.
All cages were changed once every week in a class II type A/B3 safety cabinet (NuAire, Plymouth, MN). Fur-mite samples from the experimental colony were collected every 2 wk at the time of cage change. Two biological safety cabinets were available in the housing room. One safety cabinet was used exclusively to change all cages from the fur-mite–infested colony. The second safety cabinet was used exclusively to change cages of experimental colony mice. Staff changing cages wore scrubs, gowns, head covers, shoe covers, and gloves. During cage changing of the infested colony, soiled bedding was collected from each cage and placed in a plastic container within the biological safety cabinet. This manipulation was repeated for all 70 cages.
Between all experimental colony cages, the safety cabinet work surface was sprayed with a 0.5% ivermectin spray and wiped down immediately. The spray was used as precautionary measure to minimize the possibility of transmission of fur mites to the sentinels from sources other than soiled bedding. No cages were placed inside the cabinet during the spraying or wiping, and at no time did the soiled bedding or experimental colony animals come into contact with the ivermectin spray. This solution was prepared by performing a 1:10 dilution of 5% Ivomec (Merial, Duluth, GA) with water. Ivermectin solutions were replaced with fresh solutions every 2 wk. Gloves were changed between each cage change.
Experimental colony 1.
The cages comprising experimental colony 1 were housed on a conventional rack and were divided into 2 groups. Half of the rack (35 cages, 35 animals), unexposed controls, received approximately 355 cm3 clean, nonsoiled bedding at cage change. The remaining half of the rack, exposed sentinels, received approximately 355 cm3 of a composite sample of soiled bedding from the mite-infested colony. The exposed sentinels in experimental colony 1 received 100% soiled bedding from 70 cages of mite-infested mice, thus simulating a situation in which all animals on a 70-cage rack are infested with mites.
Experimental colony 2.
The cages comprising experimental colony 2 were housed on a conventional rack and were divided into 2 groups. Half of the rack (35 cages, 35 animals), unexposed controls, received approximately 355 cm3 of clean, nonsoiled bedding at cage change. The remaining half rack, exposed sentinels, received approximately 177.4 cm3 of a composite sample of soiled bedding from the mite-infested colony and 177.4 cm3 of clean nonsoiled bedding. Therefore the exposed sentinels in experimental colony 2 received 50% soiled bedding from 70 cages of mite-infested mice and 50% clean nonsoiled bedding, thus simulating a situation in which half (35) of the mice on a 70-cage rack are infested with mites.
Experimental colony 3.
The cages comprising experimental colony 3 were housed on a conventional rack and were divided into 2 groups. Half of the rack (35 cages, 35 animals), unexposed controls, received 355 cm3 of clean, nonsoiled bedding at cage change. The remaining half rack, exposed sentinels, received approximately 59.2 cm3 of a composite sample of soiled bedding from the mite-infested colony and 236.6 cm3 of clean, nonsoiled bedding. Therefore the exposed sentinels in experimental colony 3 received 20% soiled bedding from 70 cages of mite-infested mice and 80% clean nonsoiled bedding, thus simulating a situation in which one fifth (20%) of the mice (14 animals) on a 70-cage rack are infested with mites.
Experimental colony 4.
The cages comprising experimental colony 4 were housed on a conventional rack and were divided into 2 groups. Half of the rack (35 cages, 35 animals), unexposed controls, received 355 cm3 of clean, nonsoiled bedding at cage change. The remaining half rack, exposed sentinels, received approximately 29.6 cm3 of a composite sample of soiled bedding from the mite-infested colony and 236.6 cm3 of clean, nonsoiled bedding. Therefore the exposed sentinels in experimental colony 4 received 11% soiled bedding from 70 cages of mite-infested animals and 89% clean nonsoiled bedding, thus simulating a situation in which approximately one-tenth of the mice (7 or 8 animals) are infested with mites.
A control group was included for each experimental colony to eliminate possible confounding related to the rack and rack location within the room where the mice were housed.
Contact sentinels.
