Abstract
Background
Autism spectrum disorders (ASD) involve a core deficit in social functioning and impairments in the ability to recognize face emotions. In an emotional faces task designed to constrain group differences in attention, the present study used functional MRI to characterize activation in the amygdala, ventral prefrontal cortex (vPFC), and striatum, three structures involved in socio-emotional processing, in adolescents with ASD.
Methods
Twenty-two adolescents with ASD and 20 healthy adolescents viewed facial expressions (happy, fearful, sad and neutral) that were briefly presented (250ms) during functional MRI acquisition. To monitor attention, subjects pressed a button to identify the gender of each face.
Results
The ASD group showed greater activation to the faces relative to the control group in the amygdala, vPFC and striatum. Follow-up analyses indicated that the ASD relative to control group showed greater activation in the amygdala, vPFC and striatum (p<.05 small volume corrected), particularly to sad faces. Moreover, in the ASD group, there was a negative correlation between developmental variables (age and pubertal status) and mean activation from the whole bilateral amygdala; younger adolescents showed greater activation than older adolescents. There were no group differences in accuracy or reaction time in the gender identification task.
Conclusions
When group differences in attention to facial expressions were limited, adolescents with ASD showed greater activation in structures involved in socio-emotional processing.
Keywords: Autism, Adolescents, FMRI, Faces, Emotion
Introduction
Social impairments are a primary component of autism spectrum disorders (ASD)(APA, 1994). Successful social interaction requires the capacity to identify and respond appropriately to emotional facial expressions. Individuals with ASD show facial expression recognition deficits (Losh, et al., 2009) and these deficits are thought to reflect perturbations in the neural architecture that includes the amygdala (Adolphs, 2001). Consistent with this possibility, three studies documented increased amygdala activation to faces in ASD (Dalton, et al., 2005; Kleinhans, et al., 2009; Monk, et al., 2010). However, other studies reported that individuals with ASD relative to controls show less amygdala activation to faces (Ashwin, Baron-Cohen, Wheelwright, O'Riordan, & Bullmore, 2007; Critchley, et al., 2000; Dapretto, et al., 2006; Grelotti, et al., 2005; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007; Pelphrey, Morris, McCarthy, & Labar, 2007; Pinkham, Hopfinger, Pelphrey, Piven, & Penn, 2008). Thus, at present, it is unclear whether individuals with ASD show more or less amygdala activation to face stimuli.
The inconsistencies regarding amygdala function may stem, at least partly, from differences in attention to the face stimuli between groups. Indeed, individuals with ASD show abnormalities in attention to social stimuli (Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Pelphrey, et al., 2002). One of the studies that found greater amygdala activation to faces in the ASD sample also found that gaze directed to the eyes was associated with greater amygdala activation (Dalton, et al., 2005). Since group differences in attention have the potential to contribute to differences in brain function, it is important for neuroimaging studies of face processing to limit this possibility. Monitoring eye gaze is one approach in considering attention. Another approach to decrease potential group differences in attention is to require a behavioral response and to present stimuli for a brief duration. A correct behavioral response indicates that subjects were attending to the face stimulus and the brief presentation decreases the possibility of group differences in gaze duration before a saccade.
Social deficits of ASD may be even more prominent in adolescence, and this may influence clinical presentation in ASD (Ghaziuddin, Weidmer-Mikhail, & Ghaziuddin, 1998; White, Oswald, Ollendick, & Scahill, 2009). During adolescence, the social environment of peers increases in complexity (Nelson, Leibenluft, McClure, & Pine, 2005). More sophisticated skills are required to effectively navigate social interactions, and the mismatch between these increasing demands and the abilities of ASD individuals might be particularly evident. Despite findings showing that adolescence represents a very difficult transition for youth with ASD, little work has been done to examine neural function in ASD during this period.
Moreover, among those with ASD, younger adolescents have poorer social functioning than older adolescents (McGovern & Sigman, 2005). Similarly, younger adolescents with ASD compared to their older adolescent counterparts perform worse in an emotional face recognition task (Kuusikko, et al., 2009). Thus, the transition into adolescence for those with ASD may be marked by even greater disturbance in amygdala function. No known study has examined the association of developmental variables, such as age and pubertal status, with amygdala function in ASD during adolescence.
Other structures that are responsive to socio-emotional information include the ventral prefrontal cortex (vPFC) and the striatum. The vPFC, which includes Brodmann’s Areas 11 and 47, is highly responsive to emotional stimuli (Adolphs, 2009). Although many studies found that individuals with ASD showed less activation in the vPFC in response to facial displays (Ashwin, et al., 2007; Dapretto, et al., 2006; Dichter & Belger, 2007; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2006; Pinkham, et al., 2008), another study found that those with ASD showed greater activation (Dalton, et al., 2005).
The striatum is also responsive to socio-emotional stimuli (Phillips, Drevets, Rauch, & Lane, 2003). At present, the neuroimaging findings of the striatum in ASD are mixed. In a visually guided saccade task, the ASD group showed greater striatal activation than controls (Takarae, Minshew, Luna, & Sweeney, 2007). However, in a facial expression imitation task, the ASD group showed less striatal activation than controls (Dapretto, et al., 2006). A task in which group differences in attention to faces are limited may clarify the functioning of the striatum in ASD.
The present study had two objectives. The first objective was to examine amygdala, vPFC and striatal function in ASD and control adolescents while viewing facial expressions. To reduce the likelihood that group differences in brain function were due to attention differences, subjects pressed a button to identify the gender of the faces to ensure that they were attending to the stimuli. In addition, faces were presented briefly (250ms). Thus, since it was expected that subjects would fixate on the face to identify gender and typical duration for fixation of scenes is 330ms (Rayner, 1998), the task limits group differences in attention to the faces. However, saccades may occur as quickly as 150ms and, therefore, this task does not eliminate the possibility that there could be group differences in attention. The second objective was to characterize how amygdala activation relates to developmental variables of age and puberty during adolescent development for individuals with ASD.
Following prior work (Dalton, et al., 2005; Monk, et al., 2010), our first hypothesis was that adolescents with ASD would show greater amygdala, vPFC and striatal activation to facial expressions relative to controls in a task that minimized group differences in attention. In addition, because younger adolescents with ASD are more impaired in face emotion recognition than older adolescents (Kuusikko, et al., 2009), our second hypothesis was that younger adolescents with ASD would show greater amygdala activation than older adolescents with ASD.
