Abstract
Rationale
Tachycardia-induced atrial fibrosis is a hallmark of structural remodeling of atrial fibrillation (AF). The molecular mechanisms underlying the AF-induced atrial fibrosis remain unclear.
Objective
To determine the role of angiotensin II (AngII)/AT1 receptor-coupled transforming growth factor β1 (TGF-β1)/Smads signaling pathway in the AF-induced atrial fibrosis.
Methods and Results
Rapid atrial pacing (RAP, 1000 ppm) was applied to the left atrium of rabbit heart to induce atrial fibrillation and fibrosis. Quantitative PCR and Western blot analysis revealed that RAP caused a marked increase in the expression of AngII, TGF-β1, phosphorylated Smad2/3 (P-Smad2/3), Arkadia, and hydroxyproline synthesis. But the expression of Smad7, a key endogenous antagonist of the TGF-β1/Smads-mediated fibrosis, was significantly decreased. These changes were dose-dependently reversed by AT1 receptor antagonist losartan, implicating the involvement of AF-induced release of AngII and activation of AT1-receptor specific pathway. In the adult rabbit cardiac fibroblasts, AngII increased the expression of TGF-β1, P-Smad2/3, Smad4, Arkadia, and collagen I synthesis and significantly reduced Smad7 expression. These effects of AngII were reversed by losartan, but not by the AT2 antagonist (PD123319). In addition, extracellular signal-regulated kinase (ERK) inhibitor and anti-TGF-β1 antibody also blocked the AngII-induced downregulation of Smad7. Silencing of Smad7 gene by siRNA abolished losartan's antagonism on AngII's fibrogenic effects in cardiac fibroblasts while overexpression of Smad7 blocked AngII-induced increase in collagen I synthesis.
Conclusions
AngII/AT1-receptor specific activation of Arkadia-mediated poly-ubiquitination and degradation of Smad7 may decrease the inhibitory feedback regulation of TGF-β1/Smads signaling and serves as a key mechanism for AF-induced atrial fibrosis.
Keywords: atrial remodeling, atrial fibrillation, atrial fibrosis, transforming growth factor, Smads, Angiotensin II, Arkadia
Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice1-3 and remains a major morbidity and mortality.4 Tachycardia-induced atrial fibrosis is a hallmark of AF-induced structural remodeling.1,2 Experimental studies in animal models have shown that atrial fibrosis plays an important role in the induction and perpetuation of AF.2,5,6 Atrial fibrosis causes intra- and inter-atrial inhomogeneity in conduction thus creating a substrate for local re-entry and contributing to the progressive nature of AF.6 Among the plethora of identified fibrogenic factors, the rennin-angiotensin system (RAS), especially angiotensin II (AngII), has been implicated to play an important role in the development of cardiac remodeling during AF. However, the precise downstream molecules important in the genesis of AF-induced atrial fibrosis are currently unclear. 7
AngII activates multiple intracellular second messenger molecules.8 AngII may mediate the profibrotic responses including cell growth, inflammation, fibroblast proliferation and transformation, and extracellular matrix (ECM) deposition primarily through transforming growth factor-β1 (TGF-β1).9-11 TGF-β1 is expressed in the adult heart, where it is secreted by cardiomyocytes and myofibroblasts and retains in significant amounts in ECM as a latent cytokine. TGF-β1 signal transduction pathways are initiated by binding of TGF-β1 to membrane-bound heteromeric receptor kinases (TβRI and TβRII) that transduce intracellular signals via both Smads and non-Smads pathways.12-14 The primary TGF-β1 signal transduction pathway is the highly conserved Smads pathway. Activated TβRI and TβRII receptors phosphorylate receptor regulated Smads (R-Smads, such as Smad2/3), which form homomeric complexes and heteromeric complexes with co-mediator Smad (co-Smad, such as Smad4).12-14 These active Smad complexes translocate into the nucleus, where they accumulate and bind to target genes to directly regulate their transcription. The inhibitory Smad proteins (I-Smads, such as Smad6/7) are capable of inhibiting TGF-β1 signaling.15 Smad7 binds to activated TβRI and prevents phosphorylation of Smad2/3, or recruits the ubiquitin ligases Smurf1 and Smurf2 to induce proteasomal degradation of the receptor complexes.15 Therefore, Smad7 may act as a major negative regulator forming autoinhibitory feedback loops and mediating the cross-talking with other signal pathway.
In addition, AngII can also activate the Smads pathways by a TGF-β1-independent activation of AT1 receptors and mitogen activated protein kinases (MAPKs).16 It has been reported that AngII-induced left ventricular (LV) remodeling and fibrosis are dependent on both extracellular signal-regulated kinase (ERK) and Smads activation and inhibition of either pathway is equally efficacious in restoring LV function and architecture.17 However, it is unknown whether ERK is also involved in Smads signaling in the AF-induced atrial fibrosis. It remains to be elucidated how the Smads pathways are involved in AF-induced fibrosis.
In the present study we investigate the role of Smads pathways in AF-induced atrial fibrosis by examining the expression of AngII, TGF-β1, Smads, and collagen synthesis in rabbit atria subjected to rapid-atrial pacing (RAP) and in the adult rabbit cardiac fibroblasts in the absence or presence of antagonists of AT1 receptors. Our results suggest a key causal role of AT1-receptor specific downregulation of the inhibitory Smad7 in AF-induced atrial fibrosis.
Materials and Methods
All experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals (US National Institute of Health publication No.85-23, revised 1996) and were in compliance with the guidelines specified by the Chinese Heart Association policy on research animal use, and the Public Health Service policy on the use of laboratory animals.
Animals
Male New-Zealand rabbits (2.0-2.5 kg) were randomly divided into 6 groups (n=8 for each group): normal control (N), sham control (S), rapid atrial pacing (RAP) alone (P), RAP + losartan 10 mg.kg-1 daily (D1), RAP + losartan 20 mg.kg-1 daily (D2), and RAP + losartan 30 mg.kg-1 daily (D3).
Left Atrium Rapid Pacing
Rabbits were anesthetized with 30 mg.kg-1 pentobarbital sodium (i.v.), and then intubated and ventilated with a volume-cycled ventilator (Model HX-200, TAIMENG, Chengdu, China). The left thoracic cavity was opened via sternum from the second intercostals to the fourth intercostals, and then the heart was exposed by a dilator. A custom-designed set of electrodes, comprising a pair of electrodes with a distal hook for pacing and a pair of electrodes with an interelectrode distance of 15 mm aligned proximally for recording, were sutured to the epicardial surface of the left atrium. The reason to choose left atrium for RAP is because of its higher inducibility of AF than right atrium.18 The distal ends of these electrodes leads were tunneled subcutaneously and exposed on the back, and connected to a pacemaker (output of 6V with 1.0 ms pulse duration, Guangzhou Academy of Sciences, China) in the jacket. The pacemaker was programmed to provide RAP at 1000 ppm and this pacing rate was maintained continuously for 4 weeks with a brief period of break for measurement of electrophysiological and mechanical parameters. Rabbits in normal control (Group N) were not subjected to surgery while those in sham control (Group S) were operated with the identical surgery procedure but RAP. When surgery was completed, the rabbits were given antibiotics and then allowed to recover for 5 days. Postoperative care included the administration of antibiotics and analgesics. In Groups D1, D2, and D3, oral administration of losartan (Merck & CO., Inc., USA) started at the same time as RAP, and continued for 4 weeks during the pacing period. The same amount of normal saline was given to rabbits in Groups N, S, and P.
ECG recordings
ECG was recorded before and after the pacing. During the 4-week period of pacing ECG was measured every day to ensure that the pacemakers were working properly. Atrial effective refractory period (AERP) of the left atrial appendage was measured at basic cycle lengths (BCLs) of either 120 ms or 200 ms. Five basic drive stimuli were followed by 1 single premature stimulus, and all stimuli were twice the diastolic threshold. The interval between S1 and S2 was decreased in steps of 2 ms, and AERP was determined to be the shortest S1-S2 interval resulting in a propagated atrial response.
Atrium samples
At the end of the experiment all rabbits were sacrificed and the hearts were removed and weighed immediately. Left atrium were then quickly removed and cut into three (upper, meddle, lower) sections. Each section was divided equally into four pieces. Three pieces from each section were randomly chosen to form a new part for radioimmunity assay of AngII accumulation and hydroxyproline content analysis. One part was paraffin-embedded for Masson's trichrome staining. The remaining parts were quickly frozen in liquid nitrogen and maintained at -80°C until being used for mRNA and protein analysis. The whole procedure was performed in cold conditions.
Masson Trichrome Staining for Collagen
Masson's trichrome staining of the Paraffin section prepared from bouin's fixed samples was performed as previously described.19 To quantitate atrial collagen content, images were captured with a digital camera and the red pixel content of the myocardium was measured using Adobe Photoshop 5.5 and Scion Images for Windows Beta 4.0.2 software as described.19 The analyses were performed by at least two independent investigators on coded specimens in a blinded fashion.