Seven, 6- to 8-wk-old, C3H/HeNCrl, female mice (Charles River Laboratories) were placed into 7 randomly selected cages of the mite-infested colony at week 5. At week 8, these animals were screened for fur mites by using the cellophane tape test. C3H/HeNCrl sentinels were selected because they were of a different coat color and size than were the mite-infested mice, thereby facilitating identification.
Fur-mite testing.
A cellophane tape test was the diagnostic method used to identify fur-mite infestations in live animals.5,14,25 A 4-cm2 piece of clear cellophane tape was rubbed retrograde on the head, neck, and ventrum of each mouse; affixed to a glass slide; and examined under a light microscope at 40× magnification. A positive fur-mite test was any sample that yielded at least one Myobia or Myocoptes egg or adult that was identified definitively by an experienced reader. A negative fur-mite test was any sample that yielded no Myobia or Myocoptes fur-mite eggs or adults that could be identified by an experienced reader.
Samples for testing were collected from all experimental colony animals, by 1 of 3 trained individuals, immediately prior to cage change every 2 wk for a period of 12 wk. Each group was tested 6 times after the onset of soiled bedding exposure. A 12-wk time period was selected for this study to mimic a quarterly sentinel testing program. All samples were read by 2 experienced readers who were blinded to which samples came from the soiled-bedding sentinels and which came from the clean-bedding sentinels. The blinding was achieved by placing black electrical tape over the slide labels prior to slide reading. This tape was placed by a person not involved in the collection or reading of slides. Each reader independently read all slides with the slide label covered by tape. The reader placed all slides with positive results in one slide box and those with negative results in a separate slide box. After the reader completed reading all slides, the tape was removed and the results recorded. There were no discrepancies between the results submitted from the 2 independent blinded slide readers.
At weeks 5 and 11, all cages from the mite-infested colony were rescreened to verify continued status as an infested cage. Egg and mite counts from these slide readings were documented as previously described. In addition, a sample of soiled bedding was collected from the soiled bedding collection container after cage change of the fur-mite–infested colony at week 11. This sample was examined under a light microscope for the presence of mites or eggs. Pieces of 4-cm2 cellophane tape were pressed against the sample of soiled bedding, affixed to glass slides and examined under a light microscope at 40× magnification for the presence of fur-mite adults or eggs.
At the termination of the experiment, the end of week 12, 4 mice from each experimental colony (2 unexposed controls and 2 exposed sentinels) and 4 from the fur-mite–infested colony were submitted to the University of California–Davis pathology laboratory for pelage collection and examination.
Statistical analysis.
All statistical analyses were conducted by using STATA 11.0 (StataCorp, College Station, TX). Categorical egg count data at weeks 0, 5, and 11 was analyzed by using repeated-measures logistic regression. Adult mite counts were analyzed by using the Friedman test and the Wilcoxon signed rank-sum test with a Bonferroni correction factor. An α level of P ≤ 0.05 was considered to indicate a statistically significant difference among weeks.
Results
Fur-mite–infested colony.
All of the 70 cages from the fur-mite–infested colony tested positive for fur mites within 7 d prior to the onset of the experiment (week 0) and at weeks 5 and 11 (Figure 1). The 4 mice from the infested colony that were submitted for pelage collection and examination at week 12 tested positive for fur mites by a comparative diagnostic laboratory. Several fur-mite eggs and adults were identified through microscopy of the random sample of soiled bedding collected from the mite-infested colony at week 11.
Figure 1.
(A) Adult fur mite, Myocoptes musculinus, and (B) fur-mite eggs from cellophane tape test samples taken from animals in the fur-mite–infested colony. Magnification, ×40.