Methods
Participants
Thirty-seven adolescents with ASD and 21 controls participated. In the ASD group, 8 adolescents were excluded due to excessive head movement (>3mm) and 7 did not complete the scan due to discomfort. In the control group, 1 adolescent was removed from analysis due to movement. The final sample included 22 adolescents with ASD and 20 controls (Table 1). Of the 22 adolescents with ASD, 6 were diagnosed with autism, 3 with Asperger’s syndrome and 13 with pervasive developmental disorder-not otherwise specified. All ASD participants were diagnosed based on the Autism Diagnostic Observation Schedule (ADOS) (Lord, et al., 2000), the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994) and confirmed by clinical consensus. Twelve of the 22 ASD participants were on psychotropic medication (2 were on selective serotonin reuptake inhibitors, 10 were on a medication for attention-deficit hyperactivity disorder, 4 were on an atypical antipsychotics and 1 was on an anxiolytic). A post-hoc analysis was carried out to ascertain if the medications contributed to group differences (see Results). Verbal and non-verbal cognitive functioning was obtained by administering the Peabody Picture Vocabulary Test (PPVT), Differential Ability Scales (DAS), Wechsler Intelligence Scale for Children, Stanford-Binet Intelligence Scales, or Ravens Progressive Matrices. There were no significant group differences in age, verbal cognitive functioning, gender, and handedness (Table 1). The ASD group had higher non-verbal cognitive functioning than the control group. A follow-up analysis controlled for differences in this domain. All adolescents with ASD were recruited through the University of Michigan Autism and Communication Disorders Center (UMACC) and controls were recruited through advertisements and posted flyers.
Table 1.
Subject characteristics.
| ASD (n=22) |
Control (n=20) |
Statistical Comparison | |
|---|---|---|---|
| Age, mean(SD) in years | 14.36(1.70) | 14.97(1.95) | t(40)=1.09, p=.28 |
| Age range in years | 11.17 – 16.75 | 10.25 – 18.00 | |
| Pubertal Rating Scale score(SD)1 | 2.72 (0.76) | 2.70 (0.74) | t(39)=0.10, p=.92 |
| Male-to-female ratio | 17:5 | 19:1 | X2(1)=1.44, p0.23 |
| Verbal cognitive functioning, mean(SD) | 109(18.37) | 113 (13.57) | t(40)=0.85, p=.40 |
| Non-verbal cognitive functioning, mean(SD) | 114 (13.76) | 105 (11.35) | t(40)=2.12, p=.03* |
| Handedness left-to-right ratio | 3:19 | 2:18 | X2(1)=.013, p=1.00 |
Data missing from one subject.
The Institutional Review Board approved all procedures. Families signed consent/assent forms and filled in self-report questionnaires. Since depression and anxiety are common in adolescents with ASD, we utilized the Children’s Depression Inventory (Kovacs, 1992) and the Spence Child Anxiety Scale (Spence, 1995). Parents of controls completed the Social Communication Questionnaire (SCQ) (Rutter, et al., 2003). Exclusion criteria were as follows: cognitive functioning<85, presence of a co-occurring neurological disorder or if the participants wore braces. An addition exclusion criterion for controls was SCQ>15.
Procedures
FMRI Data Acquisition
MRI images were acquired with a 3 Tesla GE Signa. Participants made responses with a button box that was linked to an IFIS system (MRI Devices, Inc., Milwaukee, WI) and attached to their right hand. The task was projected onto a screen and participants wore goggles with built-in mirrors (VisuaStim XGA, Resonance Technologies) to view the display. For the structural images, a high resolution sagittal SPGR image consisting of 110 slices of 1.4mm thickness (flip angle=15°, FOV=26cm) were acquired. For the functional images, T2*-weighted BOLD images were collected using a reverse spiral sequence (Glover & Law, 2001). The BOLD images were comprised of 40 adjacent 3mm axial slices (TR=2000ms, TE=30ms, flip angle=90°, FOV=22cm; matrix=64×64). Slices were adjacent and parallel to the AC-PC. The images were then reconstructed to maximize magnetic field homogeneity and ensure that the functional images were corrected for misalignment to the structural data.
Gender identification task (performed during FMRI acquisition)
During image acquisition, participants performed gender identification judgments on a set of emotional and neutral faces. Faces were selected from NimStim (Tottenham, et al., 2009). Sad, happy, fearful and neutral faces were presented. There were 30 trials of each emotion across two functional runs. Trials were presented in a different randomized order for each subject.
Each trial began with a fixation cross that was displayed in the center of the screen for 500ms, followed by a face that was displayed for 250ms. A black screen then replaced the face for 1500ms. During this period, participants pressed the thumb button if they saw a male face and the index finger button if they saw a female face. Following this, an inter-trial interval (ITI) that varied between 0 ms to 6000ms (at intervals of 2000ms) was included between each trial. During the ITI, a black screen was displayed and this served as the baseline. There were a total of 120 trials across the two functional runs and the duration of each run was approximately 6 minutes. E-prime (Psychological Software Tools, Pittsburgh, PA) controlled stimulus presentations and recorded responses.
Participants were instructed to respond as quickly and as accurately as possible. Prior to the MRI scan, participants completed a practice session in a mock scanner to ensure that they were comfortable with the task and testing conditions.
Emotion Recognition Task (performed after FMRI acquisition)
Following the MRI, participants performed an emotion recognition task to assess potential group differences in the ability to identify facial expressions. The face set comprised of the same stimuli that were shown in the fMRI task. There were a total of 120 trials. The faces were presented in a different randomized order for each participant. Trials began with a fixation cross in the middle of the screen for 500ms, followed by the face for 250ms and a screen which displayed these instructions: Press 1 if the face is happy, press 2 if the face is neutral, press 3 if the face is sad, and press 4 if the face is fearful. Subsequent trials were displayed only after the participant made a response. E-Prime was used for this task. Participants were instructed to respond as soon as they could discern the emotion on each face. Participants completed a short practice session prior to the emotional recognition task to ensure that they understood the instructions.
Pubertal Measure
Parents filled out the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988). While not comprehensive, this measure correlates highly with physician ratings of pubertal development (Petersen, et al., 1988).
Analyses
Functional MRI Data Analysis
The data underwent preprocessing. The skull was removed using FSL (http://www.fmrib.ox.ac.uk/fsl). Large spikes in the k-space data were filtered out. While the data were reconstructed into images, a field map correction was carried out. The reconstructed images were then corrected for difference in acquisition time for each slice. Following the slice time correction using local sinc interpolation (Oppenheim, Schafer, & Buck, 1999), the images were realigned using MCFLIRT in FSL. Images were examined to exclude cases with head motion greater than 3 mm. Using SPM5 (www.fil.ion.ucl.ac.uk/spm), T1 GRE images were co-registered to the 3D SPGR volume in order to map the functional images into a standard anatomical space. The 3D SPGR volume was then inhomogeneity-corrected and normalized using an 8 mm full width at half maximum Gaussian kernel to the SPM5 T1 template (MNI space).