Left atrium collagen content
Hydroxyproline content was measured as an index of the amount of collagen, which reflects the degree of myocardial fibrosis. The specimens were minced and then homogenized for 2 min at 4°C in sufficient deionized water to yield a 10% mixture (weight/volume). The hydroxyproline content of homogenates was assayed as described by Jamall et al.20
Radioimmunity assay of AngII accumulation
Tissues (50 mg) from each sample was homogenated in cool acetic acid, centrifuged and torrefied per the Kit instructions (125ICAMP RIA Kit, Medical college of SUN Yet-San University, Guangzhou China), and assayed with a γ-immunity indicator (FM2000, Xi'an, China).
Cell culture
Cardiac fibroblasts were isolated from left atria of adult New-Zealand rabbit heart as described previously.21,22 Cells were subcultured and passed as they reached 80-90% confluency. The purity of fibroblasts used in these experiments was 90% using routine phenotyping methods as described previously.21,22
Small interfering RNA (siRNA) transfection
Silencer siRNAs targeting Smad2, Smad3, or Smad7 were synthesized by Ambion based on the sequences of rabbit Smad2 (ID#: AAGW02032355), Smad3 (ID# AAGW02025187) and Smad7 (ID# AAGW02032373) and were used in knockdown experiments. To demonstrate that the transfection does not induce nonspecific effect on gene expression a control siRNA (CsiRNA. ID# NM001082253), which has no homology to known sequences from rabbit or humans, was used. The adult rabbit cardiac fibroblasts were cultured to approximately 80–90% confluence and then transfected with 400 pmol siRNA using Lipofectamine 2000 in a 6-well plate according to the manufacturer's instructions. Clones of Smad2, Smad3 or Smad7 siRNA that presented at least 90% inhibition of target genes were chosen for further analysis. For experimental procedures, second to third passages of each clone were used. The morphology of knockdown cells was monitored during culture under an inverted microscope. After 24 hours of transfection, cells were treated with or without AngII (1 μmol/L)/losartan (10 μmol/L) for 48 hours. Levels of Smad2/3, Smad7 and collagen I expression were determined by Western blot.
Smad7 expression plasmid and transfection
The Smad7 expression plasmid pcDNA3-FLAG-Smad7 was constructed as previously described.23 Fibroblasts were grown to 50% confluence in 100-mm dishes. After 4 hours incubation with serum-free medium, cultures were transfected with 1.0 μg pcDNA3-FLAG-Smad7 or 1.0 μg empty pcDNA3 vector in six-well plates by using Lipofectin (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. After transfection, cells were treated with AngII (1 μM) for 48 hours in media. In this experiment, total DNA in each well was adjusted to the same amount using vector DNA. All assays were performed in triplicate, and the results are presented as the means (±the standard errors) of three independent transfections.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted with TRIZOL reagent (Gibco-BRL Life Technologies). cDNA was synthesized with SYBR ExScript™ RT-PCR kit(TOYOBO, Japan) according to the protocol provided by the manufacturer. PCR primers for TGF-β1 (Forward: 5′-ACA TTG ACT TCC GCA AGG AC-3′ and reverse: 5′-TAG TAC ACG ATG GGC AGT GG-3′) and Smad7 (forward: 5′-GTG GCA TAC TGG GAG GAG AA-3′ and reverse: 5′-GAT GGA GAA ACC AGG GAA CA-3′) were designed with Primer Express software (Applied Biosystems). Glyceraldehydes-3-phosphatedehydrogease (GAPDH, forward: 5′-GCA CCG TCA AGG CTG AGA AC-3′, reverse: 5′-ATG GTG GTG AAG ACG CCA GT-3′) was used as reference to normalize input amounts of RNA for all samples. Real-time PCR was performed using a ABI7300 Real-time PCR system (Applied Biosystems, CA) with SYBR green fluorophore.24,25 All reactions were performed in at least duplicate for every sample. Threshold cycle (Ct) data were collected using the Sequence Detection Software version 1.2.3 (Applied Biosystems, CA). GAPDH was used as an internal control. mRNA-fold change relative to GAPDH was calculated with the comparative Ct method of 2-ΔΔCt. 25
Immunohistochemistry
Immunohistochemistry was performed using a microwave-based antigen retrieval technique as described previously.26 Briefly, cells after culturing in 6-chamber glass slides were stained with antibodies against Smad7 (Santa Cruz Biotechnology) using a three-layer peroxidase antiperoxidase method. For analysis of Smad7 in cultured Cardiac fibroblasts, positive stain for Smad7 was counted in 500 cells and expressed as percentage. All examinations were performed on coded slides in a blinded fashion.
Western blot
Low-molecular weight marker (Cell Signaling Technology, CST) and 50 μg of protein from samples were separated on 10% or 12% SDS gels by SDS-PAGE. Separated protein was transferred on a polyvinylidene difluoride (PVDF) membrane that was blocked at room temperature for 1 hour in Tris-buffered saline with 0.2% Tween 20 (TBS-T) containing 5% skim milk and probed with primary antibodies overnight at 4°C. The diluted concentration of the primary antibodies (Santa Cruz, California, USA): TGF-β1: 1:200; phosphorylated Smad2/3: 1:250; Smad4: 1:200; Smad7: 1:200; collagen I: 1:250; β-actin: 1:500; Secondary antibodies (Cell Signaling Technology, USA) included horseradish peroxidase (HRP)-labeled were diluted 1:1000/2000 with 0.2% TBS-T and 1% skim milk and incubated for 1 h at room temperature. Protein bands on Western blots were visualized using ECL Plus (Amersham, Arlington Heights, Illinois, USA). Relative band densities of proteins in Western blots were normalized against β-actin.
Statistical Analysis
Data were expressed as mean±stardard errors (S.E.). ANOVA and Student's t test were used to determine statistical significance. A two-tailed probability (P) of ≤0.05 was considered statistically significant.
Results
RAP-induced atrial fibrosis
Conventional ECGs were recorded in anesthetized intact rabbits. Figure 1 depicts representative ECG recordings before (Figure 1A,a) and after 4 weeks of RAP (Figure 1A,b). It is clear from the ECG recordings that RAP caused disappearance of P waves and absolutely irregular RR intervals, typical signs of sustained AF. RAP induced sustained (>24 hours) AF in 6 of 8 (6/8, 75%) animals in Group P. but none in Groups D1, D2, and D3. Epicardium stimulation induced sustained AF in 2 of 8 (2/8, 25%) animals in Group P and group D1, but none in groups D2 and D3. The pacemaker was not turned off automatically during AF, except for a brief period of break for measurement of electrophysiological and mechanical parameters. Before RAP (Group N) the baseline AERP was 107±3 and 93±2 (n=8) at BCLs of 200 ms and 120 ms, respectively. In response to 4 weeks RAP (Group P) AERP decreased significantly to 81±2 (n=8, P<0.05 vs Group N) at BCL 200 ms and 71±3 (n=8, P<0.05 vs Group N) at BCL 120 ms. In the 3 groups with different doses of losartan, AERP at BCL 200 ms was prolonged from 81±2 to 91±4 in Group D1 (n=8, P<0.05 vs Group N or Group P), 94±2 in Group D2 (n=8, P<0.05 vs Group N or Group P) and 96±2 in group D3 (n=8, P<0.05 vs Group N or Group P). Similarly, AERP at BCL 120 ms was prolonged from 71±3 ms to 83±2 ms in group D1 (n=8, P<0.05 vs Group N or Group P), 86±2 ms in group D2 (n=8, P<0.05 vs Group N or Group P) and 87±2 ms in group D3 (n=8, P<0.05 vs Group N or Group P) (Table 1). No significant difference of left ventricular ejection fraction (EF) in rabbits between pacing groups and no-pacing groups was observed (data not shown). There was no evidence of heart failure in any groups of the rabbits studied. Therefore, it is highly unlikely that the results we observed in our RAP model can be attributed to an indirect effect of RAP-induced heart failure.
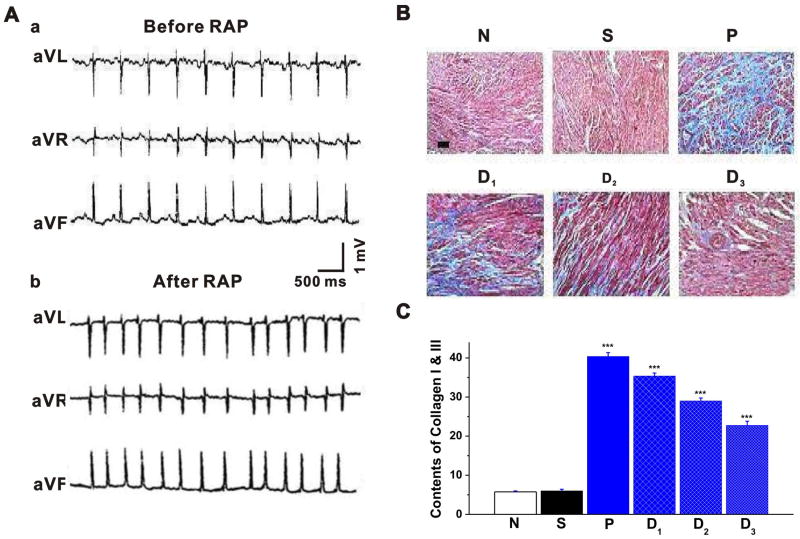
Figure 1. Rapid atrial pacing-induced atrial fibrillation and changes in collagen contents in the left atrium of rabbit heart in the absence and presence of losartan.