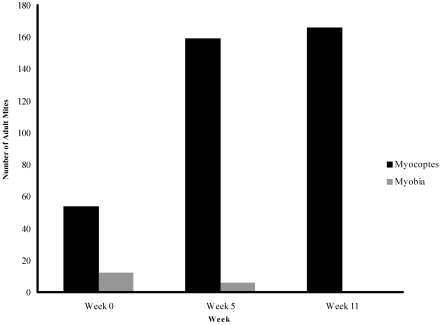
When adult mites were identified from the fur-mite–infested colony, the species was documented. At week 0, Myobia mites, Myocoptes mites, or both were identified among the infested cages. Because some cages in the infested colony tested positive for fur-mite eggs only, we were unable to conclude that all cages had a mixed infestation with Myobia and Myocoptes. At week 11, only Myocoptes mites were identified from the samples collected (Figure 2). This finding may be explained by previous reports that Myocoptes mites often crowd out Myobia mites in mixed heavy infestations.1,17
Figure 2.
Total numbers of adult fur mites identified, by species, at weeks 0, 5, and 11 from the 70 mice sampled (1 mouse from each cage) in the fur-mite–infested colony.
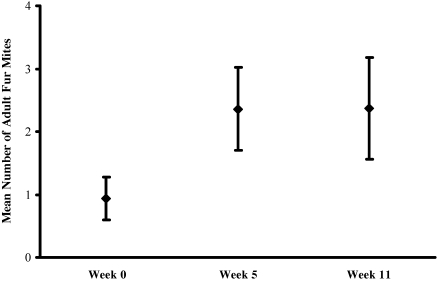
The number of eggs identified per slide among the fur-mite–infested mice was classified into 5 ordinal groups: 1 or 2, 3 to 5, 6 to 10, 11 to 20, and greater than 20. The percentage of slides in the highest egg-count category (mean egg counts greater than 20) was 57%, 79%, and 84% at weeks 0, 5, and 11, respectively. Slides collected at weeks 5 and 11 were significantly (P < 0.01) more likely to be categorized in the highest egg-count category when compared with slides collected at week 0 (Table 1). The mean number of adult mites identified per slide at weeks 5 and 11 was greater (P < 0.01) than that at week 0 (Figure 3). There was a statistically significant (P < 0.01) difference between the underlying distributions of the fur-mite counts at weeks 5 and 11 and those at week 0. The number of adult mites identified at week 5 did not differ significantly from that identified at week 11.
Table 1.
Number of slides with fur-mite eggs among the 70 mice (1 per each cage) sampled from the infested colony
| No. of slides with egg counts |
P (95% confidence interval) | ||
| Week | ≤20 | >20 | |
| 0 | 30 (43%) | 40 (57%) | Baseline |
| 5 | 14 (20%) | 56 (80%) | P < 0.01 (0.38, 1.82) |
| 11 | 11 (16%) | 59 (84%) | P < 0.01 (0.62, 2.16) |
Repeated-measures logistic regression was used to determine associated P values and 95% confidence intervals.
Figure 3.
Mean number of adult fur mites identified per slide at weeks 0, 5, and 11. One mouse from each of the 70 cages in the fur-mite–infested colony was sampled. The mean numbers of adult fur mites identified at weeks 5 and 11 were significantly (P < 0.01) different from that at week 0. Error bars represent 95% confidence intervals.
Experimental colonies.
At weeks 2, 4, 6, 8, and 10, all exposed sentinels (n = 35 from each colony) and unexposed controls (n = 35 from each colony) from the 4 experimental colonies tested negative for fur mites. At week 12, 1 of the 35 (3%) exposed sentinels from experimental colony 1, which received 100% soiled bedding, tested positive for fur-mite eggs by tape test and was confirmed positive by pelage collection and examination. The remaining exposed sentinels and unexposed controls from all experimental colonies tested negative for fur mites at week 12. The 4 randomly selected animals from each experimental colony submitted for pelage collection and examination at week 12 tested negative for fur mites.
Contact sentinels.
At week 5, 7 naïve contact sentinels were placed into 7 random cages in the fur mite infested colony. At week 8, these mice were screened for fur mites by using the cellophane tape test, and all 7 tested positive for both fur mites and eggs.