As a first step, to examine overall group differences in activation to faces in the three regions of interest, participants’ mean contrast values for all face expressions (fearful, sad, happy and neutral) combined relative to baseline (the ITI period when a black screen was displayed) were extracted from the structurally-based regions of interest (ROIs; bilateral amygdala, vPFC and striatum). These ROIs were generated using WFU Pickatlas (Maldjian, Laurienti, Burdette, & Kraft, 2002). The amygdala was the bilateral amygdala, the vPFC was derived from bilateral Brodmann’s areas 11, and 47, and the striatum consisted of the bilateral caudate and putamen. These values were then submitted to a general linear model multivariate analysis in SPSS 17. All trials with incorrect behavioral responses were excluded from the FMRI analyses.
Subsequent, statistical processing of the functional data was carried out using SPM5. General linear model and random effects analyses were utilized to assess within- and between-group effects. For each participant, conditions were modeled with the SPM5 canonical hemodynamic response function (HRF). The temporal derivative of the HRF was included (Friston, et al., 1998). A statistical image for each contrast at each voxel was generated. The contrast maps generated for each participant were then entered to test population-level hypotheses. Statistical significance was established with a small volume correction approach and family-wise error p<.05 on the ROIs (Worsley, et al., 1996).
Behavioral Data Analysis
Mean accuracy and mean reaction time were obtained for the gender identification task and the emotion recognition task.
Results
Behavioral Results
Gender identification task (performed during FMRI acquisition)
There were no significant differences between the ASD and control group in accuracy, t(39)=.66, p=0.511 and reaction time, t(39)=1.20, p=.236. (Behavioral data for one control subject were lost due to a technical malfunction.) The ASD group had a mean accuracy of 95.2% (SD=3.0) and the control group had a mean accuracy of 96.1% (SD=4.8). The mean reaction time for the ASD group was 746.2ms (SD=132.2) and for the control group it was 699.9ms (SD=111.0).
Emotion recognition task (performed following the MRI)
There were no significant differences between the ASD and control group in task accuracy, t(40)=−.073, p=0.942 and mean reaction time, t(40)=.679, p=.501. (Only subjects who are in the FMRI analysis are included in this analysis.) The ASD group had a mean accuracy of 89.8% (SD=6.9) and control group had a mean accuracy of 89.9% (SD=5.7). The mean reaction time of the correct responses for the ASD group was 1257ms (SD=278.5) and the mean reaction time for the control group was 1205ms (SD=218.6).
FMRI Results
Hypothesis 1
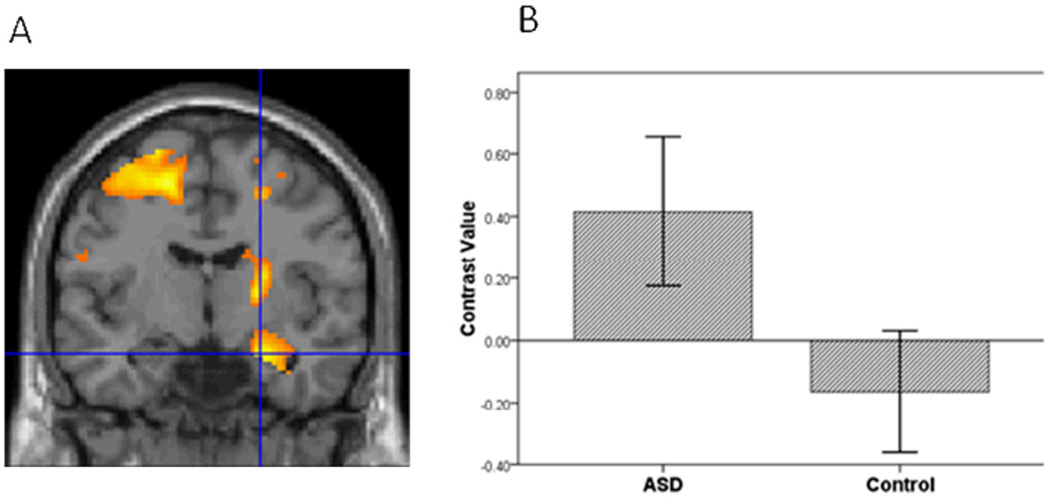
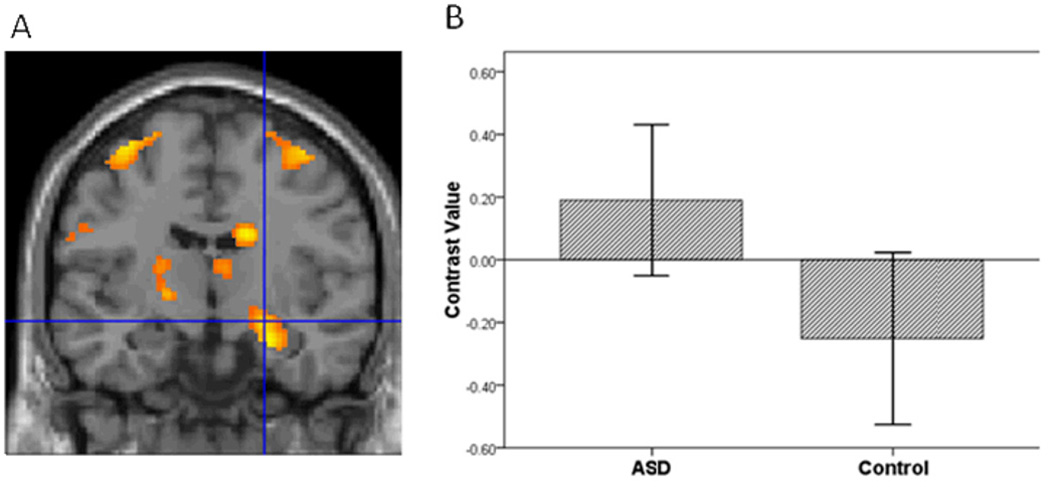
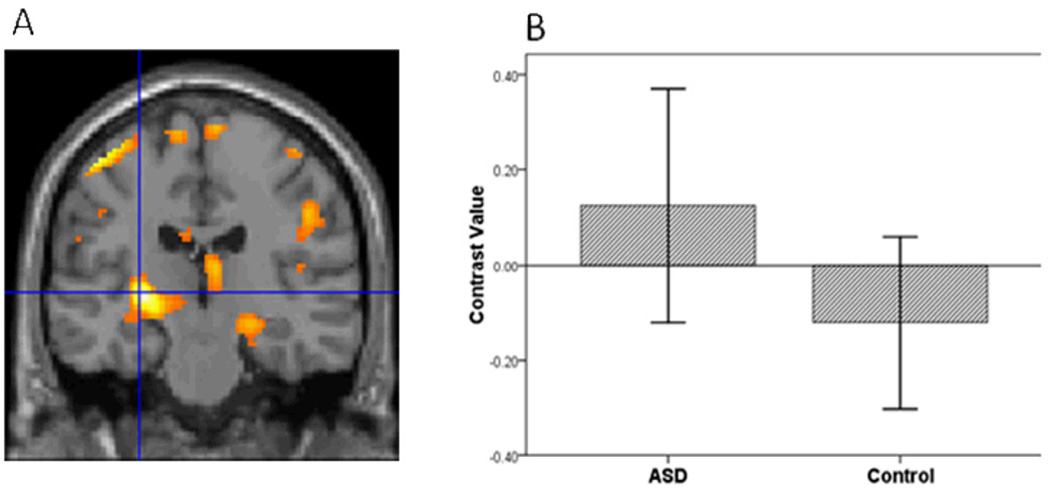
To evaluate hypothesis 1, we implemented a four step procedure. First, following seminal face processing studies of ASD (Dalton, et al., 2005; Dapretto, et al., 2006; Kleinhans, et al., 2009; Pinkham, et al., 2008), we examined group differences in activation to all face expressions relative to baseline. Mean contrast values for all face expressions were extracted from the bilateral amygdala, vPFC and striatum. Multivariate analysis showed a group difference in activation to faces, F(3,38)=4.38, p=.010. Second, to identify the brain regions that contributed to the group differences while controlling for multiple comparisons, we implemented the sequentially rejective Bonferroni procedure (Holm, 1977). Consistent with the first hypothesis, the ASD relative to the control group had greater activation in the amygdala, t(40)=2.29, p=.027, vPFC, t(40)=2.65, p=.011, and the striatum, t(40)=3.30, p=.002.