A. Representative surface ECG recordings before (a) and after (b) the rapid atrial pacing (RAP). a. ECG recordings in leads avR, avL and avF show that the rabbit was in normal sinus rhythm before RAP. The mean sinus heart rate was 173±18 bpm (n=8). b. ECG recordings in leads avR, avL and avF show that after 4 weeks of RAP the rabbit displayed an irregular atrial rhythm with irregular ventricular response: disappearance of P-wave, and absolute irregularity of the RR interval. The mean heart rate was 293±16 bpm (n=8). B. Masson trichrome-staining of rabbit atrial samples subjected to control (N), sham (N), RAP (P), and RAP in the presence of three different doses (D1, D2, and D3) of AT1 receptor antagonist losartan. Myocardium was stained red and collagens were stained blue. Bar = 50 ⎧m. C. Mean data of collagen content in the left atrium of the 6 groups (n = 8 per group). RAP caused significant deposition of collagens in the left atria, which can be attenuated by losartan in a dose-dependent manner. * P<0.05 compared with Group N, # P<0.05, ## P<0.05 compared with Group P.
Table 1. ECG parameters before and after rapid atrial pacing (RAP).
| Before RAP | After RAP | |||||
|---|---|---|---|---|---|---|
| PR (ms) | QRS (ms) | PR (ms) | QRS (ms) | AERP (ms) BCL200 ms | AERP (ms) BCL120ms | |
| Group N | 53±5 | 46±4 | 54±3 | 47±3 | 107±3 | 93±2 |
| Group S | 53±4 | 45±4 | 52±4 | 46±4 | 106±2 | 94±3 |
| Group P | 52±4 | 46±3 | - | 47±3 | 81±2* | 71±3* |
| Group D1 | 54±6 | 47±5 | 55±5 | 48±4 | 91±4*,# | 83±2*,# |
| Group D2 | 52±4 | 47±4 | 53±4 | 47±3 | 94±2*,# | 86±2*,# |
| Group D3 | 53±6 | 46±6 | 52±4 | 46±4 | 96±2*,# | 87±2*,# |
Data is presented as mean±S.E. (n=8 for each group).
P<0.05 vs Group N,
P<0.05 vs Group P.
We then examined whether RAP effectively caused fibrosis in the left atria. Hydroxyproline content was measured as an index of the amount of collagen, which reflects the degree of myocardial fibrosis. As shown in Figure 1B&C and Table 2, RAP caused a marked deposition of collagens as estimated by Masson trichrome-staining (Figure 1B&C). The hydroxyproline content in the heart of Group P was significantly higher than that in the heart of Group N or Group S. Left atrial weight (LAW) and left atrial mass index (LAMI) in Group P were significantly higher than those in Group N. These results indicate that RAP at 1000 ppm for 4 weeks caused significant atrial fibrosis.
Table 2. Effect of losartan on rappid atrial pacing (RAP)-induced AngII accumulation, hydroxyproline production in left atrium and LAW and LAMI.
| Group N | Group S | Group P | Group D1 | Group D2 | Group D3 | |
|---|---|---|---|---|---|---|
| AngII(pg/mg) | 1402±40 | 1463±36 | 2739±114* | 2744±63* | 2792±11* | 2776±64* |
| hydroxyproline | 3.22±0.27 | 3.12±0.16 | 6.38±0.40* | 4.82±0.21*,# | 4.22±0.20*,# | 3.89±0.23*,# |
| BW(g) | 2.4±0.3 | 2.2±0.1 | 2.1±0.2 | 2.2±0.3 | 2.2±0.2 | 2.1±0.2 |
| LAW(mg) | 0.72±0.14 | 0.76±0.10 | 1.10±0.26* | 0.94±0.07*,# | 0.87±0.08*,# | 0.84±0.12*,# |
| LAMI(mg/g) | 0.30±0.07 | 0.35±0.03 | 0.51±0.11* | 0.44±0.05*,# | 0.41±0.03*,# | 0.40±0.10*,# |
LAW: left atrial weight; LAMI: left atrial mass index. Data is presented as mean±S.E. (n=8 for each group).
P<0.05 vs Group N
P<0.05 vs Group P.
RAP-induced changes in AngII levels
Since AngII has been implicated an important role in cardiac fibrosis we tested whether RAP-induced atrial fibrosis is associated with any changes in AngII level in the left atria. As shown in Table 2, the accumulation of AngII as estimated by radioimmunity in the left atrium was significantly increased in Group P, D1, D2, and D3 when compared with that in the non-paced Group N (P<0.05), while it is comparable among the groups subjected to RAP (P>0.05). Therefore, RAP-induced atrial fibrosis was accompanied with a significant increase in AngII accumulation in the atrial tissues.
Effects of AT1 receptor antagonist on the RAP-induced atrial fibrosis
As shown in Table 2 and Figure 1B&C, treatment of the animals with AT1 receptor antagonist losartan caused a significant decrease in hydroxyproline content, collagen I and collagen III deposition, LAW and LAMI and attenuated the progression of RAP-induced atrial fibrosis. These data suggest that the RAP-induced atrial fibrosis may be mediated through an activation of AT1 receptors in the heart
RAP-induced changes in the expression of TGF-β1 and Smads in the left atrium
Previous studies have shown that activation of the AT1 receptors by AngII upregulates TGF-β1 expression in cardiac myocytes and fibroblasts.27,28 Therefore, we next examined whether RAP causes changes in the expression level of TGF-β1 and Smads and whether the changes are AT1 receptor-specific.
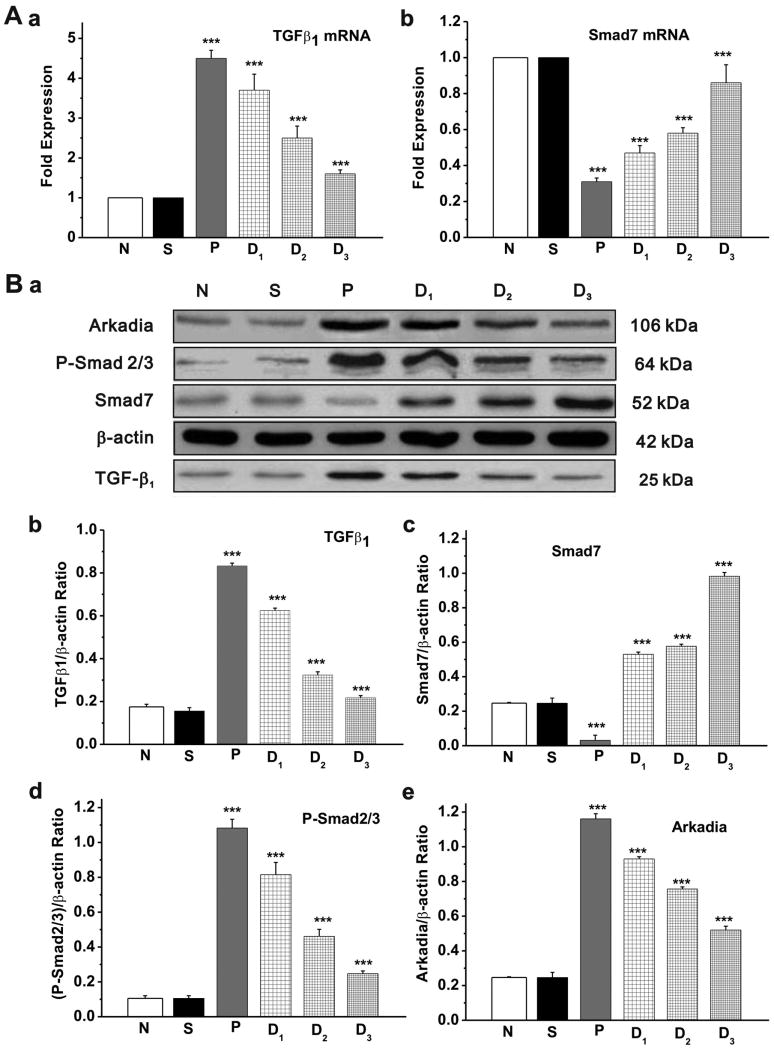
As shown in Figure 2, real-time PCR and quantitative Western blot analysis found that the expression level of TGF-β1 mRNA (Figure 2A,a) and protein (Figure 2B, a&b) was significantly increased in the left atrium of Group P compared with the non-paced Group N and Group S. In addition, the phosphorylated Smad2/3 (P-Smad2/3, Figure 2B,a&d) and the E3 ubiquitin ligase Arkadia (Figure 2B,a&e) were also increased in the left atrium among the pacing groups compared with the non-paced groups. But the expression of the inhibitory Smad, Smad7, was significantly decreased in Group P (Figure 2B,a&c). These RAP-induced changes in TGF-β1, P-Smad2/3, Arkadia, and Smad7 expression were antagonized by AT1-receptor agonist losartan in a dose-dependent manner.