Discussion
The results of this study suggest that the use of soiled bedding sentinels for the detection of fur mites is unreliable. Over a 12-wk period, 35 CRL:CD1(ICR) sentinels were exposed to 100% soiled bedding from fur-mite–infested animals. At the end of 12 wk, only 1 of these 35 (3%) animals tested positive for fur mites. In addition, the 105 mice exposed to 12 wk of 50%, 20%, and 11% soiled bedding from mite-infested animals consistently tested negative for fur mites every 2 wk for a 12-wk period.
The sample size required in routine health surveillance to detect a particular agent has been described and is correlated to the probability of detecting the infection and the assumed infection rate (prevalence).27 In the present study, because the sample size and prevalence are known, the probability of detecting the infestation can be calculated. According to this analysis, 35 mice is a sufficient sample size to detect at least one positive animal, with a probability of 97%, from a colony with a prevalence of 10%. In addition, a sample size of 140, the number of exposed sentinels in this study, is sufficient to detect at least one positive animal, with 99% probability, from a colony with a prevalence of 3%. This analysis is based on the assumption that soiled bedding serves as a perfect vehicle for the transmission of fur mites to naïve animals. Therefore, our results indicate that although a sufficient number of mice were sampled, soiled-bedding sentinels do not develop fur mite infestations at a rate sufficient to serve as an effective method of fur-mite surveillance.
The increases in fur-mite egg and adult counts noted at weeks 5 and 11 in the infested animals confirm that these animals were not self-clearing the infestations. Mite populations do undergo cyclic fluctuations every 20 to 25 d as eggs hatch.1 The increased counts of eggs and mites at week 5 and 11 may have corresponded to such fluctuations. At week 8, the 7 contact sentinels were screened for fur mites by using the cellophane tape test. All tested positive for both mites and eggs. These results confirm that the infested mice were contagious through direct contact during the study period.
Light microscopic examination of soiled bedding from infested animals indicated the presence of both mites and eggs. This discovery is interesting and suggests that despite the presence of fur mites within the bedding, the ability of mite infestations to transfer from bedding to live animals is inefficient. Environmental exposure by means of fomites may be an unlikely mode of transmission for fur mites, and actual animal-to-animal contact may be necessary for efficient transmission.
Advantages of the present study include the use of blinded slide readers to prevent observational bias as well as the use of a comparative pathology laboratory for some confirmatory diagnostic testing. An additional advantage of this study was the extensive measures taken to prevent transmission of adult fur mites or eggs to experimental animals from sources other than soiled bedding. These measures included frequent glove changes, the use of separate biological safety cabinets for infested animals, and frequent decontamination of the safety cabinet surface with dilute ivermectin. Although there is a possibility that some residual aerosolized ivermectin was present in the safety cabinet during cage change, the likelihood that this situation affected the transmission of mites to the sentinels is negligible. Several studies have examined the efficacy of topical and oral ivermectin in the treatment of fur mites.5,8,14,29,36,43 To our knowledge, no study has demonstrated aerosolized ivermectin to be an effective treatment or preventative measure for murine fur mites.
Several diagnostic methods are available for the detection of murine acariasis. Conflicting information exists regarding which diagnostic method is the most effective.1,2,5,17 An evaluation of 5 diagnostic methods for murine fur mites concluded that skin scraping was the most accurate method.5 In that study, pelage collection agreed with skin scraping results 80% of the time, whereas tape test results agreed with those from skin scraping 74% of the time.5 Others1,2,17 consider the dorsal tape test to be a very effective diagnostic method and more accurate than pelage collection and examination. This diagnostic method involves the placement of a 5.5 ×10 cm piece of transparent tape on the back of a euthanized mouse for 6 h. The tape then is examined for fur mite eggs and adults by using a light microscope.1,2,17
For our present study, we selected the cellophane tape test as the diagnostic method in order to most accurately mimic the sentinel program used at this and many other institutions. Therefore, one limitation of our study was the reliance on tape test results alone for the identification of fur-mite–positive animals. The exact sensitivity of the cellophane tape test is unknown but has been estimated to be approximately 84%.3,14 The sensitivity of this test is likely quite high with heavy mite burdens but may be reduced with low-level infestations. Attempts to reduce the likelihood of false negatives among the experimental colony animals included frequent sampling and sampling of neck, head, and ventrum of all subjects. In addition, 4 mice from each experimental colony were submitted for pelage collection and examination at the end of the 12 wk. These results were consistent with those obtained from the cellophane tape tests. Inclusion of an additional diagnostic method on all animals at the end of the study, such as pelage collection or the dorsal tape test, may have reduced the probability of false-negative results. However, we do not believe that this inclusion would affect our conclusion regarding the efficacy of a soiled-bedding–based sentinel program for fur-mite identification.
