Abstract
In a chronically compressed dorsal root ganglion (CCD) in the rat, a model of foraminal stenosis and radicular pain in human, a subpopulation of neurons with functional axons exhibits spontaneous activity (SA) that originates within the ganglion. Intracellular electrophysiological recordings were obtained from the somata of neurons of the compressed ganglion both in vitro and in vivo. The SA was classified into two types according to the presence (type I) or absence (type II) of subthreshold membrane potential oscillation. Neurons with type I SA had significantly higher somal excitability than those with type II SA. In most cases, depolarization of the membrane potential by current injection increased the discharge rates of type I - but not type II SA. Both types occasionally coexisted in the same neuron. Several lines of evidence suggested that the origin of SA in the DRG was most likely the soma for type I SA and the axon for type II. Therefore CCD neurons have multiple sites for generation of action potentials other than the terminal endings. In vivo recordings revealed the same two types of SA in a subpopulation of neurons with functionally characterized peripheral receptive fields. Thus, SA might not only produce spurious sensory input to the afferent pathways but also add to or block impulse transmission generated by natural stimulation of peripheral receptors. SA originating in the compressed ganglion is likely to interfere with sensory transmission in nociceptive and non-nociceptive neurons, thereby contributing to radicular pain, paresthesias, hyperalgesia and allodynia.
Keywords: spontaneous activity, oscillation, hyperexcitability, dorsal root ganglion, chronic compression, neuropathic pain
Introduction
Somatic primary sensory neurons are designed to “speak only when spoken to” and to do what they are told. It is the external stimulus that typically dictates what distal terminal endings should say in a train of action potentials transmitted to the CNS. But an injured or inflamed sensory neuron may develop an opinion of its own when a portion of the axon or cell body (soma) becomes sufficiently hyperexcitable to generate ectopic, “spontaneous” action potentials (SA) (Wall and Devor, 1983; Bennett and Xie, 1988; Song et al., 1999; Liu et al., 2000; Xu et al., 2000; Xie et al., 2005). The SA, and the corresponding abnormal sensations (paresthesias), may arise from the cell body or axon of a neuron that develops either an intrinsic capacity to generate action potentials such as an oscillatory ionic mechanism in the membrane, or develops an abnormal sensitivity to bodily derived stimuli extrinsic to the neuron such as core temperature, changes in posture or chemical factors released from neighboring cells (Devor and Seltzer, 1999). Depending on the sensory submodality of the neuron, the SA may maintain a state of central sensitization, evoke innocuous sensations such as tingling or elicit outright pain (LaMotte, 2006). For many pathological conditions that contribute to neuropathic and inflammatory pain, there is limited information about whether the SA is axonal or somal, intrinsically or extrinsically evoked; and little is known of the electrophysiological and sensory properties of neurons that express SA in vivo.
To address these issues, the present study investigated the SA generated in intact neurons by a chronic compression of the dorsal root ganglion (CCD) – a rat model of foraminal stenosis and radicular pain in humans (Hu and Xing, 1998; Song et al., 1999; Zhang et al., 1999; Ma and LaMotte, 2005). The electrophysiological properties of spontaneously active neuronal somata were first recorded in vitro from the intact DRG. These properties could be differentiated into two categories according to whether the SA originated from the soma or the axon. During electrophysiological recordings obtained from visualized neuronal somata on the surface of the ganglion, in vivo, the same two types of SA were found to exist in neurons with functionally characterized peripheral receptive fields and were shown to alter sensory transmission. Thus, SA may contribute to paresthesiae, allodynia, hyperalgesia and pain during CCD and possibly other inflammatory/neuropathic disorders.
Materials and Methods
Surgical procedure for rod implantation
Adult female Sprague Dawley rats weighing 150–250 g were housed in groups of three or four in a climate-controlled room under a 12 h light/dark cycle. The use and handling of animals were approved by the Institutional Animal Care and Use Committee of the Yale University School of Medicine and were in accordance with guidelines provided by the National Institutes of Health and the International Association for the Study of Pain.
In CCD rats (n = 45), under pentobarbital sodium anesthesia (Nembutal, 50 mg/kg i.p.), the transverse process and intervertebral foramina of L4 and L5 were exposed unilaterally as previously described (Song et al., 1999). A stainless steel L shaped rod (0.63 mm in diameter and 4 mm in length) was inserted into each foramen, one at L4 and the other at the L5 ganglion. The incision was closed in layers. Five to 10 d after surgery, the correct placement of each implanted rod was confirmed when the ganglion was harvested for recording. Because all the rods were in the correct position (Song et al., 1999; Yao et al., 2003), all DRGs from CCD animals were accepted for the experiment. Thirty-eight unoperated rats were used as controls.
In vitro intracellular electrophysiological recording from somata in the intact DRG
Twenty-five CCD and 20 control rats were used for intracellular recording from visualized somata in intact DRG neurons, in vitro, as previously described (Zhang et al., 1999; Ma et al., 2006). Briefly, under pentobarbital anesthesia (50 mg/kg i.p.), the L4 and L5 DRGs with their corresponding dorsal roots, spinal nerves and sciatic nerve above the mid-thigh level were removed from the animal and transferred to a chamber perfused with oxygenated artificial CSF (ACSF). The ACSF contained (in mm): 130 NaCl, 3.5 KCl, 24 NaHCO3, 1.25 NaH2PO4, 1.2 MgCl2, 1.2 CaCl2, and 10 Dextrose. The solution was bubbled with 95% O2 and 5% CO2 and had a pH of 7.4 and an osmolarity of 290–310 mOsm.
The recording chamber was mounted on the stage of an upright microscope (BX50-WI, Olympus Optical, Tokyo, Japan). The bath solution was preheated to 36 ± 1°C by means of an in-line heater with a servo-controller (TC-344A, Warner Instruments Inc., Hamden, CT). The intracellular recording electrodes were fabricated from borosilicate glass (World Precision Instruments, Sarasota, FL) and pulled on a Flaming/Brown micropipette puller (P-97, Sutter Instrument, Novato, CA). Electrodes were filled with 1.0 M KCl (impedance: 40∼80 MΩ) and positioned by a micromanipulator (MIS-5000, Burleigh Instruments, Fisher, NY). Electrical pulses were delivered through either of two suction electrodes, one applied at the end of dorsal root and the other at the end of the sciatic nerve.
Each neuron under study was classified by the size of its soma and the conduction velocity of its dorsal root axon. The somal size, obtained visually from a mean of its longest and shortest diameter, was categorized as small (S, < = 30 μm), medium (M, 31∼45 μm) or large (L, >45 μm) (Zhang et al., 1999; Ma et al., 2003). A dorsal-root axon was classified as respectively as either a C-, Aδ-, or Aα/β-fiber according to whether its respective dorsal root conduction velocity (CVdr) was <1.2 m/s, < 7.5 or >7.5 m/s (Harper and Lawson, 1985; Zhang et al., 1999; Ma et al., 2006).