Figure 2. Expression of TGF-β1 and Smads in the left atrium of rabbit heart.
A. Quantitative analysis of transcriptional expression of TGF-β1 and Smad7 by real-time RT-PCR. a. The TGF-β1 mRNA levels relative to GAPDH expression ratio (TGF-β1/GAPDH in arbitrary unit) was normalized to that of Group N and Group S and the relative expression levels (in fold expression) were calculated (* P<0.05 vs Group N, # P<0.05 vs Group P, n=5 for each group). b. The relative Smads mRNA expression ratio (Smad7/GAPDH in arbitrary unit) in left atria was normalized to that of Group N and Group S and the relative expression levels (in fold expression) were calculated (* P<0.05, ** P<0.01, *** P<0.001 vs Group S, # P<0.05 vs Group P, n=5 for each group). B. a. Representative Western blot gel depicts the protein expression of TGF-β1, Smad7, P-Smad2/3, and Arkadia. b-e. Mean value of the protein expression level of TGF-β1 (b), Smad 7 (c), P-Smad2/3 (d) and Arkadia (e) in control (Group N), sham (Group S), RAP (Group; P) and RAP in the presence of three different doses (Group D1, D2, D3) of AT1 receptor agonist losartan. RAP caused a significant increase in expression of TGF-β1, P-Smad2/3, and Arkadia but decreased the expression of Smad7. Losartan reversed the effect of RAP on the expression of these proteins in a dose-dependent manner (* P<0.05, ** P<0.01, *** P<0.001 vs Group S, # P<0.05 vs Group P, n=5 for each group).
The above results from the whole-animal model strongly suggest that RAP may cause atrial fibrosis through an AngII/AT1 receptor specific activation of TGF-β1/Smads pathway. The downregulation of Smad7 may be a key for the increased activation of P-Smad2/3 that is responsible for the increased atrial fibrosis during AF. To further confirm our observations in the in vivo study and gain more information on how Smad7 is downregulated during RAP, we examined whether stimulation of the growth-arrested adult rabbit cardiac fibroblast with AngII (10-6 mol/L) for 48 hours would cause similar changes in the Smads signaling.
AngII induced TGF-β1 and Smads expression in cultured cardiac fibroblasts
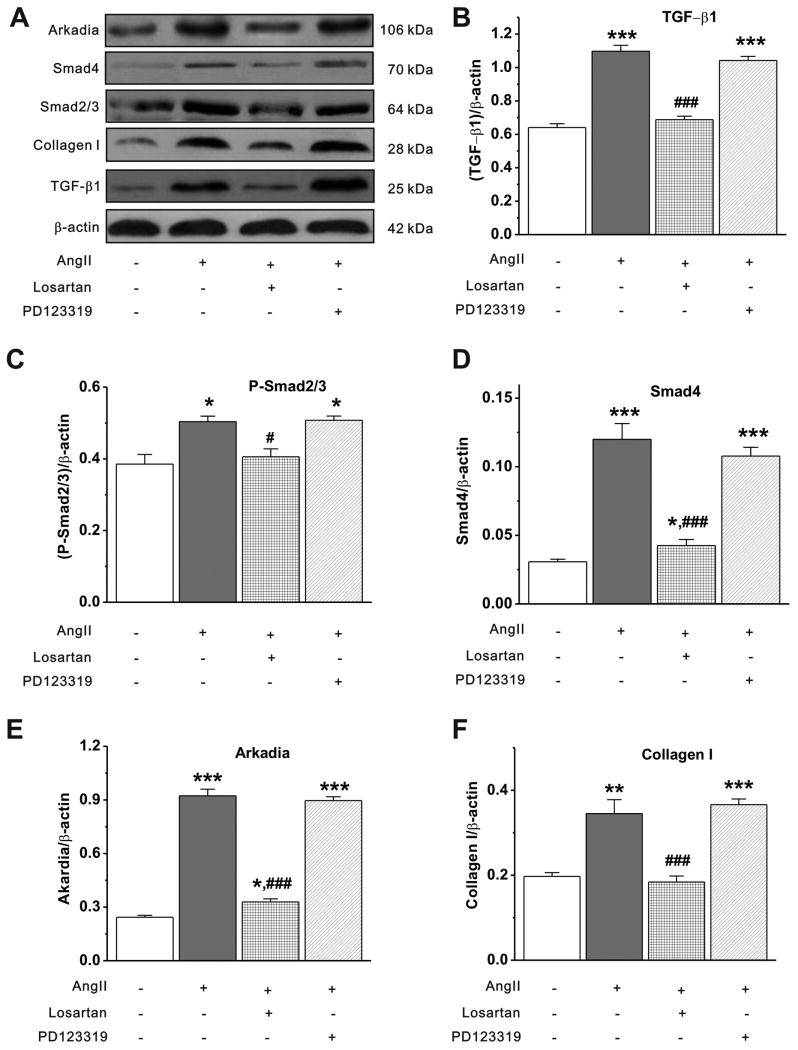
The primary cardiac fibroblasts (passage 2) were serum starved for 24 hours, followed by treatments with a) dimethylsulfoxide (DMSO, 0.005%) as control group; b) AngII (1 μmol/L); c) AngII (1 μmol/L)+losartan (10 μmol/L); and d) AngII (1 μmol/L)+PD123319 (AT2 antagonist, 100 μmol/L with 0.005% DMSO) for 48 hours. Cells were treated with losartan or PD123319 at 1 hour before AngII stimulation. Either losartan (10 μmol/L) alone or PD123319 (100 μmol/L) alone failed to significantly affect Smad7 expression. The protein expression of TGF-β1, P-Smad2/3, Smad4, Arkadia and collagen I were determined by Western blot analysis. Continuous stimulation of cardiac fibroblasts with AngII for 48 hours caused a significant increase in the expression of TGF-β1, P-Smad2/3, Smad4, Arkadia, and collagen I (Figure 3). AT1 antagonist losartan (10-5 mol/L) inhibited the effects of AngII on TGF-β1 protein expression, Smad2/3 phosphorylation and collagen I expression. The AT2 receptor antagonist PD123319, however, had no effects on the AngII-induced changes in TGF-β1 and Smads expression, indicating an AT1 receptor-specific mechanism in the activation of the TGF-β1/Smads signaling pathway.
Figure 3. AngII induced TGF-®1 and Smads expression in cultured cardiac fibroblasts.
A. Representative Western blot gel depicts the protein expression of TGF-β1, P-Smad2/3, Smad4, Arkadia, and collagen I. B-F. Mean value of the protein expression level of TGF-β1 (B), P-Smad2/3 (C), Smad4 (D), Arkadia (E) and collagen I (F) under control, AngII alone, AngII+losartan, and AngII+PD123319 conditions (mean±S.E., n=4, * P<0.05, ** P<0.01, *** P<0.001 vs control; # P<0.05, ### P<0.001 vs AngII).