Another potential limitation of the present study was the selection of a 12-wk follow-up period. This duration may have been insufficient to capture the potential transmission of acariasis through soiled-bedding exposure. A longer follow-up period may have increased the number of exposed sentinels that developed fur-mite infestations. However, such an outcome would not have practical utility for institutions relying on quarterly sentinel testing for information on colony health status.
Our results disagree with those of earlier studies34,38 that confirmed that soiled bedding based sentinels may be effective for the identification of fur-mite infestations in colony animals. Compared with our present study, one previous study differed in that fewer cages were used (4 cages), the mice were followed for a longer period of time (19 wk), and fur-mite infestations in sentinel animals were diagnosed by using pelage collection and examination.38 In our present study, the use of the cellophane tape test as the primary diagnostic method has been identified as a potential limitation. However, at the end of our 12-wk exposure period, 2 exposed cages from each of the experimental colony groups (11%, 20%, 50%, and 100% soiled bedding) were submitted to a comparative pathology lab for pelage collection and examination, and all animals were found to be negative for fur mites. Therefore, the primary difference in design between the earlier study38 and our present study is the duration of soiled-bedding exposure.
Historically, our institution has relied on quarterly cellophane tape tests of soiled-bedding sentinels for information on ectoparasite infestations of colony animals. One sentinel animal is placed for every 70 cages of colony animals, and soiled bedding from each colony cage is transferred to the sentinel cage at cage change. The design of this experiment was intended to mimic our current sentinel program and evaluate its efficacy in providing accurate information on the ectoparasite status of colony animals. On the basis of this study, a 12-wk soiled-bedding sentinel program does not serve as a sensitive means for identifying fur-mite–infestations in colony animals. This conclusion is true even when the infestation rate approaches 100%. In most outbreak situations, it would be very unlikely to have 100% (70 cages) of animals infested with fur mites. In the most recent fur mite outbreak at our institution, only 20% to 50% of cages on any one rack were diagnosed as infested. Institutions using similar sentinel testing strategies should consider alternatives to soiled bedding sentinels, perhaps including periodic testing of colony animals or the use of naïve contact sentinels. Future research is necessary to evaluate the most effective sentinel program for the evaluation of ectoparasite status in laboratory animal facilities. In addition, importations of nonvendor mice from institutions relying solely on a soiled-bedding sentinel program should be viewed with caution. Mice imported from nonvendor facilities should be tested and treated for acariasis in quarantine before entrance into facilities with colony animals is permitted.
Sentinel programs by their very nature rely on exposure of the sentinel to colony animals through direct contact, contact with bedding, or environmental contact. One advantage of such a program is the efficiency of sampling a single animal that reflects the health status of the group. Our study is the first to demonstrate that for fur mite detection, a soiled-bedding sentinel program is ineffective and unreliable.
Acknowledgments
We thank the University of California–Davis Comparative Pathology Laboratory for sharing technical expertise and Jamie Austin for providing statistical consultation.