Electrophysiological recordings were collected with bridge mode under current clamp using an AxoClamp-2B (Molecular Devices, Palo Alto, CA), stored digitally via a Digidata 1322A interface, and analyzed off-line with pClamp 8 software (Molecular Devices). The electrode resistance was balanced by a bridge circuit in the amplifier. A neuron was accepted for study only when it exhibited a resting membrane potential (RMP) more negative than −45 mV. Current steps, each 100 ms duration, were delivered through the intracellular recording electrode in increments of 0.05 nA from −0.5 to 4 nA. The input resistance (Rin, MΩ) was calculated from the slope of a steady-state I-V plot obtained from responses to a series of hyperpolarizing currents steps from −0.5 to −0.05 nA. The current threshold (Rheobase, nA) was defined as the minimal depolarizing current required to evoke an action potential. The voltage threshold (VT, mV) was defined as the first point on the rising phase of an action potential at which the change in voltage exceeded 50 mV/ms.
In some cases (n = 11 cells from 10 CCD rats), collagenase P (10 mg/ml in ACSF; Roche Diagnostics, Mannheim, Germany) was applied locally to the surface of DRG for 10 min and repeated two to three times separated by 10 min washout, until the somata to be studied were fully separated from the surrounding connective tissue. A preliminary study in our laboratory found that Collagenase P at this concentration did not have a significant effect on the membrane properties of DRG neurons from CCD or control animals (data not shown). The soma was then pulled out from the surface of ganglion by a glass suction pipette with a fire-polished tip (inner diameter close to the soma) with the stem axon still connected. The soma was impaled with a sharp-electrode again. After recording the action potential and CV, the electrode was withdrawn and the neuron was pulled away farther from the surface of ganglion, until the stem axon was broken. Then the soma was impaled with the sharp-electrode for a third time (for details, see Fig. 5). By this method, we could determine whether the site of origin of spontaneous activity was in (or very close to) the soma or in the axon.
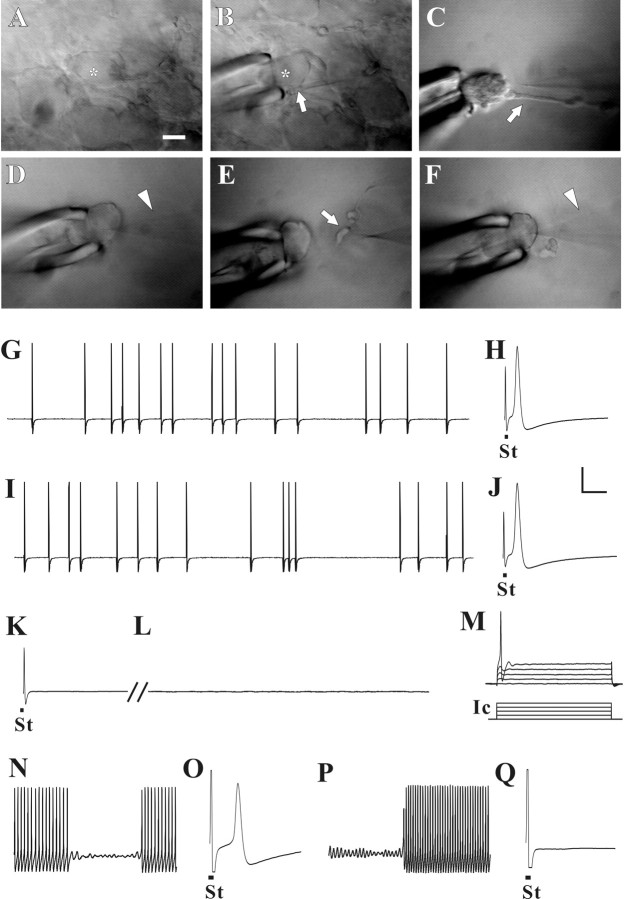
Figure 5.
Acute axotomy provided direct evidence that type I SA originated in or near the soma and that type II SA originated in the axon. In each experiment, intracellular recording by sharp electrode was first obtained from the soma of a DRG neuron (A, marked with asterisk) to determine the type of SA. This particular neuron exhibited type II SA (G). An axonal CV of 20 m/s was obtained by electrical stimulation (St) of the dorsal root (H). The electrode was withdrawn, and, after local application of collagenase, a fire-polished pipette and gentle suction was used to pull the soma (asterisk, B) away from the surface of ganglion (C) while leaving the axon intact (arrow in B and C). Intracellular recording was obtained again from soma with the sharp electrode (D, arrowhead) to confirm the presence of SA (I) and CV (J). Then, the electrode was withdrawn and the soma pulled farther from the surface of ganglion, thereby severing the axon (E, arrow). Sharp-electrode (arrowhead) intracellular recording was obtained from the soma for a third time (F) to confirm the absence of an action potential evoked by stimulation of the dorsal root (K) and to assess the presence or absence of SA and somal excitability. Although SA was no longer present in this neuron (L), an action potential could be elicited by current injection (Ic) from the recording electrode (M). In another DRG neuron, type I SA could be recorded from the soma (N), and a CV of 21 m/s was obtained by stimulation of the dorsal root (O). After the axon was severed, as described, type I SA remained (P), but the CV could no longer be obtained (Q). Scale bar: A–F, 20 μm. Vertical calibration bar: G–N, 20 mV; Ic in J, 1 nA. Horizontal calibration bar: H, J, K, O, Q, 2 ms; G, I, L, 100 ms; M, 20 ms; N, P, 50 ms.
In vivo intracellular electrophysiological recording from visualized somata on the surface of DRG
In vivo recordings were performed in 26 CCD and 18 control animals. Under pentobarbital anesthesia (initial dose of 50 mg/kg i.p. followed by 20 mg · kg−1 · h−1 as needed), the L5 transverse process was removed to expose the L4 spinal nerve, and a laminectomy was performed at the levels of L1-L6. The L4 DRG was exposed, and the L4 dorsal roots were transected just before their entry to the spinal cord. Oxygenated ACSF was dripped periodically on to the surface of the ganglia during the surgical procedure. Under a dissecting microscope, the sheath covering the surface of the DRG (perineurium and epineurium) was carefully removed using fine forceps and scissors. The animal was then transferred to a platform attached to the recording table. The spinal process was clamped at the T12 and S1 regions, and a pool was formed by suturing the skin to a metal ring. The ganglion was then placed on a spoon-shaped platform under the microscope (Olympus, BX50WI). The two platforms, one holding the ganglion and the other the animal, were mechanically isolated from each other allowing the lower limb to be manipulated during the search for receptive fields without transmitting unwanted mechanical stimuli to the electrophysiologically recorded neuron. The DRG was continuously perfused at a rate of 3∼4 ml/min with oxygenated ACSF. The temperature of the ACSF around the ganglion was maintained at ∼35°C by a heater and controller (Warner Instrument, TC-344A).
Intracellular recordings were obtained from neurons on the surface of DRG, visualized by means of reflected-light epi-illumination. The insertion of a cross-positioned analyzer and polarizer served to reduce glare and enhance the contrast of the image. This in vivo intracellular recording preparation is an improved version of the one we previously described in which the DRG was externalized and placed in a recording chamber while retaining partial connectivity via a separated portion of nerve with the lower hindlimb (Ma et al., 2003). The present preparation allowed the DRG and nerve to remain in situ thereby minimizing any collateral, surgical damage that might reduce the chances of finding the receptive fields for a DRG neuron.
Classification of the receptive field properties of DRG neurons.