AngII-induced downregulation of Smad7 and increased ubiquitin-dependent protein degradation
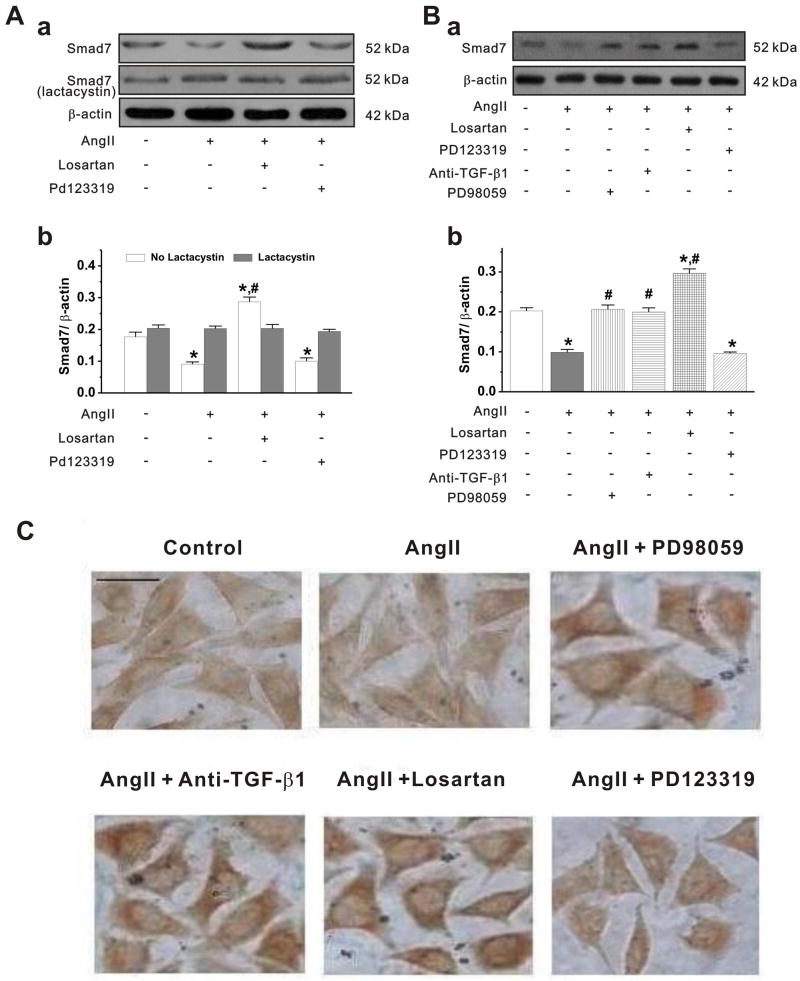
As shown in Figure 4A, the Smad7 protein expression in cardiac fibroblasts was significantly reduced by AngII stimulation, which could be antagonized by AT1 receptor antagonist losartan, but not by AT2 receptor antagonist PD123319. These findings are in agreement with the findings in the in vivo model (see Figure 2). Since AngII also caused an increase in Arkadia (see Figures 2 and 3) and previous evidence indicated that Arkadia may act as an E3 ubiquitin ligase and play a key role in the regulation of TGF-β/Smad signaling through degradation of signal molecules,29 we investigated whether the ubiquitin-dependent protein degradation through Arkadia is involved in the AT1-receptor specific downregulation of Smad7 expression. As shown in Figure 4A, pretreatment of the cardiac fibroblasts with a specific proteasome inhibitor lactacystin (10-4 mmol/L) for 3 hours before AngII stimulation, AngII-caused Smad7 downregulation was reversed, suggesting that Arkadia-mediated ubiquitin-proteasome protein degradation of Smad7 may be responsible for the AT1-receptor specific downrefulation of Sma7.
Figure 4. AT1 receptor antagonist induced Smad7 expression in cardiac fibroblasts.
A. a. The fibroblasts were treated without (-) or with (+) specific proteasome inhibitor lactacystin (10-4 mmol/L) for 3 h before cells treated with AngII, losartan and PD123319. b. Mean value (mean±SEM) of Smad7 protein expression obtained from densitometric analysis and expressed as ratio of Smad7 /β-actin under the corresponding conditions as indicated in a In the absence of lactacystin, AngII decreased Smad7 expression, which could be reversed by losartan, but not by PD123319. In contrast, in the presence of lactacystin, AngII caused no changes in Smad7 (n = 6, * p<0.001 vs control; # p<0.001 vs AngII). B. a. Representative Western blot gel depicts the effects of anti-TGF-β1 antibody and ERK1/2 inhibitor on AngII-induced Smad7 expression. Cardiac fibroblasts were incubated with AngII (10-6 mmol/L), AngII+losartan (10-5 mmol/L), AngII+PD123319 (10-5 mmol/L), AngII+anti-TGF-β1 antibody (10 μg/ml), or AngII+ PD98059 (10-4 mmol/L) for 48 h. Losartan, PD123319, anti-TGF-β1 antibody, and PD98059 were added 1 hour before the addition of AngII. b. The mean values (mean±S.E.) from 5–6 separate experiments (*P<0.001 vs control, # P<0.001 vs AngII). C. Immunohistochemistry staining of Smad7. Bar = 50 um. Cells treated with the same conditions as Western bolt described in B.
AT1 receptor specific downregulation of Smad7 through both TGF-β1 and ERK pathways
Since AngII/AT1 receptors activate multiple intracellular signaling pathways including the TGF-β1 and ERK1/2 pathways that participate in the regulation of Smads, we further tested whether both TGF-β1 and ERK1/2 pathways are involved in the regulation of Smad7 expression. As shown in Figure 4B and C, treatment of adult rabbit cardiac fibroblasts with anti-TGF-β1 antibody or the ERK inhibitor PD98059 both blocked the AngII-induced downregulation of Smad7 expression. Smad7 expression was increased by blockade of endogenous TGF-β1 to an almost identical level as that by ERK inhibitor. Blockage of AT1 receptor by losartan caused nearly one-fold increase in Smad7 expression (Fig. 4B) compared with blockade of endogenous TGF-β1 or inhibition of ERK. Blockade of AT2 receptor by PD123319, however, had no effect on the expression of Smad7 in these cells. Similar results were found in immunohistochemistry staining of Smad7 in the primary cardiac firoblasts (Figure 4C).
Effects of Smad7 gene silencing by siRNA on AngII-induced collagen synthesis in cardiac fibroblasts
To further determine the relative role of Smad2/3 and Smad7 in the AF/AngII-induced fibrosis, silencer siRNAs targeting Smad2, Smad3, or Smad7 were transfected into the primary cardiac fibroblasts (passage 2-3). Cells were serum starved for 24 hours, and then transfected with 400 pmol of CsiRNA, Smad2 siRNA, Smad3 siRNA, or Smad7 siRNA under the same conditions for 24 hours. The cells were then treated with dimethylsulfoxide (DMSO, 0.005%) as control group, AngII (10-6 mmol/L), losartan (10-6 mmol/L), or AngII (10-6 mmol/L)+losartan (10-5 mmol/L, Cells were treated with losartan at 1 hour before AngII stimulation) for 48 hours.
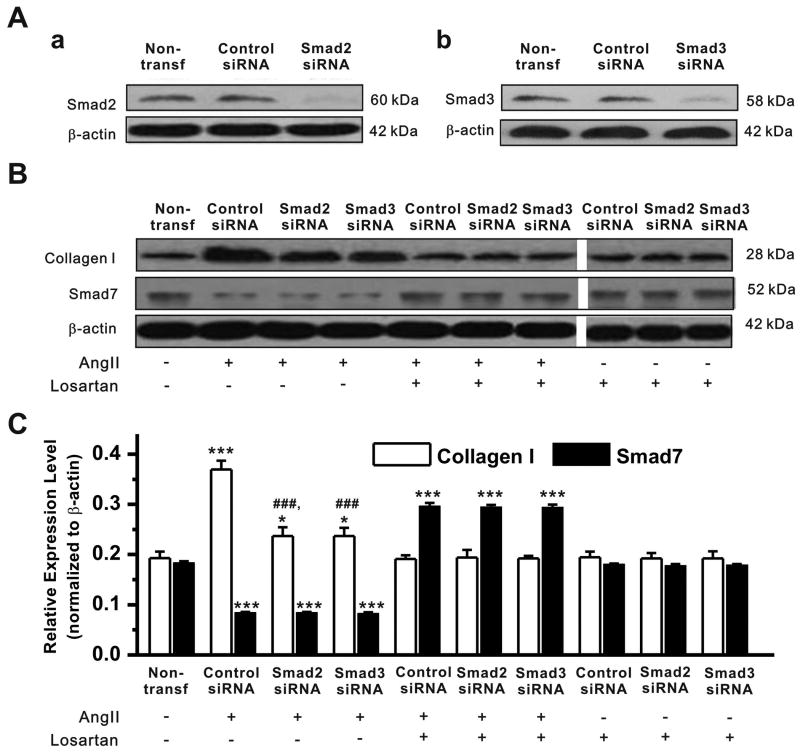
Transfection with siRNAs targeting Smad2, Smad3, or siSmad7 significantly down-regulated the expression level of Smad2, Smad3, or Smad7 (>90%) as measured by qRT–PCR (data not shown) and Western blotting analyses (Figure 5Aa,b and Figure 6A,a, respectively).
Figure 5. Effect of knockdown of Smad2 or Smad3 on Smad7 and collagen I expression in cardiac fibroblasts.
A. Cardiac fibroblasts were transfected with control siRNA, Smad2 siRNA, or Smad3 siRNA. a. Representative Western blot gel depicts the protein expression of Smad2 in cells without transfection (non-transf.), or with transfection of control siRNA or Smad2 siRNA. Smad2 siRNA successfully knocked down the expression of Smad2. b. Representative Western blot gel depicts the protein expression of Smad3 in cells without transfection (non-transf.), or with transfection of control siRNA or Smad3 siRNA. Smad3 expression was successfully knocked down by Smad3 siRNA. B. Representative Western blot gels depicts the protein expression of collagen I and Smad7 in cardiac fibroblsts transfected with control siRNA, Smad2 siRNA or Smad3 siRNA after the cells were treated with AngII (10-6 mmol/L), losartan (10-5 mmol/L), or AngII+losartan. C. The mean values (mean±S.E., n=10) of relative expression levels (normalized to β-actin) of collagen I and Smad7 under corresponding conditions (* P<0.05, *** P<0.001 vs non-transfection; ### P<0.001 vs Control siRNA).