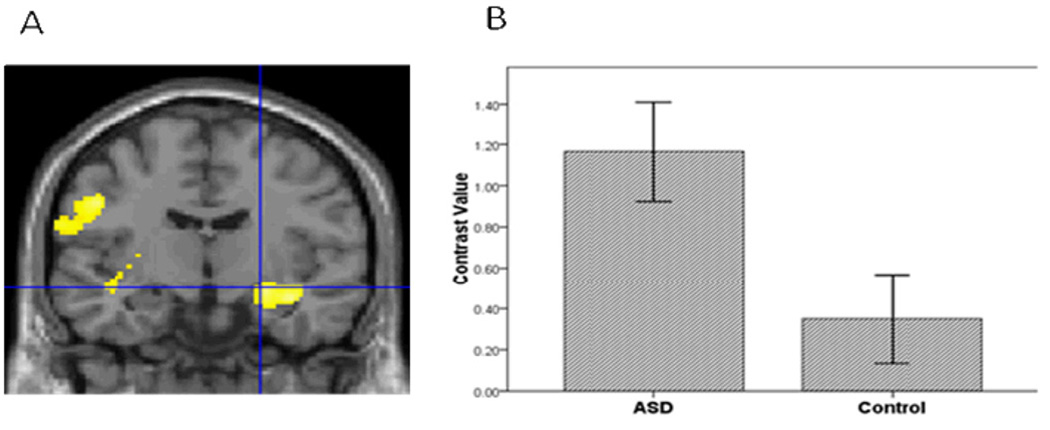
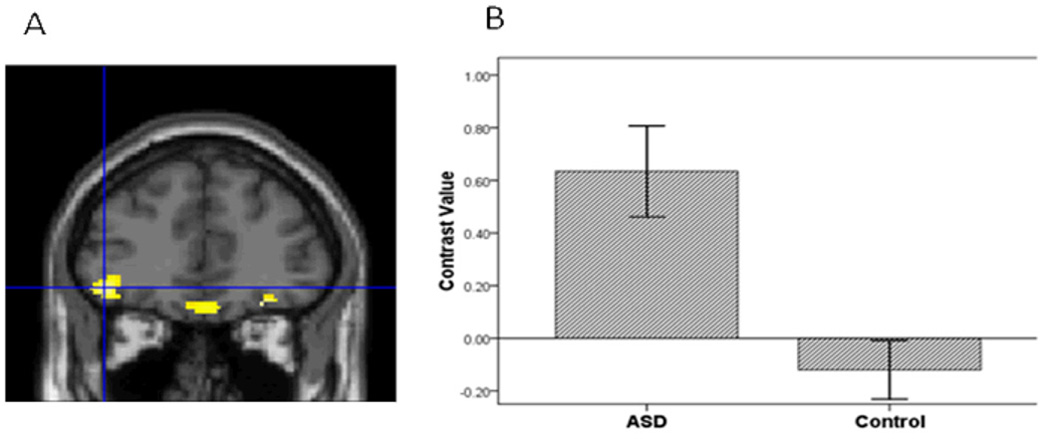
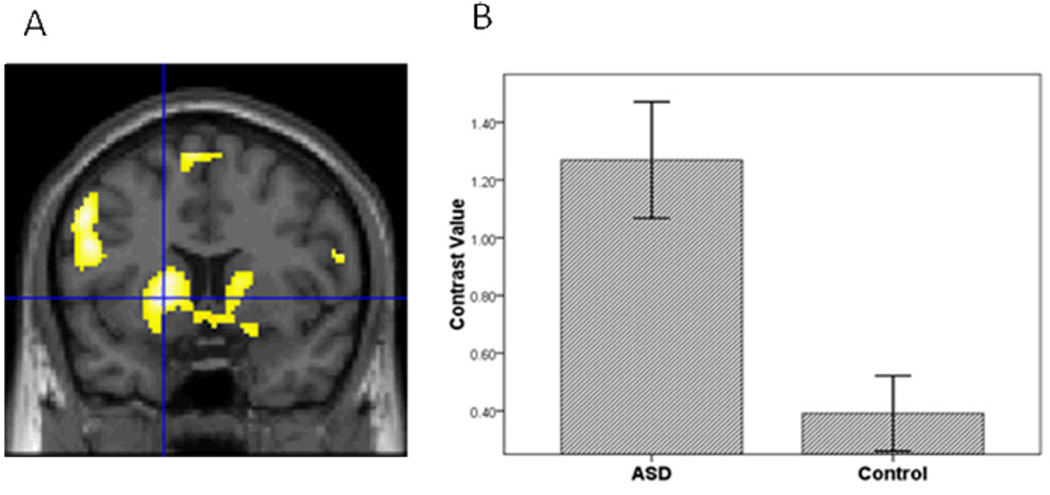
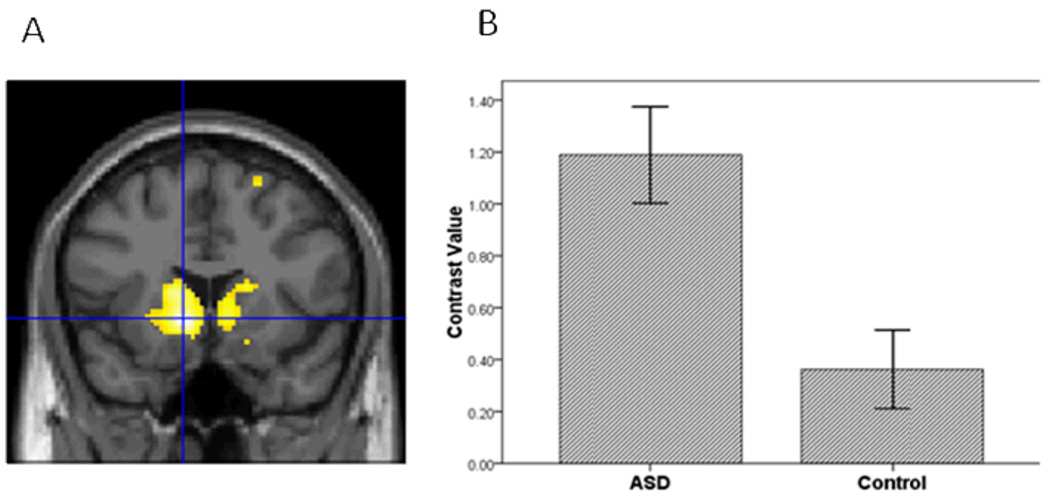
Third, to further evaluate hypothesis 1, we conducted a voxel-wise ROI analysis in SPM5 to examine group differences in amygdala, vPFC and striatal activation to specific emotions vs. baseline in each hemisphere. For sad vs. baseline, adolescents with ASD relative to controls demonstrated greater activation in all ROIs (Table 2; Figures 1, 2, and 3). In addition, for happy vs. baseline, the ASD group showed greater striatal activation relative to controls (Table 2; Figure 4).