The receptive properties of DRG neurons were classified, using hand-held stimulators, according to standard criteria (Burgess and Perl, 1967; Leem et al., 1993; Lawson et al., 1997). Muscle spindle afferents were classified by their characteristic responses to changes in muscle length, probing of the muscle belly and slight taps to the tendon. Mechanoreceptive cutaneous afferents were classified as either low- or high-threshold (LTM or HTM) depending, respectively, on whether they were activated by innocuous stimulation with a soft brush, hair movement or blunt, gentle pressure or, conversely, required activation by mild pinching. The HTMs were additionally classified according to whether they also responded to noxious heat stimulation (51°C, 5 s) as delivered by a servo-controlled Peltier thermode (LaMotte et al., 1991), and/or noxious cold (ice-water, 4°C, 20 s). Because mechanical stimuli were used as the primary search stimuli for most of the cases in our study, the search procedure was biased against identifying the properties of mechanically insensitive units.
Criteria for classifying a neuron as spontaneously active.
For each neuron isolated for study, a continuous recording was obtained for 3 min without the delivery of any external stimulus. If spontaneous ongoing discharge occurred during this period, the neuron was classified as spontaneously active only if the activity could not be attributable to normal, ongoing activation of peripheral receptors. For example, the tonic activity of a muscle spindle afferent was easily manipulated by changing the length of the muscle; similarly, on-going activity in a low-threshold, cold-sensitive afferent could be inhibited by gently warming the skin (Leem et al., 1993). Any “injury discharge” that appeared on occasion immediately after electrode insertion, and lasted no longer than 30 s, was ignored. Because we have never observed SA with a firing rate of <0.1 Hz in the CCD model, it seems unlikely that we might have misclassified a neuron with a low rate of SA as silent during the initial 3 min of recording; even exceptionally low rates (Wu et al., 2001) would likely result in at least one impulse during our observation period,
Statistical analyses
Data values are presented as means with SEMs. Student's t tests (SigmaStat version 2.03; SPSS, San Rafael, CA) were used to determine the statistical significance of differences between means obtained from two different groups of neurons. One-way ANOVAs followed by post hoc pairwise comparisons (Student–Newman–Keuls method) (SigmaStat 2.03) were used to determine the statistical significance of differences between means obtained from three or more experimental groups. χ2 tests were used to assess differences in the percentages of neurons with SA. A probability of 0.05 was chosen as the criterion for significance.
Results
In vitro electrophysiological recordings
CCD neurons exhibited two types of SA
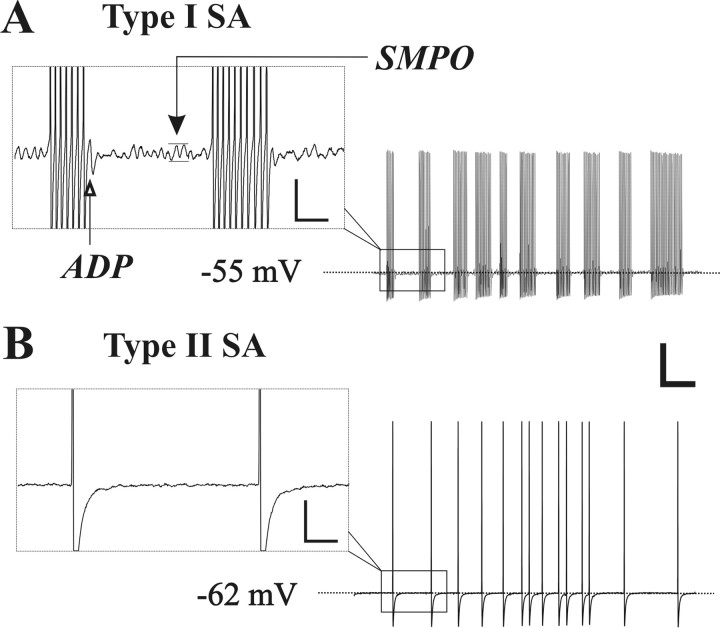
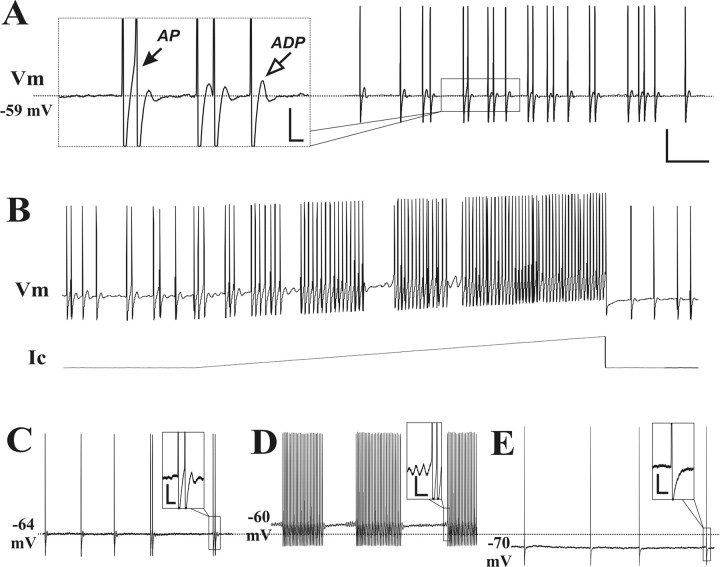
In vitro, sharp-electrode intracellular recordings were obtained from 344 neurons in the chronically compressed DRG and 102 neurons in control ganglia from unoperated rats. Thirty-seven (10.8%) of the CCD neurons exhibited SA. Expressed as a proportion of the total number recorded in each size category, SA was found in 11.5% (21 of 182) large-sized, 12.9% (13 of 101) medium-sized, and 4.9% small-sized (3 of 61) neurons. In contrast, only one control neuron (1%), with a large-sized soma, exhibited SA. The SA could be classified into two distinct types according to the presence (type I) or absence (type II SA) of a subthreshold membrane potential oscillation (SMPO) >2 mV in amplitude (Fig. 1). The distinction between two types of SA was usually clear because the oscillatory amplitude of the resting membrane potential in these neurons was either >2 mV or <1 mV (which was close to the recording noise level) with very few cases falling in between. Another characteristic of type I SA was an afterdepolarization (ADP) that followed each single action potential or burst of action potentials (Fig. 1A). In most cases, the ADP was not detectable for type II SA (Fig. 1B).
Figure 1.
Two types of SA, in CCD neurons, distinguished according to the presence or absence of SMPO. A, Intracellular recording from the soma of a large-diameter Aβ neuron exhibiting a bursting pattern of spontaneous firing and SMPO (black arrow in the inset) that is characteristic of type I SA. The open arrow indicates the presence of an ADP after the occurrence of a burst of action potentials. B, Another large-diameter Aβ neuron exhibited type II SA that was irregularly firing and characterized by the absence of SMPO and ADP. Calibration: A, B, 20 mV, 100 ms; insets, 5 mV, 20 ms.
Among the 34 large- and medium-sized SA neurons from CCD animals, 20 were type I (58.8%, 9 large- and 11 medium-sized) and 14 were type II (41.2%, 12 large and two medium-sized). The latter neurons with type II SA included two neurons that exhibited ADPs but were classified as type II on the basis of the absence of SMPO.