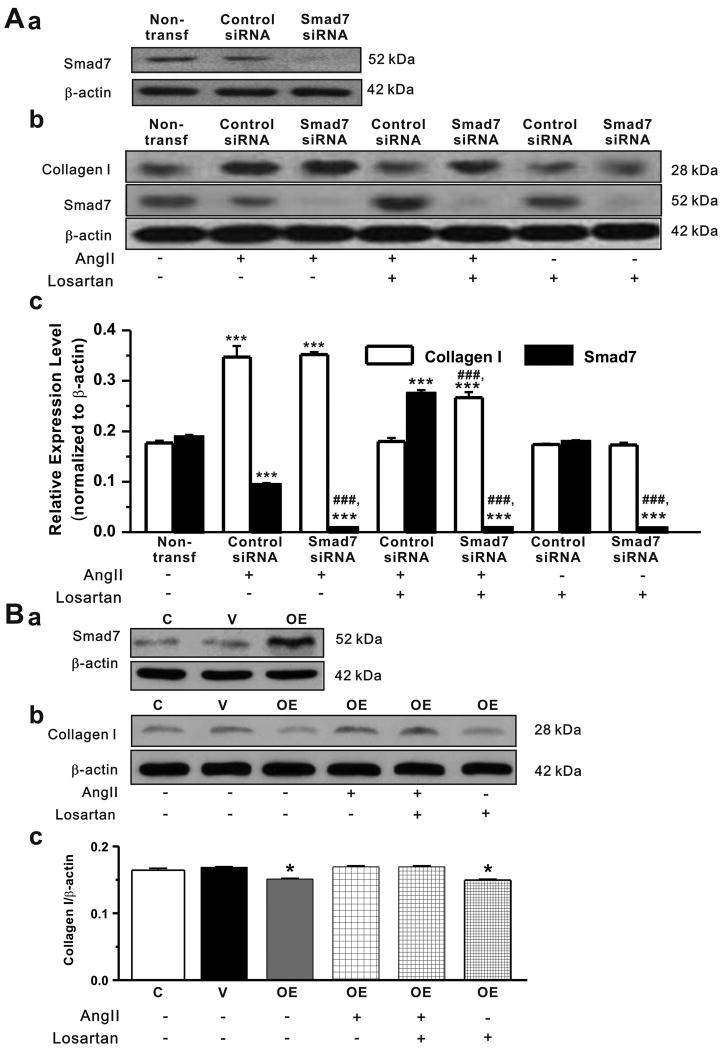
Figure 6. Effects of Smad7 knockdown or overexpression on collagen I expression in cardiac fibroblasts.
A. Cardiac fibroblasts were transfected with control siRNA and Smad7 siRNA. a. Representative Western blot gel depicts the protein expression of Smad7 in cells without transfection (non-transf.), or with transfection of control siRNA or Smad7 siRNA. Smad7 siRNA successfully knocked down the expression of Smad7. b. Representative Western blot gels depicts the protein expression of collagen I and Smad7 in cardiac fibroblsts transfected with control siRNA or Smad7 siRNA when exposed to AngII (10-6 mmol/L), losartan (10-5 mmol/L), or AngII+losartan. c. The mean values of expression ratio of collagen I or Smad7 over β-actin under corresponding conditions (mean±S.E., n=7, *** P<0.001 vs non-transf; ### P<0.001 vs control siRNA). B. a. Representative Western blot gel depicts the protein expression of Smad7 in cardiac fibroblasts transfected with empty pcDNA3 vector (V) or pcDNA3-FLAG-Smad7 (OE). Overexpression of Smad7 caused a significant increase in Smad7 protein expression. Representative Western blot gels (b) and the mean values (mean±SEM) of collagen I protein expression (c) obtained from densitometric analysis and expressed as ratio over β-actin in cardiac fibroblasts treated with either AngII (10-6 mmol/L), losartan (10-5 mmol/L), or AngII+losartan. Overexpression (OE) of Smad7 caused a significant decrease in collagen I expression under basal conditions (in the absence of AngII) compared to the control (C, without transfection) cells and the cells transfected with empty vectors (V) (n=7, * P<0.05 vs no transfection or empty vector).
As shown in Figure 5A,B and C, AngII caused a significant increase in collagen I expression (0.369±0.017 vs 0.193±0.012, n=10, P<0.001) and a decrease in Smad7 expression (0.184±0.003 vs 0.084±0.002, n=10, P<0.001) in cardiac fibroblasts transfected with control siRNA (Control siRNA). When Smad2 and Smad3 genes were silenced by siRNA the AngII- induced increase in collagen I expression in the fibroblasts was significantly less than that in the fibroblasts transfected with control siRNA (0.237±0.017 for Smad2 siRNA and 0.236±0.016 for Smad3 siRNA vs 0.369±0.017 for Control siRNA, n=10, P<0.001, respectively). But knockdown of Smad2 or Smad3 did not completely block the AngII-induced increase in collagen I expression (0.237±0.017 and 0.236±0.016 for Smad2 siRNA and Smad3 siRNA, respectively, vs 0.193±0.012 for non-transf., n=10, P<0.05) and had no effect on the AngII-induced decrease in Smad7 expression. Losartan along had no effects on collagen I and Smad7 expression in these cells. But the AngII-induced increase in collagen I expression was completely blocked by losartan. In the presence of losartan, AngII caused a significant increase (instead of decrease) in Smad7 expression.
In the cells transfected with control Smad7 siRNA, as shown in Figure 6A,b and c, AngII induced a significant increase in collagen I (0.35±0.02 vs 0.18±0.01 in non-transfected cells, n=10, P<0.001) and decrease in Smad7 expression (0.094±0.001 vs 0.189±0.004 in the non-transfected cells, n=10, P<0.001) which was comparable to that in the fibroblasts under control conditions (see Figure 3F, and Figure 4). When Smad7 gene were silenced by siRNA, Smad7 expression was significantly decreased in the cells (0.009±0.001, n=7, P<0.001 vs Control siRNA and nontransfection). Under basal conditions without AngII stimulation siRNA knockdown of Smad7 slightly increased the collagen I synthesis but it was not statistically significant (0.176±0.004 vs 0.192±0.002, respectively, n=5, P>0.05). Losartan along had no effects on collagen I expression in these cells but partially blocked the AngII-induced increase in collagen I expression (0.27±0.01, n=7, P<0.001 vs Control siRNA and non-transfection, respectively).
These results suggest that although upregulation of Smad2/3 may contribute to the AngII-induced increase in the collagen I synthesis the downregulation of Smad7 may play a major role in the AF/AngII-induced atrial fibrosis.
Effects of overexpression of Smad7 on AngII-induced collagen synthesis in cardiac fibroblasts
In order to further confirm whether downregulation of Smad7 plays a causal role in the AF/AngII-induced atrial fibrosis, we examined the effects of overexpression of Smad7 on the AngII-stimulated expression of collagen I in the isolated cardiac fibroblasts.
As shown in Figure 6B, overexpression (OE) of Smad7 caused a significant decrease in collagen I expression under basal conditions (in the absence of AngII) compared to the control cells (C, without transfection) and the cells transfected with empty vectors (V) (n=7, * P<0.05 vs non-transfection or empty vector). Overexpression of Smad7 also antagonized the AngII-induced increase in collagen I expression (0.169±0.002, n=7, vs 0.345±0.033, n=4, P<0.001, see Figure 3F) and masked the losartan's antagonism on AngII's fibrogenic effects (0.169±0.001, n=7, vs 0.184±0.014, n=4, P=0.176, see Figure 3F). In the presence of losartan the expression level of collagen I (0.150±0.001, n=7) is comparable to that under basal condition (0.151±0.001, n=7, P=0.493). These results strongly suggest that downregulation of Smad7 is required for the AngII-induced increase in collagen I synthesis and the reduced expression of inhibitory Smad7 may play a causal role in the AF-induced atrial fibrosis.