References
- 1.Baker DG. 2007. Arthropods, p 565–579 Fox GB, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research, 2nd ed St Louis (MO): Academic Press [Google Scholar]
- 2.Baker DG. 2007. Parasites of rats and mice, p 359–363 Baker DG. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing [Google Scholar]
- 3.Bornstein DA, Scola J, Rath A, Warren HB. 2006. Multimodal approach to treatment for control of fur mites. J Am Assoc Lab Anim Sci 45:29–32 [PubMed] [Google Scholar]
- 4.Brielmeier M, Mahabir E, Needham JR, Lengger C, Wilhelm P, Schmidt J. 2006. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: a field study. Lab Anim 40:247–260 [DOI] [PubMed] [Google Scholar]
- 5.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 6.Charles River [Internet] 2010. Prevalent rodent infectious agents (PRIA) PCR panel: a quarantine testing tool. [Cited 19 May 2010]. Available at: http://www.criver.com/SiteCollectionDocuments/rm_ld_r_PRIA_PCR_Panel.pdf
- 7.Compton SR, Homberger FR, Paturzo FX, Clark JM. 2004. Efficacy of 3 microbiological monitoring methods in a ventilated cage rack. Comp Med 54:382–392 [PubMed] [Google Scholar]
- 8.Conole J, Wilkinson MJ, McKellar QA. 2003. Some observations on the pharmacological properties of ivermectin during treatment of a mite infestation in mice. Contemp Top Lab Anim Sci 42:42–45 [PubMed] [Google Scholar]
- 9.Cundiff DD, Riley LK, Franklin CL, Hook RR, Jr, Besch-Williford C. 1995. Failure of a soiled bedding sentinel system to detect cilia-associated respiratory bacillus infection in rats. Lab Anim Sci 45:219–221 [PubMed] [Google Scholar]
- 10.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 11.Dillehay DL, Lehner ND, Huerkamp MJ. 1990. The effectiveness of a microisolator cage system and sentinel mice for controlling and detecting MHV and Sendai virus infections. Lab Anim Sci 40:367–370 [PubMed] [Google Scholar]
- 12.Fraser J, Joiner GN, Jardine JH, Galvin TJ. 1974. The use of pelleted dichlorvos in the control of murine acariasis. Lab Anim 8:271–274 [DOI] [PubMed] [Google Scholar]
- 13.Friedman S, Weisbroth SH. 1977. The parasitic ecology of the rodent mite, Myobia musculi. IV. Life cycle. Lab Anim Sci 27:34–37 [PubMed] [Google Scholar]
- 14.Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: a method to eradicate murine fur mites. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 15.Iijima OT, Takeda H, Komatsu Y, Matsumiya T, Takahashi H. 2000. Atopic dermatitis in NC/Jic mice associated with Myobia musculi infestation. Comp Med 50:225–228 [PubMed] [Google Scholar]
- 16.Ike F, Bourgade F, Ohsawa K, Sato H, Morikawa S, Saijo M, Kurane I, Takimoto K, Yamada YK, Jaubert J, Berard M, Nakata H, Hiraiwa N, Mekada K, Takakura A, Itoh T, Obata Y, Yoshiki A, Montagutelli X. 2007. Lymphocytic choriomeningitis infection undetected by dirty-bedding sentinel monitoring and revealed after embryo transfer of an inbred strain derived from wild mice. Comp Med 57:272–281 [PubMed] [Google Scholar]
- 17.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120 Fox JG, Andersen LC, Loew FM, Quimby FW. Laboratory animal medicine. New York (NY): Academic Press [Google Scholar]
- 18.Johnston NA, Trammell RA, Ball-Kell S, Verhulst S, Toth LA. 2009. Assessment of immune activation in mice before and after eradication of mite infestation. J Am Assoc Lab Anim Sci 48:371–377 [PMC free article] [PubMed] [Google Scholar]
- 19.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos MA, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th2 lymphocytes. Scand J Immunol 43:604–612 [DOI] [PubMed] [Google Scholar]
- 20.Jungmann P, Guenet JL, Cazenave PA, Coutinho A, Huerre M. 1996. Murine acariasis: I. Pathological and clinical evidence suggesting cutaneous allergy and wasting syndrome in BALB/c mouse. Res Immunol 147:27–38 [DOI] [PubMed] [Google Scholar]