The mean amplitudes of the SMPO and the ADP were significantly greater for those with type I SA (4.10 ± 0.20 and 2.40 ± 0.21 mV, respectively) than for neurons with type II SA (0.90 ± 0.05 and 0.26 ± 0.11 mV). The discharge frequency was significantly higher for type I SA (69.5 ± 19.3 Hz, range 5.1 ∼ 188.1 Hz) than for type II (13.5 ± 2.9 Hz, range 0.6 ∼ 49.8 Hz).
The SA of all three small-diameter CCD neurons (discharge frequencies of 0.6, 15.5 and 29.2 Hz, respectively) and the single large-sized, control neuron (79.1 Hz) were classified as the type I variety. Because of the small sample size, these neurons were not included in the present analyses.
As described in previous studies of CCD and certain animal models of peripheral nerve injury, the firing patterns of SA recorded from DRG neurons could be classified into three major categories: tonic (regular/continuous), bursting, and irregular (Hu and Xing, 1998; Devor and Seltzer, 1999; Zhang et al., 1999; Ma et al., 2003). The interspike interval (isi) was constant for the tonic SA but highly variable for the irregular. Bursting SA was characterized by a fixed isi during each burst and a fixed or variable isi between bursts. Based on these criteria, we found that the incidence of each of the three firing patterns differed for the two types of SA. Of the 20 neurons with type I SA, 60% exhibited bursting, 20% tonic and 20% an irregular pattern. In contrast, only 7% of the 14 type II neurons exhibited bursting, 7% tonic and 86% irregular. Thus, although all three patterns were found in both types of SA, the most common pattern of firing was bursting for type I and irregular for type II.
Type I and II SA neurons had different electrophysiological properties
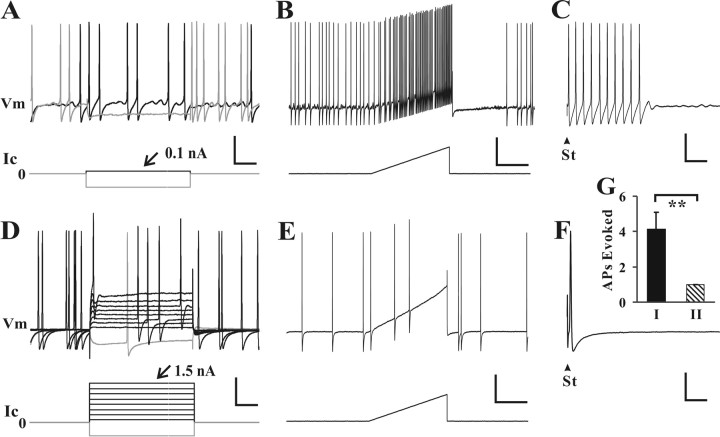
The somata of neurons with type I and type II SA exhibited differences in excitability. For example, when stimulated by current injection, the rheobase of a neuron with type I SA was very close to zero (Fig. 2A). In contrast, the rheobase of a typical neuron with type II SA was much greater than zero, similar to that of a silent (non-SA) CCD neuron (Fig. 2D).
Figure 2.
The somata of neurons with type I SA were more excitable than those with type II SA. A–F, Typical responses to current injection delivered through the recording electrode were obtained in two large-diameter Aβ neurons, one exhibiting type I SA (A–C) and the other, type II SA (D–F). Step increments in current (Ic) were delivered through the recording electrode until an action potential was evoked (A, D) followed by a current ramp (B, E). The rheobase current required to elicit an action potential (number on top of the current traces) was lower and the number of APs evoked by the ramp considerably greater in the neuron with type I SA. Also, type I SA, but not type II, was inhibited by a hyperpolarization of the membrane potential, Vm (voltage response shown as the lighter voltage trace superimposed on the darker in A and D). Refer to Figure 3 for mean values of somal membrane properties of neurons with type I and II SA. When testing the conduction velocity, electrical stimulation (St) of the dorsal root sometimes evoked multiple action potentials in the neuron with type I SA (C) but always only a single action potential in the neuron with type II SA (F). G, Mean number of action potentials (APs) evoked in neurons with type I (filled bar) and type II SA (hatched bar). Vertical calibration bars: A–F, 20 mV (Vm), 1 nA (Ic). Horizontal calibration bars: A, D, 20 ms; B, E, 200 ms; C, F, 10 ms. **p < 0.01, t test.
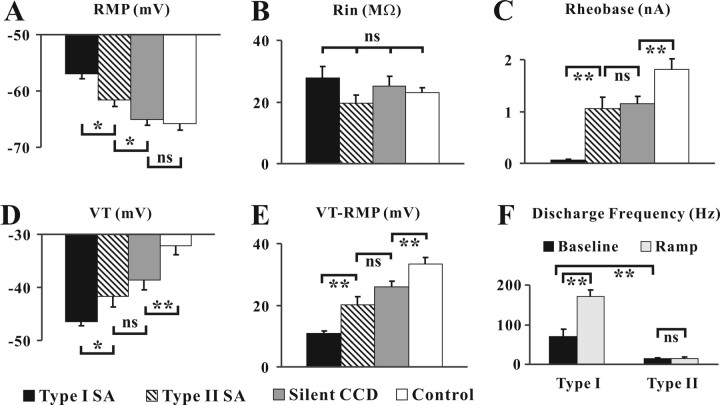
A series of measurements of somal excitability were obtained for each of four groups of neurons: control (naive) neurons (n = 32) and CCD neurons that were silent (n = 33), exhibited type I SA (n = 20), or exhibited type II SA (n = 14). Combining the data for medium- and large-sized neurons, mean values for each of the groups were obtained for the membrane potential RMP, input resistance, rheobase, voltage threshold (VT), the difference between RMP and VT, and the discharge frequency during a current ramp (Fig. 3). Compared with neurons in the other three groups, neurons with type I SA exhibited a significantly more depolarized mean RMP, lower mean rheobase, and a more hyperpolarized mean voltage threshold that was closer to RMP (Fig. 3A,C–E). No significant difference between groups was found for the mean input resistance (Fig. 3B). The mean discharge frequency of basal discharge of type I SA was significantly greater than type II SA and increased to a significantly greater degree by a depolarizing ramp of current (Figs. 2B,E, 3F). Note that the lower baseline rate of the type II SA was unaffected by the ramp (Figs. 2E, 3F). In neurons with type I SA both the discharge rate and the amplitude of SMPO could be enhanced by RMP depolarization and reduced by hyperpolarization. Therefore, type I, but not type II SA, was dependent on somal excitability.
Figure 3.
Measures of somal excitability in neurons with or without each type of SA. A–E, Somal membrane properties including RMP (A), Rin (B), rheobase (C), voltage threshold (VT; D) and the difference between VT and RMP (VT-RMP; E) were obtained from CCD neurons with type I (filled bars) or type II SA (hatched bars), CCD neurons without SA (Silent CCD; gray bars), and naive neurons (Control; open bars). F, Discharge frequency before (baseline) and during the depolarizing current ramp recorded from neurons with two types of SA. Neurons with type I SA exhibited a significantly greater somal excitability compared with CCD neurons with type II SA or without SA each of which was more excitable than control neurons. *0.01 < p < 0.05; **p < 0.01; ns, no significant difference (one-way ANOVA for A–E, t test for F).