Discussion
In this study we examined the role of AngII/AT1 receptor/Smads signaling pathway in the AF-induced atrial fibrosis. The major novel finding of this study include: 1) our real-time PCR and quantitative Western blot analysis revealed that RAP-induced AF caused a significant downregulation of Smad7 expression in rabbit atria and increased P-Smad2/3 activity and atrial fibrosis; 2) the AF-induced downregulation of Smad7 and upregulation of P-Smad2/3 and collagen production were dose-dependently reversed by AT1 receptor antagonist losartan but not by the AT2 receptor antagonist PD123319; 3) in isolated adult rabbit cardiac fibroblasts losartan also caused an upregulation of Smad7 expression and decreased the level of P-Smad2/3, Smad4, and collagen I; 4) blockade of TGF-β1 or inhibition of ERK both upregulated Smad7 expression, implicating both TGF-β1 and ERK signaling pathways may be involved in the AT1 receptor-specific regulation of Smad7 expression; 5) both RAF and AngII increased Arkadia protein expression and degradation of Smad7, which could be reversed by AT1 receptor antagonist, but not by AT2 receptor antagonist; 6) blockage of proteasome could diminish AngII-induced degradation of Smad7; 7) Silencing of Smad7 gene by siRNA abolished losartan's antagonism on AngII's fibrogenic effects in cardiac fibroblasts while overexpression of Smad7 blocked AngII-induced increase in collagen I synthesis. These results strongly suggest that AT1 receptor-specific downregulation of Smad7 through increased Arkadia-medicated protein degradation may be a novel mechanism for the AF-induced atrial fibrosis.
AF and atrial fibrosis
A significant feature of AF-induced structural remodeling is tachycardia-induced atrial fibrosis,1,2 which plays an important role in the induction and perpetuation of AF.2,5,6 Biopsy and autopsy from patients and animal models with AF have displayed the presence of atrial fibrosis.30 There exist, however, controversial opinions whether structural changes in the atria are due to tachycardia or related to underlying diseases.31,32 In this study we demonstrated that RAP alone induced profound changes in gene expression of collagens and fibrogenic factors in the heart, strongly suggesting that tachycardia during AF may cause atrial remodeling due to atrial fibrosis. In addition to the increase in the hydroxyproline content and left atrium weight, a significant deposition of cardiac collagen I and III was observed in the atria subjected to rapid pacing. These results also support previous observations that atrial fibrosis consisting of the collagen type I and III is a typical feature of AF, especially in the permanent form of AF.33 Atrial fibrosis causes intra and inter-atrial inhomogeneity in conduction thus creating a substrate for local re-entry and contributing to the progressive nature of AF.6
Several animal models of chronic sustained AF have been developed to assess the atrial electrical remodeling and arrhythmias of AF. In dogs model of pacing-induced AF,34 AERP was decreased and the animals became more vulnerable to AF. It has been reported that rapid pacing induces AF with much higher incidence at the left atrial site than at the right atrium and any other sites, partly due to the inhomogeneous dispersion of AERP.18 In our rabbit model of left atrial RAP, AERP of Group P was statistically shortened compared with the baseline. After 4 weeks treatment with AT1 receptor antagonist, AERPs of Group D1, D2, D3 were shortened to a significantly less extend than Group P in a dose-dependent manner. The shortening of AERP was correlated to atrial fibrosis. The structural abnormalities of the atria, especially their increased size, likely contributed to their continued vulnerability to electrical stimulation, suggesting that atrial electrical remodeling has a close relationship with atrial fibrosis.
AngII/TGF-β1/Smads pathways in AF-induced atrial fibrosis
Both AngII and TGF-β1 have been found to stimulate the progression of cardiac fibrosis during cardiac hypertrophy and heart failure.27,35,36 AngII participates in the development of AF -induced myocardial fibrosis through activation of AT1 and AT2 receptors.9-11 AT1 receptor antagonism significantly attenuates fibrosis process of atrial fibrillation in dogs.37 In the present study we found that application of losartan decreased the deposition of cardiac collagens in a dose-dependent manner, suggesting that activation of AT1 receptors may be an important mechanism for AF-induced atrial fibrosis. Recent studies indicate that AngII and TGF-β1 do not act independently from one another but rather act as part of a network that promotes cardiac remodeling.27 AngII mediates the expression of TGF-β1 in vitro38 and in vivo39 in various cell types including cardiac fibroblasts. Serum level of TGF-β1 was increased in patients with AF and it was down-regulated after defibrillation therapy.40 Changes in genes regulating TGF-β1 function and signaling were observed in patients with permanent AF 41 and in canine AF models.42 TGF-β1 is a known pro-fibrotic agent and its enhanced expression has been shown to increase myocardial fibrosis.12,43 Overexpression of constitutively active TGF-β1 in mouse caused only atrial inter-stitial fibrosis but not the ventricles.44 It seems that atria are more susceptible to TGF-β1 influences than ventricles. In the present study, we found that TGF-β1 was significantly up-regulated in left atria of the pacing group (Group P) as compared to the non-pacing controls (Group N and Group S in Figure 2). This corresponds well with an excessive atrial collagen synthesis observed in Group P with AF (Table 1 and Figure 1). Consistent with our in vivo observations, we found that AT1 receptor antagonist, but not AT2 receptor antagonist, inhibited the AngII-induced increase in TGF-β1 expression in isolated adult rabbit cardiac fibroblasts (Figure 3), implicating an AT1 receptor-specific mechanism for the AngII activation of TGF-β1 signaling pathway.
Although it has been demonstrated that TGF-β1/Smad pathway is involved in the cardiac fibrosis in myocardial infarction, it is not clear whether and how TGF-β1/Smad pathway is involved in the AF-induced atrial fibrosis. Binding of TGF-β1 to its two membrane-bound receptor kinases TβRI and TβRII initiates a series intracellular signals via both non-Smad pathways and Smad-mediated transcriptional regulation.12-14 The signaling of TGF-β1 is finely regulated at different levels. Inhibitory Smads, including Smad6 and Smad7, are key regulators of TGF-β1 signaling through negative feedback loops. They can form stable complexes with activated TβRI and block the phosphorylation of R-Smads (Smad2/3), or recruit ubiquitin E3 ligases, such as Smurf1/2, resulting in the ubiquitination and degradation of the activated TβRI. Smad6/7 can also inhibit TGF-β1 signaling in the nucleus by interacting with transcriptional repressors, such as histone deacetylases, or disrupting the formation of the TGF-β1-induced functional Smad-DNA complexes.15 Smad7 is in turn regulated by various mechanisms such as Arkadia-mediated ubiquitination and degradation (see below). Therefore, Smad7 may act as a major negative regulator in the autoinhibitory feedback loops and mediate the cross-talking with other signal pathway. Deregulation of Smad7 expression has been associated with various human diseases, such as inflammatory disease and carcinogenesis. Overexpression of Smad7 has been shown to antagonize TGF-β-mediated fibrosis, carcinogenesis, and inflammation, suggesting a therapeutic potential of Smad7 to treat these diseases.45 In the present study, we found that the RAP-induced stimulation of AngII/AT1/TGF-β1 pathway caused a significant downregulation of Smad7 expression, which might result in a marked increase in P-Smad 2/3 and collagen I and III synthesis in the atria subjected to RAP.15 Therefore, a reduction in Smad7 may be the major mechanism for the AF-induced atrial fibrosis.
Mechanisms underlying AF-induced downregulation of Smad7 expression
In the present study, we found that the RAP-induced AngII release and AT1 receptor activation caused a significant reduction of Smad7 expression in the atria. We also demonstrated that Smad7 expression was significantly up-regulated by AT1 receptor antagonist losartan in a dose-dependent manner. Since both anti-TGF-β1 antibody and ERK inhibitor PD98059 were almost equally efficacious in increasing Smad7 expression in adult rabbit cardiac fibroblasts the AT1 receptor specific downregulation of Smad7 expression may involve both TGF-β1 and ERK signaling pathways.16 In addition, we found that RAP-induced stimulation of AngII/AT1 receptor increased Arkadia expression in the left atria and AT1 receptor antagonist diminished Arkadia expression in rabbit cardiac fibroblasts. Proteasome inhibitor prevented the AngII-induced Smad7 downregulation (Figure 4A). These results implicated that activation of the Arkadia-ubiquitin-proteasome pathway may be responsible for the AT1 receptor specific downregulation of Sma7 during AF. Furthermore, overexpression of Smad7 blocked AngII-induced increase in collagen I synthesis and silencing of Smad7 gene by siRNA abolished losartan's antagonism on AngII's fibrogenic effects in cardiac fibroblasts while knockdown of Smad2 or Smad3 did not completely inhibit the AngII-induced increase in collagen I synthesis, implicating a causal role for Smad7 downregulation, but not upregulation of Smad2/3, in the AF/AngII-induced atrial fibrosis.-
In summary, the current study provided compelling in vivo and in vitro experimental evidence that tachycardia during AF may activate the AngII/AT1 receptor/TGF-β1 and ERK/Smads signaling pathways and AT1 receptor-specific downregulation of the I-Smads (Smad7) may serve as a key mechanism for the AF-induced atrial fibrosis. These results may provide new insights into the understanding of the mechanisms for AF-induced atrial fibrosis and myocardial remodeling and valuable information for novel therapeutic targets of AF.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by the Science Fund Committee of Guangdong Province of China Grant (#06021342, to X.G.); National Center for Research Resources (#P-20 RR-15581, to D.D.) and National Heart, Lung, and Blood Institute Grant (#HL63914, to D.D.), American Diabetes Association Innovation Award (#7-08-IN-08 to D.D.), and National Basic Research Program of China Grant (#2009CB521903, to D.D.).