Table 2.
Differences in activation between adolescents with ASD and controls. Statistical threshold was set at p≤.05 small volume-corrected for each of the ROIs in each hemisphere. There were no areas in which the control group showed significantly greater activation than the ASD group. For cluster size, p=.05 uncorrected.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Contrast | Side | BA | Cluster size |
t | p corrected | x | y | z |
| Amygdala | Sad vs. Baseline | L | 24 | 2.69 | .050 | −20 | −2 | −14 | |
| R | 101 | 3.23 | .015 | 24 | −10 | −12 | |||
| vPFC | Sad vs. Baseline | L | 47 | 157 | 4.07 | .028 | −44 | 40 | −10 |
| R | 11 | 98 | 3.91 | .041 | 28 | 40 | −18 | ||
| R | 11 | 63 | 3.91 | .042 | 4 | 34 | −18 | ||
| Striatum | Sad vs. Baseline | L | 1144 | 4.35 | .008 | −18 | 8 | 0 | |
| Happy vs. Baseline | L | 992 | 4.62 | .004 | −10 | 16 | −4 | ||
| R | 840 | 4.30 | .009 | 10 | 10 | −2 | |||
L=Left; R=Right; BA=Brodmann’s Area; vPFC=ventral prefrontal cortex
Figure 1.
A. Relative to the controls, the ASD group had greater amygdala activation in the contrast of Sad vs. Baseline. Threshold for this and subsequent figures was p=.005 for the images. B. To illustrate the activation for this and subsequent bar graphs, mean contrast values were extracted from the entire ROI within hemisphere. Contrast values represent the difference in mean activation in the ROI for a given contrast for all subjects averaged together in each group. Error bars for all figures represent standard errors of the means.
Figure 2.
The ASD group compared to controls had greater vPFC activation in the contrast of Sad vs. Baseline.
Figure 3.
The ASD group compared to controls had greater striatal activation in the contrast of Sad vs. Baseline.
Figure 4.
The ASD group compared to controls had greater striatal activation in the contrast of Happy vs. Baseline.
Fourth, to compare group differences in activation to between expressions, analyses focused on expressions and hemispheres where group differences were found in step 3. Relative to controls, the ASD group showed greater activation to sad vs. happy and a trend for greater activation in the contrast of sad vs. neutral in the right amygdala (Table 3; Figures 5–6). The ASD group also showed greater activation in the left striatum to sad vs. neutral (Table 3; Figure 7).
Table 3.
Differences in activation between adolescents with ASD and controls between expressions. No other contrast approached significance.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Contrast | Side | BA | Cluster size |
t | p corrected | x | y | z |
| Amygdala | Sad vs. Happy | R | 65 | 3.32 | .012 | 24 | −8 | −20 | |
| Sad vs. Neutral | R | 27 | 2.53 | .066 | 24 | −10 | −12 | ||
| Striatum | Sad vs. Neutral | L | 53 | 4.35 | .008 | −28 | −22 | 0 | |
Figure 5.
The ASD group compared to controls had greater amygdala activation in the contrast of Sad vs. Happy.
Figure 6.
The ASD group compared to controls had a trend for greater amygdala activation in the contrast of Sad vs. Neutral.
Figure 7.
The ASD group compared to controls had greater striatal activation in the contrast of Sad vs. Neutral.
Appendix Tables 1–2 provide activation in the ROIs for the ASD and control groups separately. In addition, Appendix Table 3 provides activation for the fusiform. Finally, Appendix Table 4 includes a whole brain analysis.
Appendix Table 1.