Neurons with type I SA also exhibited a significant decrease in accommodation. Current injection at rheobase level (100 ms duration) (Fig. 2A,D) evoked an average of 11.7 ± 1.4 spikes in neurons with type I SA but only 2.1 ± 0.4 in those with type II SA (p < 0.001, t test). When stimulating the dorsal root, multiple spikes could be evoked in neurons with type I SA (Fig. 2C,G), whereas almost all the neurons of type II SA responded with only a single action potential (Fig. 2F,G) as did control neurons.
The differences in these electrophysiological properties for neurons with type I and II SA were not particular for a given size of neuron because they were equally present in neurons of both large and medium diameter (data not shown). There were no significant differences in measures of excitability, such as rheobase and accommodation, for CCD neurons of the same somal size category that were silent as opposed to those exhibiting type II SA (Fig. 3A–E) although both types were more excitable than control neurons in accordance with previous findings (Zhang et al., 1999; Song et al., 2003; Ma et al., 2006). Among the 20 neurons with type I SA, five showed an inflection in the falling phase of action potential and were presumably nociceptive (Koerber et al., 1988; Ritter and Mendell, 1992; Ma et al., 2003). In contrast, none of the 14 type II SA neurons showed the inflection. Therefore neurons with type I SA included both putative nociceptors and non-nociceptors, whereas type II SA was only found in putative non-nociceptive neurons.
The two types of SA were occasionally present in the same DRG neuron
In two cases, types I and II SA coexisted in the same DRG neuron. In these neurons, although the SA was not associated with obvious SMPO (barely exceeded 1 mV and therefore originally classified as type II), each action potential was followed by an ADP that sometimes triggered another action potential (Fig. 4A,C). The increase in discharge rate and the amplitude of SMPO during the depolarizing current ramp (Fig. 4B) were suggestive of the presence of type I SA. Depolarization in these (two) neurons induced SMPO and burst firing of type I SA, whereas hyperpolarization eliminated type I SA leaving type II SA unaffected (Fig. 4C–E). SMPO could also be induced in three other neurons with type II SA during the depolarizing current injection. However, no additional action potentials were triggered during depolarization. Therefore the frequency of SA discharge remained the same as measured at resting membrane potential.
Figure 4.
Coexistence of the two types of SA in the same neuron. A, The first of each group of spontaneous action potentials in a large-diameter neuron was not preceded by an oscillation of the membrane potential (Vm), a characteristic of type II SA as shown on left in an enlargement of the boxed area of the trace on right. However, the action potential (AP) was followed by an ADP (open arrow) that sometimes triggered another action potential (black arrow), a feature of type I SA. B, The vigorous discharges and SMPO evoked by a depolarizing current ramp (Ic) is another characteristic of type I SA (same neuron as in A). C–E, Another large-diameter neuron exhibited both type I and II SA at resting potential (−64 mV). Depolarization to −60 mV (D) induced SMPO and firing, a characteristic of neurons with type I SA, whereas a hyperpolarization to −70 mV (E) eliminated type I SA, leaving type II SA unaffected. Vertical calibration bars: unexpanded traces in A–E, 20 mV; insets of A and C–E, 5 mV; Ic in B, 1 nA. Horizontal calibration bars: A, B, 100 ms; C–E, 200 ms; insets, 10 ms.
Type I SA most likely originated in the soma whereas type II SA originated in the axon
Indirect evidence for the site of origin of each type of SA.
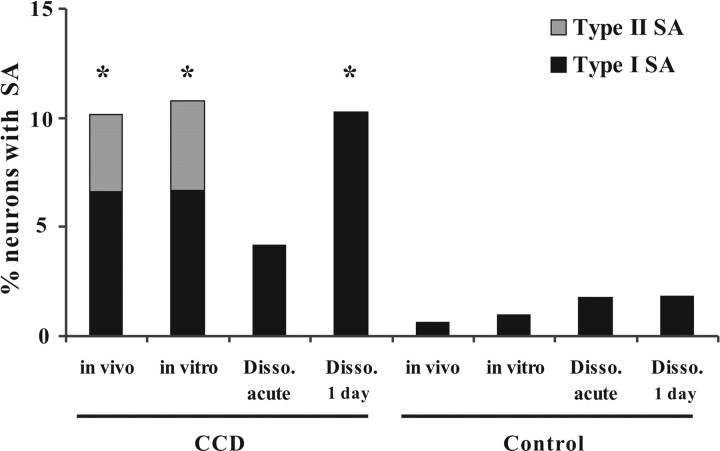
For neurons with type I SA, the presence of SMPO, a depolarized RMP, a close-to-zero rheobase, close-to-RMP VT, and the increasing of discharge frequency during somal depolarization were suggestive of the SA originating in the soma. Conversely, for neurons of type II SA, the lack of SMPO, a higher rheobase and VT, and the absence of a change in discharge frequency in response to somal depolarization, were suggestive of a more remote origin, most likely in the axon – but still within the DRG because CCD-induced SA has been shown to originate within the DRG (Song et al., 1999). Although we observed two types of SA in the intact DRG preparation, only type I SA was present in dissociated CCD neurons deprived of their axons and cultured for 3∼30 h (Ma and LaMotte, 2005, their Fig. 6). The absence of type II SA in dissociated cells also supports the notion that type I SA originates in the soma and type II in the axon.
Figure 6.
Percentage of neurons with each type of SA in CCD and control rats. Type II SA was present in CCD neurons recorded both in vivo and in the intact dorsal root–DRG nerve preparation in vitro but not in acutely dissociated (Disso.) or 1 d cultured CCD neurons or in control neurons. Type I SA was found in all conditions. *p < 0.05 compared with control groups, χ2 test.
Direct evidence for the site of SA generation.
In experiments with 11 DRG neurons, 4 of which were first found to exhibit exhibiting type I SA and 7 others type II SA, collagenase P was applied locally to the surface of DRG to loosen the soma and separate it from surrounding tissue (Fig. 5A,B). The soma of the previously recorded neuron was then pulled away from the surface of ganglion, via gentle suction with a micropipette, without mechanically stressing or severing its axon (Fig. 5C). The soma was again penetrated by the recording electrode to confirm both the presence of the SA and the CV (and thus the functional integrity of the axon) (Fig. 5D). After again withdrawing the electrode, the soma was pulled sufficiently far away from the surface of the DRG to break the stem axon at a site that was in close proximity to the soma (< 50 μm) (Fig. 5E). The sharp-electrode was again used to obtain intracellular recording from the soma to test for the presence of SA, the absence of a CV (to confirm the axotomy) and the capability of the cell to produce an action potential in response to injected current (Fig. 5F). All the 7 DRG neurons with type II SA lost their SA after axotomy yet still exhibited action potentials in response to depolarizing current steps delivered through the recording electrode to the soma (Fig. 5G–M). In contrast, 3 of the 4 neurons with type I SA still exhibited spontaneous firing after axotomy (Fig. 5N–Q); the other neuron ceased exhibiting SMPO and SA during the pulling/axotomy procedure but still responded to current injection. These findings provided direct evidence that type I SA originated in the soma and that type II SA originated in the axon. They also supported the notion that the SA in these “super-acutely dissociated” neurons was intrinsic to the soma and not stimulus evoked, for example, by chemical factors or mechanical stresses applied to the soma by surrounding, intraganglionic tissue.
An additional experiment was performed to determine whether type II SA was generated within the DRG: After initial recording, the nerve and dorsal root was transected close to the DRG and the neuron was recorded for the second time using a sharp electrode. Five of six large-diameter Aβ neurons tested continued to exhibit type II SA. The results are consistent with previous findings in our laboratory that CCD-induced SA originated within or close to the DRG (Song et al., 1999).