- 21.Koszdin KL, DiGiacomo RF. 2002. Outbreak: detection and investigation. Contemp Top Lab Anim Sci 41:18–27 [PubMed] [Google Scholar]
- 22.Laltoo H, Van Zoost T, Kind LS. 1979. IgE antibody response to mite antigens in mite-infested mice. Immunol Commun 8:1–9 [DOI] [PubMed] [Google Scholar]
- 23.Lipman NS, Homberger FR. 2003. Rodent quality assurance testing: use of sentinel animal systems. Lab Anim (NY) 32:36–43 [DOI] [PubMed] [Google Scholar]
- 24.Manuel CA, Hsu CC, Riley LK, Livingston RS. 2008. Soiled-bedding sentinel detection of murine norovirus 4. J Am Assoc Lab Anim Sci 47:31–36 [PMC free article] [PubMed] [Google Scholar]
- 25.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 26.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43 [DOI] [PubMed] [Google Scholar]
- 27.National Research Council 1991. Principles of rodent disease prevention, p 5–7 Companion guide to infectious diseases of mice and rats. Washington (DC): National Academy Press [Google Scholar]
- 28.Otto G, Tolwani RJ. 2002. Use of microisolator caging in a risk-based mouse import and quarantine program: a retrospective study. Contemp Top Lab Anim Sci 41:20–27 [PubMed] [Google Scholar]
- 29.Papini R, Marconcini A. 1991. Treatment with ivermectin in drinking water against Myobia musculi and Myocoptes musculinus mange in naturally infected laboratory mice. Angew Parasitol 32:11–13 [PubMed] [Google Scholar]
- 30.Pence BC, Demick DS, Richard BC, Buddingh F. 1991. The efficacy and safety of chlorpyrifos (Dursban) for control of Myobia musculi infestation in mice. Lab Anim Sci 41:139–142 [PubMed] [Google Scholar]
- 31.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur-mite–infested mice. Eur J Immunol 36:2434–2445 [DOI] [PubMed] [Google Scholar]
- 32.Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci 44:26–28 [PubMed] [Google Scholar]
- 33.Reuter JD, Dysko RC. 2003. Quality assurance–surveillance monitoring programs for rodent colonies, p 1–13 Reuter JD, Suckow MA. Laboratory animal medicine and management. Ithaca (NY): International Veterinary Information Service [Google Scholar]
- 34.Ricart Arbona RJ, Lipman NS, Wolf F. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 35.Scharmann W, Heller A. 2001. Survival and transmissibility of Pasteurella pneumotropica. Lab Anim 35:163–166 [DOI] [PubMed] [Google Scholar]
- 36.Skopets B, Wilson RP, Griffith JW, Lang CM. 1996. Ivermectin toxicity in young mice. Lab Anim Sci 46:111–112 [PubMed] [Google Scholar]
- 37.Smith PC, Nucifora M, Reuter JD, Compton SR. 2007. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp Med 57:90–96 [PubMed] [Google Scholar]
- 38.Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327 [PubMed] [Google Scholar]
- 39.Watson DP. 1961. The effect of the mite Myocoptes musculinus (CL Koch 1840) on the skin of the white laboratory mouse and its control. Parasitology 51:373–378 [DOI] [PubMed] [Google Scholar]
- 40.Watson J. 2008. New building, old parasite: mesostigmatid mites—an ever-present threat to barrier facilities. ILAR J 49:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]
- 42.Welter A, Mineo JR, de Oliveira Silva DA, Lourenco EV, Vieira Ferro EA, Roque-Barreira MC, Maria da Silva N. 2007. BALB/c mice resistant to Toxoplasma gondii infection proved to be highly susceptible when previously infected with Myocoptes musculinus fur mites. Int J Exp Pathol 88:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wing SR, Courtney CH, Young MD. 1985. Effect of ivermectin on murine mites. J Am Vet Med Assoc 187:1191–1192 [PubMed] [Google Scholar]
- 44.Yamaguchi T, Maekawa T, Nishikawa Y, Nojima H, Kaneko M, Kawakita T, Miyamoto T, Kuraishi Y. 2001. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J Dermatol Sci 25:20–28 [DOI] [PubMed] [Google Scholar]