Activation for the ASD group. Statistical threshold was set at p<.05 small volume-corrected for each of the ROIs (amygdala, vPFC and striatum) using SPM5. For brevity, only the largest activation in each region was reported for this and subsequent tables. Contrasts not reported were not significant at the threshold.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Contrast | Side | BA | Cluster size |
t | p corrected | x | y | z |
| Amygdala | Sad vs. Baseline | L | 61 | 5.52 | .000 | −20 | −2 | −14 | |
| R | 104 | 4.91 | .000 | 24 | −10 | −12 | |||
| Fearful vs. Baseline | L | 61 | 4.11 | .002 | −28 | −4 | −20 | ||
| R | 101 | 4.03 | .002 | 22 | −10 | −12 | |||
| Happy vs. Baseline | L | 54 | 4.00 | .002 | −20 | −4 | −14 | ||
| R | 58 | 2.90 | .031 | 24 | 2 | −20 | |||
| Neutral vs. Baseline | L | 61 | 5.48 | .000 | −22 | −8 | −10 | ||
| R | 94 | 3.53 | .008 | 28 | −2 | −22 | |||
| Sad vs. Happy | R | 80 | 3.09 | .020 | 24 | −8 | −20 | ||
| vPFC | Sad vs. Baseline | L | 47 | 425 | 5.77 | .000 | −36 | 26 | 2 |
| R | 11 | 450 | 4.76 | .004 | 28 | 40 | −18 | ||
| R | 11 | 88 | 4.14 | .024 | 4 | 34 | −18 | ||
| Fearful vs. Baseline | L | 47 | 392 | 5.68 | .000 | −42 | 16 | −4 | |
| R | 47 | 338 | 4.40 | .011 | 36 | 30 | −2 | ||
| Happy vs. Baseline | L | 47 | 282 | 4.52 | .008 | −30 | 20 | −2 | |
| R | 47 | 402 | 5.62 | .000 | 36 | 26 | 4 | ||
| Neutral vs. Baseline | L | 47 | 87 | 5.06 | .002 | −36 | 20 | 0 | |
| L | 47 | 98 | 4.44 | .011 | −36 | 30 | −2 | ||
| R | 47 | 309 | 4.72 | .005 | 32 | 30 | −6 | ||
| Striatum | Sad vs. Baseline | L | 1336 | 6.89 | .000 | −18 | 8 | 0 | |
| R | 1377 | 6.42 | .000 | 18 | 4 | 10 | |||
| Fearful vs. Baseline | L | 1347 | 5.35 | .000 | −30 | −4 | 0 | ||
| R | 1266 | 4.71 | .003 | 18 | 4 | 8 | |||
| R | 11 | 3.61 | .049 | 28 | −36 | 6 | |||
| Happy vs. Baseline | L | 1308 | 7.46 | .000 | −18 | 8 | 0 | ||
| R | 1305 | 7.28 | .000 | 14 | 8 | 0 | |||
| Neutral vs. Baseline | L | 1255 | 6.18 | .000 | −24 | −4 | −6 | ||
| R | 1204 | 5.3 | .001 | 24 | −6 | 12 | |||
| Sad vs. Neutral | L | 80 | 4.44 | .006 | −28 | −22 | 0 | ||
Appendix Table 2.
Activation for the Control group. Statistical threshold was set at p <.05 small volume-corrected for each of the ROIs (amygdala, vPFC and striatum) using SPM5. Contrasts not reported were not significant at the threshold.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Contrast | Side | BA | Cluster size |
t | p corrected | x | y | z |
| Amygdala | Sad vs. Baseline | L | 61 | 7.29 | .000 | −22 | −2 | −14 | |
| R | 104 | 5.92 | .000 | 26 | 2 | −20 | |||
| Fearful vs. Baseline | L | 61 | 6.91 | .000 | −26 | −2 | −14 | ||
| R | 104 | 7.46 | .000 | 18 | −8 | −14 | |||
| Happy vs. Baseline | L | 58 | 7.32 | .000 | −20 | −4 | −14 | ||
| R | 104 | 4.72 | .001 | 20 | −2 | −14 | |||
| Neutral vs. Baseline | L | 61 | 9.05 | .000 | −24 | −2 | −14 | ||
| R | 104 | 6.14 | .000 | 20 | −2 | −14 | |||
| Neutral vs Happy | L | 45 | 3.13 | .017 | −24 | −8 | −18 | ||
| vPFC | Sad vs. Baseline | L | 47 | 306 | 7.82 | .000 | −36 | 26 | 4 |
| R | 47 | 502 | 8.89 | .000 | 38 | 22 | 0 | ||
| Fearful vs. Baseline | L | 47 | 353 | 11.11 | .000 | −42 | 16 | −4 | |
| R | 47 | 464 | 10.10 | .000 | 38 | 18 | −4 | ||
| Happy vs. Baseline | L | 47 | 183 | 6.40 | .000 | −32 | 26 | 4 | |
| R | 47 | 447 | 9.68 | .000 | 36 | 26 | 2 | ||
| Neutral vs. Baseline | L | 47 | 315 | 6.98 | .000 | −36 | 18 | −2 | |
| R | 47 | 488 | 7.75 | .000 | 34 | 30 | −8 | ||
| Striatum | Sad vs. Baseline | L | 1385 | 7.45 | .000 | −20 | −4 | 14 | |
| R | 1399 | 7.88 | .000 | 18 | 2 | 10 | |||
| R | 11 | 4.18 | .012 | 28 | −36 | 6 | |||
| Fearful vs. Baseline | L | 1249 | 8.44 | .000 | −28 | −4 | 8 | ||
| R | 1349 | 8.03 | .000 | 20 | 2 | 10 | |||
| Happy vs. Baseline | L | 1357 | 8.63 | .000 | −26 | 2 | −6 | ||
| R | 1358 | 8.94 | .000 | 16 | 2 | 10 | |||
| Neutral vs. Baseline | L | 1381 | 8.76 | .000 | −24 | −6 | −8 | ||
| R | 1397 | 7.58 | .000 | 20 | −4 | 14 | |||
Appendix Table 3.
Activation in the fusiform. Statistical threshold was set at p<.05 small volume-corrected for the left and right fusiform (from WFU Pickatlas) using SPM5. There were no areas in which the control group showed significantly greater activation than the ASD group. For the cluster size, the p value was .05 uncorrected. Contrasts which were not included were not significant at the corrected threshold. Controls vs. ASD revealed no significant differences.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group comparison |
Contrast | Side | BA | Cluster size |
t | p corrected | x | y | z |
| ASD vs. Controls |
Sad vs. Baseline | L | 20 | 94 | 3.67 | .046 | −46 | −38 | −16 |
| ASD | Sad vs. Baseline | L | 19 | 405 | 7.67 | .000 | −22 | −58 | −12 |
| R | 19 | 377 | 8.66 | .000 | 26 | −56 | −14 | ||
| Fear vs. Baseline | L | 37 | 372 | 5.79 | .000 | −48 | −58 | −16 | |
| R | 37 | 377 | 6.84 | .000 | 34 | −58 | −14 | ||
| Happy vs. Baseline | L | 19 | 371 | 5.53 | .000 | −30 | −70 | −12 | |
| R | 19 | 377 | 7.47 | .000 | 22 | −60 | −12 | ||
| Neutral vs. Baseline | L | 19 | 393 | 5.59 | .000 | −30 | −64 | −14 | |
| R | 19 | 375 | 7.47 | .000 | 22 | −60 | −12 | ||
| Control | Sad vs. Baseline | L | 19 | 400 | 12.65 | .000 | −28 | −62 | −14 |
| R | 19 | 377 | 14.21 | .000 | 26 | −56 | −14 | ||
| Fear vs. Baseline | L | 19 | 400 | 12.65 | .000 | −28 | −62 | −14 | |
| R | 37 | 377 | 14.21 | .000 | 26 | −56 | −14 | ||
| Happy vs. Baseline | L | 19 | 397 | 9.97 | .000 | −32 | −62 | −16 | |
| R | 37 | 377 | 13.95 | .000 | 36 | −50 | −14 | ||
| Neutral vs. Baseline | L | 19 | 401 | 11.29 | .000 | −30 | −64 | −14 | |
| R | 37 | 377 | 12.63 | .000 | 34 | −50 | −16 | ||
Appendix Table 4.