In vivo electrophysiological recordings
CCD neurons, recorded in vivo, exhibited two types of SA and other properties similar to those recorded in vitro
In vivo, intracellular recording was obtained from 227 DRG neurons on the surface of the chronically compressed ganglion. SA was found in 23 of these neurons (10.1%) including 13 with large-, 9 with medium- and 1 with small-diameter somata. The electrophysiological properties of DRG neurons recorded in vivo were similar to those recorded in vitro both in the present study and in earlier studies (Zhang et al., 1999; Ma et al., 2005). The SA from CCD neurons recorded in vivo could be classified into two types according to the presence or absence of SMPO. Eight large, six medium and one small-diameter somata were classified as exhibiting type I SA. The other 5 large and 3 medium-diameter somata were categorized as expressing type II SA. The incidence of SA in control and CCD animals was similar for the in vivo and in vitro preparations (Fig. 6). Excluding data for the one small neuron, the mean diameter of the somata was not significantly different for neurons with type I (48.9 ± 1.7 μm) and type II SA (47.9 ± 2.6 μm).
In accordance with results obtained in vitro, neurons with type I SA exhibited significantly different properties from those of neurons with type II SA, including higher amplitudes of SMPO (2.49 ± 0.28 vs 0.64 ± 0.08 mV for type I and type II SA, respectively, p < 0.01, t test) and ADP (2.72 ± 0.29 vs 0.05 ± 0.05 mV, p < 0.01), a higher discharge frequency (35.3 ± 6.1 vs 9.0 ± 6.2 Hz, p < 0.05), a more depolarized RMP (−55.9 ± 1.5 vs −64.6 ± 2.2 mV, p < 0.01), lower rheobase (0.16 ± 0.03 vs 1.15 ± 0.23 nA, p < 0.01) and a reduced VT-RMP (10.2 ± 0.9 vs 25.4 ± 3.7 mV, p < 0.05), although no significant difference was found in voltage threshold (−45.8 ± 1.9 vs −39.2 ± 4.4 mV) or input resistance (11.7 ± 2.3 vs 17.9 ± 3.2 MΩ). In 155 neurons from control animals, only one large neuron exhibited SA (0.6%); it was type I.
The proportions of SA firing patterns and the differences in the incidence of each pattern for the two types of SA were similar to those recorded in vitro. Of the 20 neurons with type I SA, 64% exhibited bursting, 22% tonic and 14% irregular discharge. In contrast, of the 14 neurons with type II SA, only 14% had a bursting pattern, 14% had tonic firing and 72% exhibited an irregular discharge. Thus, the patterns of SA in vivo, as in vitro, were predominantly bursting for type I and irregular for type II.
SA of both types could interfere with the transmission of sensory information from peripheral receptors
The receptive properties of all the CCD neurons recorded in vivo and the incidence of SA within each type of sensory modality are summarized in Table 1. Of the 204 neurons recorded, 38.8% had muscle spindles, 30%, cutaneous low-threshold mechanoreceptors, and 8.8% nociceptors. Muscle spindle afferents had the highest incidence of SA and nociceptive afferents the lowest. The sensory properties could not be determined for the remaining 48 neurons (22.5%), including 3 with SA. The absence of a receptive field might have resulted from a variety of factors such as axonal damage during the surgical preparation (e.g., neurons innervating the skin or muscle of the back) or an inappropriate search strategy as a result of our focus on mechanically responsive neurons (see Materials and Methods).
Table 1.
Receptive field properties of chronically compressed DRG neurons with and without SA, determined in vivo
| Modality | Non-SA | Type I SA | Type II SA | SA% |
|---|---|---|---|---|
| Non-nociceptive | ||||
| MS | 73 | 11 | 4 | 17.0% |
| ALTM-RA, hair. | 32 | 1 | 1 | 5.9% |
| ALTM-RA, glab. | 19 | 2 | 9.5% | |
| ALTM-SA | 13 | 0.0% | ||
| Nociceptive | ||||
| AHTM | 13 | 1 | 7.1% | |
| CHTM | 6 | 0.0% | ||
| Unknown | ||||
| A-type | 35 | 1 | 1 | 5.4% |
| C-type | 13 | 1 | 7.1% | |
| Total | 204 | 15 | 8 | 10.1% |
The table provides the number of neurons of each sensory modality (or unknown modality) that was silent (Non-SA) or exhibited type I SA or type II SA (the SA% representing the total percentage of neurons with SA). MS, Muscle spindle; ALTM-RA, ALTM-SA, A-type low-threshold mechanoreceptor, rapidly adapting and slowly adapting, respectively; hair., hairy skin; glab., glabrous skin; AHTM, CHTM, A-type and C-type high-threshold mechanoreceptor, respectively. Because the primary method of searching for receptive fields was mechanical stimulation, our samples were biased toward mechanosensitive nociceptors. AHTMs and CHTMs also included those that responded to nociceptive heat and/or cold stimuli.
We were able to identify the peripheral receptive field properties of 13 neurons with type I SA and 7 with type II, all of them “A-type,” i.e., with large or medium-diameter neurons with conduction velocities indicative of myelinated axons.
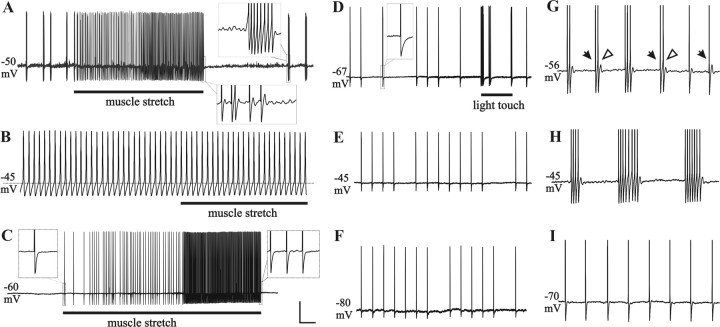
Action potentials generated from the axon or soma in the DRG will either “add to” or “collide with” (thereby blocking) action potentials generated by the natural stimulation of peripheral receptors. Clearly the highest rates of SA would produce the greatest amount of interference with sensory transmission. For example, after CCD, neurons with type I SA, including those with muscle spindles, had the highest rates (Fig. 7A–C).
Figure 7.