Non-standard Abbreviations and Acronyms
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- AngII
angiotensin II
- AT1 receptor
angiotensin II receptor type 1
- AT2 receptor
angiotensin II receptor type 2
- BCL
basic cycle lengths
- Co-Smad
co-mediator Smad
- I-Smad
inhibitory Smad
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- LAW
left atrial weight
- LAMI
left atrial mass index
- MAPKs
mitogen activated protein kinases
- P-Smad
phosphorylated Smad
- RAP
rapid-atrial pacing
- RAS
rennin-angiotensin system
- R-Smads
regulatory Smad
- siRNA
small interfering RNA
- TGF-β1
transforming growth factor-β1
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaauw Y, Crijns HJ. Atrial fibrillation: insights from clinical trials and novel treatment options. J Intern Med. 2007;262:593–614. doi: 10.1111/j.1365-2796.2007.01885.x. 1. [DOI] [PubMed] [Google Scholar]
- 2.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 3.Wyse DG, Gersh BJ. Atrial fibrillation: a perspective: thinking inside and outside the box. Circulation. 2004;109:3089–3095. doi: 10.1161/01.CIR.0000132611.01101.DC. [DOI] [PubMed] [Google Scholar]
- 4.Beyerbach DM, Zipes DP. Mortality as an endpoint in atrial fibrillation. Heart Rhythm. 2004;1:B8–18. doi: 10.1016/j.hrthm.2004.04.018. discussion. [DOI] [PubMed] [Google Scholar]
- 5.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–S27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Aldhoon B, Melenovsky V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiol Res. 2009 doi: 10.33549/physiolres.931651. [DOI] [PubMed] [Google Scholar]
- 8.Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med. 2006;84:975–983. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 9.Boldt A, Scholl A, Garbade J, Resetar ME, Mohr FW, Gummert JF, Dhein S. ACE-inhibitor treatment attenuates atrial structural remodeling in patients with lone chronic atrial fibrillation. Basic Res Cardiol. 2006;101:261–267. doi: 10.1007/s00395-005-0571-2. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama Y, Atarashi H, Kobayashi Y, Takano T. Angiotensin-converting enzyme inhibitors are not effective at inhibiting further fibrous changes in the atria in patients with chronic atrial fibrillation: speculation from analysis of the time course of fibrillary wave amplitudes. Jpn Heart J. 2004;45:93–101. doi: 10.1536/jhj.45.93. [DOI] [PubMed] [Google Scholar]
- 11.Chrysostomakis SI, Karalis IK, Simantirakis EN, Koutsopoulos AV, Mavrakis HE, Chlouverakis GI, Vardas PE. Angiotensin II type 1 receptor inhibition is associated with reduced tachyarrhythmia-induced ventricular interstitial fibrosis in a goat atrial fibrillation model. Cardiovasc Drugs Ther. 2007;21:357–365. doi: 10.1007/s10557-007-6053-z. [DOI] [PubMed] [Google Scholar]
- 12.Buxton IL, Duan D. Cyclic GMP/protein kinase G phosphorylation of Smad3 blocks transforming growth factor-beta-induced nuclear Smad translocation: a key antifibrogenic mechanism of atrial natriuretic peptide. Circ Res. 2008;102:151–153. doi: 10.1161/CIRCRESAHA.107.170217. [DOI] [PubMed] [Google Scholar]
- 13.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Crowe DL, Castillo C, Wuenschell C, Chai Y, Warburton D. Smad7 is a TGF-beta-inducible attenuator of Smad2/3-mediated inhibition of embryonic lung morphogenesis. Mech Dev. 2000;93:71–81. doi: 10.1016/s0925-4773(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 17.de Boer RA, Pokharel S, Flesch M, van Kampen DA, Suurmeijer AJ, Boomsma F, van Gilst WH, Van Veldhuisen DJ, Pinto YM. Extracellular signal regulated kinase and SMAD signaling both mediate the angiotensin II driven progression towards overt heart failure in homozygous TGR(mRen2)27. J Mol Med. 2004;82:678–687. doi: 10.1007/s00109-004-0579-3. [DOI] [PubMed] [Google Scholar]
- 18.Moriguchi M, Niwano S, Yoshizawa N, Kitano Y, Kojima J, Inuo K, Saitou J, Izumi T. Inhomogeneity in the appearance of electrical remodeling during chronic rapid atrial pacing: evaluation of the dispersion of atrial effective refractoriness. Jpn Circ J. 2001;65:335–340. doi: 10.1253/jcj.65.335. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan D, Hrapchak B. Theory and practice of histotechnology. Batelle Press; Ohio: 1980. [Google Scholar]
- 20.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 21.Ju H, Hao J, Zhao S, Dixon IM. Antiproliferative and antifibrotic effects of mimosine on adult cardiac fibroblasts. Biochim Biophys Acta. 1998;1448:51–60. doi: 10.1016/s0167-4889(98)00114-1. [DOI] [PubMed] [Google Scholar]
- 22.Peterson DJ, Ju H, Hao J, Panagia M, Chapman DC, Dixon IM. Expression of Gi-2 alpha and Gs alpha in myofibroblasts localized to the infarct scar in heart failure due to myocardial infarction. Cardiovasc Res. 1999;41:575–585. doi: 10.1016/s0008-6363(98)00264-8. [DOI] [PubMed] [Google Scholar]
- 23.Nakao A, Roijer E, Imamura T, Souchelnytskyi S, Stenman G, Heldin CH, ten DP. Identification of Smad2, a human Mad-related protein in the transforming growth factor beta signaling pathway. J Biol Chem. 1997;272:2896–2900. doi: 10.1074/jbc.272.5.2896. [DOI] [PubMed] [Google Scholar]
- 24.Britton FC, Wang GL, Huang ZM, Ye L, Horowitz B, Hume JR, Duan D. Functional characterization of novel alternatively spliced ClC-2 chloride channel variants in the heart. J Biol Chem. 2005;280:25871–25880. doi: 10.1074/jbc.M502826200. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem. 2000;275:40904–40909. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- 27.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Wang DH, Yao A, Zhao H, DiPette DJ. Regulation of ANG II receptor in hypertension: role of ANG II. Am J Physiol. 1996;271:H120–H125. doi: 10.1152/ajpheart.1996.271.1.H120. [DOI] [PubMed] [Google Scholar]
- 29.Cunnington RH, Nazari M, Dixon IM. c-Ski, Smurf2, and Arkadia as regulators of TGF-beta signaling: new targets for managing myofibroblast function and cardiac fibrosis. Can J Physiol Pharmacol. 2009;87:764–772. doi: 10.1139/Y09-076. [DOI] [PubMed] [Google Scholar]
- 30.Laurent G, Moe GW, Hu X, Pui-Sze SP, Ramadeen A, Leong-Poi H, Doumanovskaia L, Konig A, Trogadis J, Courtman D, Strauss BH, Dorian P. Simultaneous right atrioventricular pacing: a novel model to study atrial remodeling and fibrillation in the setting of heart failure. J Card Fail. 2008;14:254–262. doi: 10.1016/j.cardfail.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 32.Shirani J, Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: Implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc Pathol. 2000;9:95–101. doi: 10.1016/s1054-8807(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 33.Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukaya H, Niwano S, Satoh D, Masaki Y, Niwano H, Kojima J, Moriguchi M, Izumi T. Inhomogenic effect of bepridil on atrial electrical remodeling in a canine rapid atrial stimulation model. Circ J. 2008;72:318–326. doi: 10.1253/circj.72.318. [DOI] [PubMed] [Google Scholar]
- 35.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima H, Kumagai K. Reverse-remodeling effects of angiotensin II type 1 receptor blocker in a canine atrial fibrillation model. Circ J. 2007;71:1977–1982. doi: 10.1253/circj.71.1977. [DOI] [PubMed] [Google Scholar]
- 38.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 40.Seko Y, Nishimura H, Takahashi N, Ashida T, Nagai R. Serum levels of vascular endothelial growth factor and transforming growth factor-beta1 in patients with atrial fibrillation undergoing defibrillation therapy. Jpn Heart J. 2000;41:27–32. doi: 10.1536/jhj.41.27. [DOI] [PubMed] [Google Scholar]
- 41.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kaab S, Hinterseer M, Kartmann H, Kreuzer E, Dugas M, Steinbeck G, Nabauer M. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 42.Cardin S, Libby E, Pelletier P, Le BS, Shiroshita-Takeshita A, Le MN, Leger J, Demolombe S, Ponton A, Glass L, Nattel S. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ Res. 2007;100:425–433. doi: 10.1161/01.RES.0000258428.09589.1a. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Mehta JL, Li D, Joseph L, Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 45.Halder SK, Beauchamp RD, Datta PK. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res. 2005;307:231–246. doi: 10.1016/j.yexcr.2005.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.