Group differences in activation for whole brain. Statistical threshold was set at p<.05 cluster-level using SPM5.
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group comparison | Contrast | Side | Region/BA | Cluster size |
t | p value | x | y | z |
| ASD vs. Controls | Sad vs. Baseline |
R | Insula/13 | 324 | 4.38 | .023 | 42 | −32 | 20 |
| L | Striatum | 263 | 4.35 | .046 | −18 | 8 | 0 | ||
| L | Inferior frontal gyrus/9 |
375 | 4.32 | .013 | −54 | 10 | 38 | ||
| L | Middle temporal gyrus/21 |
257 | 3.98 | .049 | −56 | −40 | −4 | ||
| Happy vs. Baseline |
L | Striatum | 656 | 4.62 | .001 | −10 | 16 | −4 | |
| Neutral vs. Baseline |
R | Medial frontal gyrus/9 |
256 | 4.15 | .045 | 8 | 40 | 24 | |
Hypothesis 2
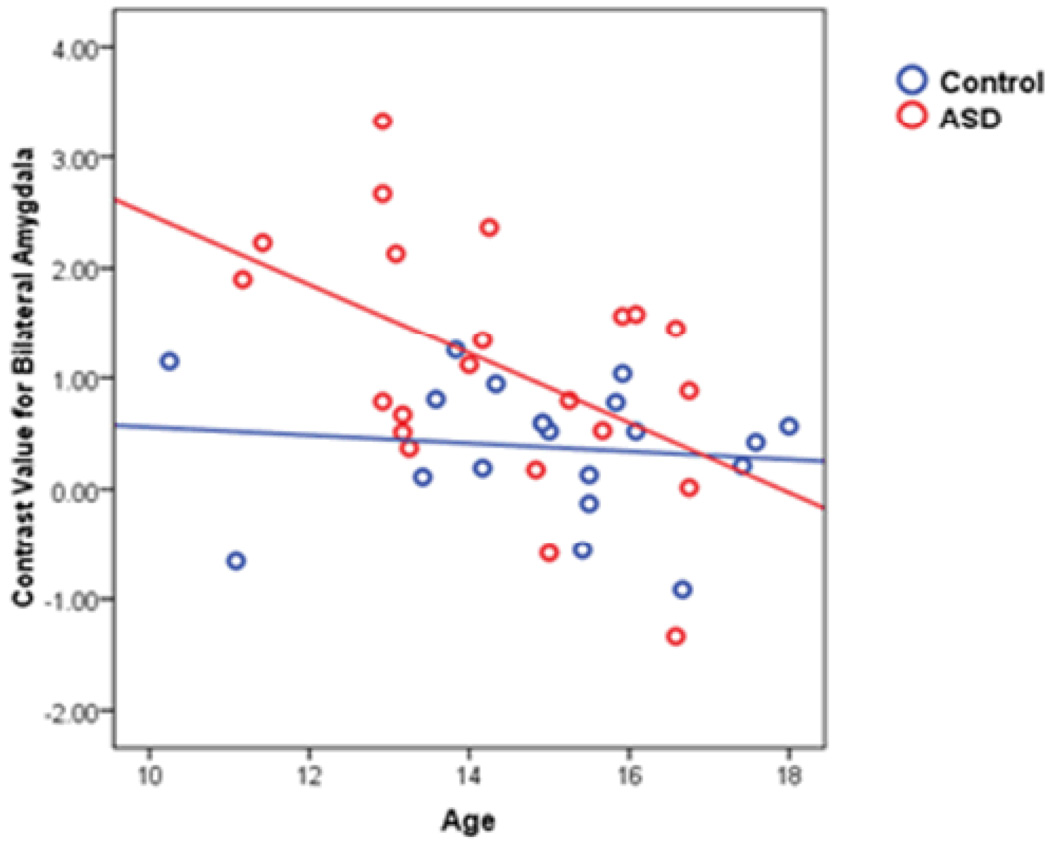
Bilateral amygdala activation (mean activation from the ROI) to all expressions vs. baseline negatively correlated with age in the autism sample, Spearman rho=−.46, p=.033, and pubertal status, Spearman rho=.46, p=.036 (pubertal status from one subject was not collected). In the Sad vs. Baseline comparison, there was a negative association between age and bilateral amygdala activation (Figure 8). Developmental measures did not correlate with the other contrasts in which group differences were found (Sad vs. Happy, Sad vs. Neutral). Among controls, amygdala activation did not correlate with age or pubertal status.
Figure 8.
Amygdala activation and age were negatively correlated in the ASD group, Spearman’s rho=−0.46, p=0.031. The correlation among controls was −.20, p=0.40. Circles represent each subject’s mean activation for the entire bilateral amygdala in the contrast of Sad vs. Baseline.
Follow-Up Analysis of Medication
To examine whether medications influenced the results, adolescents with ASD who were on at least one psychotropic medication were removed from the group analysis. The remaining 10 adolescents with ASD not on medication were compared to the 20 controls. The ASD group continued to have greater activation relative to controls in all the same contrasts and ROIs described above (p<.05 uncorrected).
Follow up Analyses on Depression and Anxiety
Relative to controls, the ASD group showed a trend for higher depression symptoms, t(40)=1.95, p=.059. When depression was added as a nuisance covariate, the group differences reported above remained significant (p<.05 uncorrected). For anxiety, there were no group differences in the measure, and therefore, further analyses were not conducted.
Discussion
In an emotional faces task that limited group differences in attention, adolescents with ASD exhibited greater activation than controls in neural structures associated with processing socio-emotional stimuli. Consistent with our hypotheses, the ASD group showed greater bilateral activation in the amygdala, vPFC and striatum. In addition, within the ASD group, there was a negative association between developmental variables (age and pubertal status) and amygdala activation, such that the younger adolescents had more pronounced activation than the older adolescents. Comparable performance in the gender identification task during FMRI acquisition suggests that both groups were attending similarly to the faces. Finally, the results were independent of depression and anxiety.
Our findings demonstrate that when attention to faces appears comparable, adolescents with ASD show greater activation in key emotion/face processing structures relative to controls. Two possible interpretations are offered. The first is that the facial expressions are more ambiguous for those with ASD. From early in development, individuals with ASD attend less to faces (Osterling & Dawson, 1994). A lack of experience with faces may contribute to impairments in face emotion recognition (Losh, et al., 2009). Thus, individuals with ASD are less able to decipher expressions and may not know how to respond appropriately (i.e., the stimuli are ambiguous). Notably, ambiguity engages the amygdala, vPFC and striatum (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005). Therefore, impaired emotion recognition in ASD may lead to a less definitive interpretation of the stimuli and the neural manifestation of this ambiguity may be expressed by an increased activation in these structures. However, in contrast to prior work (Losh, et al., 2009), we did not find group differences in the face emotion recognition task. This inconsistency may be due to the nature of the face stimuli in the present study. That is, the facial expressions were particularly identifiable and, thus, the task lacked sensitivity. Nevertheless, in the present study, FMRI may have been more capable of detecting a neural signal of ambiguity than the behavioral measure.