In vivo recordings of type I SA or type II SA in CCD neurons with functionally characterized peripheral receptive fields. A–I, Typical recordings of type I SA are shown for two muscle spindles afferents (A–C for one neuron, G–I for another), and of type II SA for a cutaneous, low-threshold mechanoreceptive (guard hair) neuron (D–F). All three neurons were large-diameter, A-types (with myelinated axons). Recordings were first obtained at RMP (A, D, G), and then current was injected to depolarize (B, E, H) and hyperpolarize (C, F, I) the soma. SMPO and ADP were present in type I SA (see insets in A) but not type II SA (inset in D). Despite the ongoing spontaneous discharges, these neurons responded to natural stimuli of their receptive fields (bar indicates stimulus duration in A–D). Depolarization of the somal membrane potential to approximately −45 mV by injecting positive current (0.2 nA) increased the discharge rate and changed the pattern of type I SA, e.g., from bursting to continuous in B. During depolarization, the soma no longer responded to stimulation of the receptive field. In the same neuron, hyperpolarization of the somal membrane potential (C) by injecting negative current (−0.5 nA) eliminated the type I SA as well as the SMPO and ADP (insets in C), whereas action potentials could still be evoked by stimulating the receptive field. In the neuron with type II SA, depolarization to −45 mV (E; 0.36 nA) or hyperpolarization to −80 mV (F; −0.4 nA) had no obvious effect on the pattern or frequency of SA. G–I provides another example of interactions between peripheral input and type I SA. Action potentials were truncated to save space. Throughout the recording period, the hindlimb and muscle length was maintained in a fixed position. During hyperpolarization (I), action potentials generated from the peripheral muscle spindle receptor were observed in the absence of extra, interfering action potentials generated from the soma. At RMP (G), individual action potentials generated from the muscle (filled arrows) could sometimes trigger extra spikes from the soma (open arrows). As the duration of bursts increased during depolarization (H), the pattern of peripheral input could no longer be observed in the soma. Vertical calibration bar: A–E, 20 mV; G–I, insets, 10 mV. Horizontal calibration bar: A, C, 1 s; B, 20 ms; D–F, 300 ms; G–I, insets, 40 ms.
In addition, action potentials generated from the periphery could trigger extra action potentials originating in the neuronal somata with SMPO. Thus, for neurons exhibiting both types of SA, the source of input action potentials could be the natural stimulation of the receptive field (Fig. 7G–I), an electrical stimulation of the nerve or root (Fig. 2C), or SA intrinsically generated from the axon or the soma (Fig. 4).
To investigate the relationship between action potentials generated from the soma and the periphery, we altered the excitability of the soma by injecting depolarizing or hyperpolarizing current. During depolarization, type I SA was enhanced (Fig. 7B,H), sometimes to the point that it totally blocked the input produced by stimulating the receptive field (Fig. 7B). In contrast, type I SA was inhibited during hyperpolarization, leaving only the action potentials originating from the periphery (Fig. 7C,I). Peripherally generated activity in neurons with type II SA was usually unaffected by the changes in the somal RMP produced by current injection (Fig. 7D–F). It's worth noting that action potential activity recorded from the soma is not necessarily indicative of activity that occurs at the central terminals of the recorded neurons.
Discussion
Multiple sites for the generation of SA in CCD neurons
Under normal conditions, action potentials in a primary sensory neuron are generated from the peripheral nerve ending and conducted axonally into the CNS. However, after injury or inflammation to the nerve or ganglion, the DRG or the peripheral nerve may become a source of ectopic SA (Devor and Seltzer, 1999; LaMotte, 2006).
In the sciatic-nerve axotomy model, SA was generated from both the DRG and the neuroma (Wall and Devor, 1983; Amir et al., 2005). The DRG could also develop SA in the absence of axotomy, as observed after CCD (Hu and Xing, 1998; Song et al., 1999). Type II SA, as presently described, disappeared from the soma after an axotomy ∼50 μm from the axon hillock but remained after transection of the dorsal root and nerve. Thus, it probably originated from the axon at a site within or close to the DRG. Because type II SA was not modulated by changes in the RMP, the site of electrogenesis, for example at the T-junction (Amir et al., 2005) or elsewhere, must be sufficiently far away to disallow the influence of electrical events in the soma. Indeed, the stem axons of certain large-sized somata with myelinated axons may be hundreds of micrometers in length (Ito and Takahashi, 1960; Ha, 1970; Amir and Devor, 2003a). As the site of electrogenesis may also be mechanically sensitive (Chen and Devor, 1998), mechanical probing of the DRG, although not applicable in the present study because of the mechanical stability requirements for sharp electrode recording, could be combined with extracellular or intra-axonal recording to search for sites of SA generation.
Based on present findings, it is likely that type I SA originates within the soma and not the stem axon. The incidence of type I SA in dissociated neurons that were deprived of their stem axons roughly approximates that observed in the intact CCD ganglion recorded both in vitro and in vivo (Fig. 6). After a spinal nerve injury, dissociated DRG neurons, including those fluorescently labeled from muscle and skin were also found to express SMPO that sometimes triggered SA (Liu et al., 2002). Our finding that type I SA remains after the cell body is pulled away from the ganglion suggests that it is not maintained by chemical factors released from neighboring cells or mechanical stresses on the cell (Rydevik et al., 1989). It was still possible that type I SA might originate from the axon hillock particularly if not completely dissociated or separated from the soma.
The occasional coexistence of type I and II SA suggests the possibility of two ectopic sources of SA in one DRG neuron. The sites of electrogenesis for the two types of SA must be different because the modulation of RMP only affected type I but not type II SA in these neurons. Unlike control neurons, chronically compressed neurons could have multiple sites of action potential generation in addition to the peripheral nerve ending. These additional sites provide the possibility of interactions between inputs from peripheral receptors and the ectopic SA in the axon or soma.
Functional significance of spontaneous ectopic generators in the soma and axon
Using reflected-light epi-illumination microscopy, the improved in vivo recording preparation allowed us to identify more receptive fields for DRG neurons than before (Ma et al., 2003). The electrophysiological properties of neurons within each sensory modality were similar to those previously described (Koerber et al., 1988; Leem et al., 1993; Lawson et al., 1997; Ma et al., 2003). Our in vivo recordings suggest that SA from both soma and axon may interfere with the transmission of action potentials from the peripheral sensory terminals to the CNS. For example, a continuous- or long-duration bursting pattern of type I SA could totally “block” the action potentials from peripheral receptors whereas a relatively low frequency of type II SA might just add to the peripheral input. Because of the possibility of a conduction failure at the T-junction, we cannot predict the actual pattern of action potentials delivered into the spinal cord (Luscher et al., 1994; Amir and Devor, 2003a,b). Although the majority of SA neurons were muscle spindles and cutaneous low-threshold mechanoreceptors, we did find type I SA in some Aδ- and C-neurons in vitro and in vivo. These neurons each exhibited an inflection on the falling phase of the action potential – a characteristic of nociceptors. The smaller sample of nociceptive neurons with C- as opposed to A-fibers in the present in vivo experiment may have resulted from the difficulty in maintaining recording from small-sized neurons for the duration of time required to identify the location of a high-threshold receptive field using noxious stimulation. Nevertheless, the overall incidence of SA among small-sized, C-neurons in vivo (including those with unknown receptive fields) was only 5%, similar to what we found in vitro (4.9%). In an in vivo study of a modified spinal nerve injury model (Djouhri et al., 2006), SA was found in ∼35% of nociceptive C-neurons in the L4 DRG associated with an inflamed spinal nerve. It is uncertain at this point, whether these differences in the incidence of SA in C-nociceptive neurons relate to differences in the nature of the injurious stimulus (chromic-gut ligation vs rod implantation), the location of the injury (nerve vs ganglion) or to other factors.
Both Types of SA could contribute to abnormal innocuous sensations, pain and enhanced pain states, by adding to or altering the transmission of sensory information normally delivered from peripheral receptors to higher order neurons in the somatic afferent pathway. Ectopic discharges in nociceptive neurons may elicit a chronic state of central sensitization (Ji and Woolf, 2001). Ectopic discharges in low-threshold mechanoreceptive afferent neurons might elicit annoying paresthesiae. In addition, a hypothesis to test is that CCD causes a novel expression of neuropeptides and/or neurotrophic factors in low-threshold neurons with ectopic discharge. Such discharge might then act to enhance the excitability of second order neurons thereby inducing and maintaining a state of central sensitization (Woolf and Doubell, 1994; Ma and Woolf, 1996; Neumann et al., 1996; Fukuoka et al., 2001).
Possible mechanisms of spontaneous activity originating from the soma versus the axon
Very little is known about whether there are differences in somal and axonal mechanisms of generating SA. For type I SA with a bursting or irregular pattern, it was proposed that an oscillation triggered the first spike of each burst of discharges (Amir et al., 1999, 2002b). The mechanism for SMPO might be attributable to the interaction between a tetrodotoxin (TTX)-sensitive Na+ conductance and a passive voltage-independent K+ conductance (Pedroarena et al., 1999; Amir et al., 2002a; Enomoto et al., 2006). However, it is not clear which currents might contribute to the different patterns of SA, or to the oscillation-induced triggering of action potentials. CCD produces an upregulation of TTX-sensitive and resistant Na+ currents and the hyperpolarization-activated currents, and a downregulation of transient potassium currents (Yao et al., 2003; Tan et al., 2006). Those changes may induce a decrease in the current- and voltage-thresholds and accommodation, which could potentially contribute to the generation of SA.
Mechanisms contributing to SMPO and SA in the soma might also apply to the axon (Kapoor et al., 1997; Baker, 2000). However, the mechanisms are likely to differ for the soma and axon given the different patterns of firing for the two types of SA and the lack of afterdischarge in type II SA. The type II SA we observed is similar to that originating from a neuroma (Wall and Devor, 1983; Amir et al., 2005) except for the absence of axotomy after CCD. Type II SA might have been induced in the axon by excitatory substances such as inflammatory mediators (Ma et al., 2006) and chemokines (White et al., 2005) released from neighboring neurons and non-neuronal cells in a chronically compressed DRG. Another possibility is that a partial demyelination of the axon, although not blocking the conduction of action potentials from the periphery, produced hyperexcitability and SA. Although we have no direct evidence for a causal relationship between SA and partial demyelination in the CCD model, previous studies have shown that demyelinated axons could produce SA possibly via a mechanism involving axonal SMPO, elevated periaxonal K+ concentration and persistent Na+ currents (Calvin et al., 1977; Felts et al., 1995; Kapoor et al., 1997; Baker, 2000; Nonaka et al., 2000).
In conclusion, spontaneous action potentials can be generated from both the somata and the axons of chronically compressed DRG neurons. Type I SA originated in or near a hyperexcitable soma that exhibited SMPO. Type II SA originated from the axon and was not accompanied by somal SMPO. The same two types of SA were recorded in vivo in a subpopulation of neurons with functionally characterized peripheral receptive fields. These observations demonstrate that SA can originate from multiple locations in a DRG neuron under pathological conditions and may alter the transmission of sensory information from peripheral receptors, in vivo.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS14624. We thank Dr. David Donnelly and Dr. Sally Lawson for helpful information generously provided.
References
- Amir R, Devor M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys J. 2003a;84:2181–2191. doi: 10.1016/S0006-3495(03)75024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Devor M. Extra spike formation in sensory neurons and the disruption of afferent spike patterning. Biophys J. 2003b;84:2700–2708. doi: 10.1016/S0006-3495(03)75075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Liu CN, Kocsis JD, Devor M. Oscillatory mechanism in primary sensory neurones. Brain. 2002a;125:421–435. doi: 10.1093/brain/awf037. [DOI] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci. 2002b;22:1187–1198. doi: 10.1523/JNEUROSCI.22-03-01187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Kocsis JD, Devor M. Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci. 2005;25:2576–2585. doi: 10.1523/JNEUROSCI.4118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Axonal flip-flops and oscillators. Trends Neurosci. 2000;23:514–519. doi: 10.1016/s0166-2236(00)01645-3. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol (Lond) 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin WH, Howe JF, Loeser JD. Ectopic repetitive firing in focally demyelinated axons and some implications for trigeminal neuralgia. In: Anderson D, Matthews B, editors. Pain in the trigeminal region. Amsterdam: Elsevier/North-Holland; 1977. pp. 125–136. [Google Scholar]
- Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain. 1998;2:165–178. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzacki R, editors. Textbook of pain. Ed 4. London: Churchill Livingstone; 1999. pp. 129–164. [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci. 2006;26:3412–3422. doi: 10.1523/JNEUROSCI.5274-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Kapoor R, Smith KJ. A mechanism for ectopic firing in central demyelinated axons. Brain. 1995;118:1225–1231. doi: 10.1093/brain/118.5.1225. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H. Axonal bifurcation in the dorsal root ganglion of the cat: a light and electron microscopic study. J Comp Neurol. 1970;140:227–240. doi: 10.1002/cne.901400206. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol (Lond) 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S-J, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Ito M, Takahashi I. Impulse conduction through spinal ganglion. In: Katsuki Y, editor. Electrical activity of single cells. Tokyo: Igakushoin; 1960. pp. 159–179. [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Li YG, Smith KJ. Slow sodium-dependent potential oscillations contribute to ectopic firing in mammalian demyelinated axons. Brain. 1997;120:647–652. doi: 10.1093/brain/120.4.647. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- LaMotte RH. Spontaneous ectopia. In: Schmidt RF, Willis WD, editors. Encyclopedia of pain. Berlin: Springer; 2006. [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai E. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurons in guinea-pig. J Physiol (Lond) 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Liu CN, Devor M, Waxman SG, Kocsis JD. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol. 2002;87:2009–2017. doi: 10.1152/jn.00705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Streit J, Quadroni R, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994;72:622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Ma C, Greenquist KW, LaMotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Honmou O, Sakai J, Hashi K, Kocsis JD. Excitability changes of dorsal root axons following nerve injury: implications for injury-induced changes in axonal Na+ channels. Brain Res. 2000;859:280–285. doi: 10.1016/s0006-8993(00)01979-x. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Pose IE, Yamuy M, Chase MH, Morales FR. Oscillatory membrane potential activity in the soma of a primary afferent neuron. J Neurophysiol. 1999;82:1465–1476. doi: 10.1152/jn.1999.82.3.1465. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Rydevik BL, Myers RR, Powell HC. Pressure increase in the dorsal root ganglion following mechanical compression. Closed compartment syndrome in nerve roots. Spine. 1989;14:574–576. doi: 10.1097/00007632-198906000-00004. [DOI] [PubMed] [Google Scholar]
- Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- Song XJ, Vizcarra C, Xu D-S, Rupert RL, Wong Z-N. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol. 2003;89:2185–2193. doi: 10.1152/jn.00802.2002. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol. 2006;95:1115–1123. doi: 10.1152/jn.00830.2005. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain: increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21(RC140):1–5. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Huang Mae LY, Zhao ZQ. Activation of silent mechanoreceptive cat C and Aδ sensory neurons and their substance P expression following peripheral inflammation. J Physiol (Lond) 2000;528:339–348. doi: 10.1111/j.1469-7793.2000.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Donnelly D, Ma C, LaMotte RH. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23:2069–2074. doi: 10.1523/JNEUROSCI.23-06-02069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]