The second interpretation is that the facial expressions are more distressing for individuals with ASD and this distress might be driving greater activation in these structures. (The two interpretations are possibly interrelated. Heightened distress could make individuals avoid faces more, which would lead to reduced familiarity and, therefore, social signals from faces could be more ambiguous. Alternatively, distress may emerge from the perceived ambiguity of the stimuli.) Indirect evidence for the possibility that facial expressions are distressing for individuals with ASD comes from work showing that ASD is associated with greater skin conductance response than controls to faces with direct gaze (Joseph, Ehrman, McNally, & Keehn, 2008). Contrary to this interpretation, we did not find group differences in anxiety. Nevertheless, the experience of distress in the ASD sample may not have been effectively captured in our anxiety measure. Further work is necessary to test these two models.
The finding that younger adolescents had greater amygdala activation than older adolescents indicates that marked developmental changes occur in the neural processing of socio-emotional stimuli among adolescents with ASD. This is consistent with work showing that younger adolescents have poorer social functioning (McGovern & Sigman, 2005) and emotion recognition than older adolescents (Kuusikko, et al., 2009). The present findings also parallel structural imaging work, which found that an ASD sample between 7.5 and 12.5 years of age had larger amygdala volume than controls, but the group difference disappeared with age (Schumann, et al., 2004). Biological and social changes associated with the transition to adolescence may underlie the changes in the amygdala and socioemotional difficulties. Targeted interventions that impede this cascade of events may help youth with ASD transition more successfully into adolescence.
Four limitations to this study are noted. First, no developmental measures other than age and a puberty questionnaire were used. Including biological measures of puberty as well as measures of social life may provide further insight into how these variables interact for youth with ASD. Second, a high proportion of the recruited ASD sample was not included in the analyses (due to movement and discomfort in the MRI). This could limit the generalizability of our findings. Third, although the task limited the opportunity for group differences in attention, there was no direct measure of eye gaze on the faces. Therefore, it is not possible to definitively state that attention between groups was comparable. Fourth, although group differences in activation were found between Sad and Happy faces in the amygdala and Sad and Neutral faces in the striatum, the comparison of Sad vs. Neutral faces was only a trend (p=.066 small volume corrected) in the amygdala. Therefore, we are unable to make definitive statements about the role of the amygdala in processing emotional social stimuli in ASD.
Future investigations could incorporate a longitudinal approach to more accurately chart developmental change in brain function. Moreover, such an approach could eventually identify brain correlates that would predict more severe clinical symptoms. Also, through additional measures of face expertise, puberty and social interaction, a tighter coupling between developmental events and changes in brain function could be formed. In addition, group differences in the vPFC and the developmental results were only found with baseline as the comparison. Contrasts with baseline indicate that the ASD group and younger adolescents, respectively, show greater activation to faces, but the results may not be specific to faces. Future research could include complex nonsocial stimuli to evaluate the specificity of the response. Furthermore, because the task involved many presentations of a limited number of emotions, there was the potential for habituation. Habituation may have reduced the sensitivity of the task to differentiate the response between emotions. To reduce the possibility of habituation, additional emotions, along with nonsocial stimuli, could be included to increase the variety of stimuli. In addition, the present findings were strongest for sad faces. Through the use of psychophysiological measures and recording subject facial expressions that occur in response to the face stimuli, future work could more fully evaluate how adolescents with ASD process sad faces differently and why the processing is associated with greater amygdala activation. Finally, coupled with work showing that youth with ASD are more socially impaired in early adolescence (McGovern & Sigman, 2005), the present study suggests that socially-based interventions that afford an opportunity for neural adaptation may facilitate the transition to adolescence.
Key Points
Subjects with ASD show facial expression recognition deficits and these deficits are thought to reflect perturbations in neural structures that include the amygdala, vPFC, and striatum.
Prior work is inconsistent about whether individuals with ASD show increased or decreased activation in these structures when viewing face stimuli. The inconsistencies may stem, at least partly, from differences in attention to the face stimuli between groups.
Using an emotional faces task that minimizes group differences in attention, the present study found that adolescents with ASD exhibited greater activation than controls in neural structures associated with processing emotional faces (amygdala, vPFC cortex and striatum). In addition, within the ASD group, the younger adolescents had more pronounced activation than the older adolescents.
The present findings suggest that neural correlates of ASD may be characterized as heightened activation of socio-emotional structures when group differences in attention are minimized. The heightened activation of the amygdala is most pronounced when youth with ASD are transitioning into adolescence.
ACKNOWLEDGEMENTS
We thank the families who participated.
This research was supported by Autism Speaks (C.S.M.) and NIH (U19 HD035482 to C.L.; MH066496 to C.L.).
Footnotes
Conflict of interest statement: Drs. Lord and Risi receive royalties from a publisher of diagnostic instruments described in this paper. They give all profits generated by the University of Michigan Autism and Communication Disorders Center (UMACC) in regards to this paper and all other UMACC projects to a charity. No other author has a conflict of interest.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The Social Brain: Neural Basis of Social Knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35(3):1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Weidmer-Mikhail E, Ghaziuddin N. Comorbidity of Asperger syndrome: a preliminary report. J Intellect Disabil Res. 1998;42(Pt 4):279–283. doi: 10.1111/j.1365-2788.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16(9):1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Human Brain Mapping. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1977;6:65–70. [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14(6):947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory (CDI) Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Kuusikko S, Haapsamo H, Jansson-Verkasalo E, Hurtig T, Mattila ML, Ebeling H, et al. Emotion recognition in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(6):938–945. doi: 10.1007/s10803-009-0700-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, et al. Neuropsychological profile of autism and the broad autism phenotype. Archives of General Psychiatry. 2009;66(5):518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft R. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2002;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. J Child Psychol Psychiatry. 2005;46(4):401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, et al. Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry and Neuroscience. 2010;35(2):105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Oppenheim A, Schafer R, Buck J. Discrete-time signal processing. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards MH, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading and information processing: 20 years of research. Psychological Bulletin. 1998;124(3):372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Berument S, Le Couteur A, Lord C, Pickles A. Social Communication Questionnaire (SCQ) Los Angeles, Calif.: 2003. [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH. The Social Worries Questionnaire. Social skills training: Enhancing social competence with children and adolescents. Windsor: NFER-Nelson; 1995. [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research. 2007;156(2):117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29(3):216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]