Abstract
Rhes, the Ras Homolog Enriched in Striatum, is an intermediate-size GTP binding protein. Although its full functions are not yet known, it has been shown to affect signaling and behaviors mediated by G protein-coupled receptors. Here we have tested whether Rhes affects behaviors mediated by opioid receptors. Wild type and rhes-deficient mice were administered morphine and tested for analgesia in formalin and tail flick tests. Rhes−/− mice showed significantly enhanced analgesia in both tests relative to rhes+/+ mice. Furthermore, rhes−/− mice did not display tolerance to repeated morphine administration and displayed significantly less withdrawal than rhes+/+ mice. These findings indicate that Rhes is involved in behaviors mediated by mu opioid receptors and in the adaptive response to repeated morphine administration.
Keywords: RASD2, opioid, tolerance, analgesia, dependence, GTP-binding protein
Rhes (Ras Homolog Enriched in Striatum, RASD2) is a GTP-binding protein that is highly expressed in the striatum of rodents and has a high degree of homology with the Ras superfamily of small GTPase proteins [7]. It was originally characterized by using subtractive hybridization for genetic sequences differentially expressed in striatum [7, 29]. The rhes gene codes for a 266 amino acid protein of intermediate size between Ras-like GTPases and α subunits of heterotrimeric G proteins. Rhes is most closely related to AGS1/Dexras1, showing 62% identity [7]. Both proteins contain an elongated C-terminus, defining them as the founding members of a novel subfamily of the Ras superfamily [7]. Furthermore, both genes are regulated by hormones, rhes by thyroid hormone and AGS1/dexras1 by dexamethasone [11, 30].
In vitro investigations of Rhes have shown its ability to attenuate Gs and, more recently, Gi/o signaling after agonist stimulation of G protein coupled receptors (GPCR). In PC12 cells, Rhes was shown to attenuate reporter gene activation by the Gs-coupled thyroid stimulating hormone receptor, but not by the M2 muscarinic receptor [31]. However, in HEK293 cells expressing Rhes and M2, attenuation by Rhes of agonist-initiated inhibition of CaV2.2 was demonstrated. This effect is mediated by Gβγ subunits liberated from Gi/o and demonstrates that Rhes, like AGS1/Dexras1, can affect Gi/o-mediated signaling [28]. In addition, the efficacy of Gi/o activation through D2 receptors is lower in rhes−/− mice compared with rhes+/+ counterparts, suggesting that the presence of Rhes protein is important in normal signal transduction by this dopamine receptor [5].
Rhes has been shown to be preferentially expressed in neurons of the striatum [5, 7, 9, 31], and in vivo studies point to its role in dopaminergic signaling and behavior. Lesion of the nigrostriatal pathway results in a decrease in rhes mRNA and protein expression in the striatum [9, 10]. Rhes−/− mice show increased D1 agonist-initiated locomotor activation, increased D2 antagonist-initiated catalepsy, and increased D1 signaling through adenylyl cyclase (AC) relative to rhes+/+ mice [5]. These studies suggest that absence of Rhes leads to an increase in dopamine receptor activation of AC, or similarly a decrease in inhibitory tone through AC. However, rhes−/− mice also show a decrease in dopamine D1 receptor-mediated grooming behavior relative to rhes+/+ mice [19], a behavior thought to be mediated by activation of the phospholipase C pathway [4, 18], thus suggesting that Rhes may differentially affect signaling pathways.
Although dopamine systems have been emphasized in early behavioral and cellular studies of Rhes due to the enrichment of both dopamine receptors and Rhes in striatum, there is no a priori reason to assume that only dopamine receptors are modulated by Rhes. Indeed, opioid receptors are also highly expressed in striatum [14], and striatum has been shown to play a role in analgesia [1, 12]. Here we have tested whether these GPCRs are also modulated by Rhes. We hypothesized that because there is increased excitatory tone through AC in rhes−/− mice and a decrease in the ability to activate Gi/o [5], these mice would show decreased morphine analgesia relative to wild type mice. To test this hypothesis, we tested analgesia in two different models of pain and found, surprisingly, that rhes−/− showed enhanced analgesia relative to rhes+/+ mice. In addition, we tested whether Rhes affects the development of tolerance and dependence upon repeated morphine administration.
Methods
Subjects
Rhes+/+ and rhes−/− mice were generated as previously described [24] and backcrossed for ten generations onto the C57/BL6 background. Male mice from heterozygous and homozygous matings were used for all experiments. Genotypes were verified by using PCR of tail biopsies, and mice were used at 2–4 months of age. Mice were group housed (n=4/cage) in a climate-controlled vivarium on a 12-h light/dark cycle (lights on at 0600). Food and water were provided ad libitum with the exception of testing times. All animal experiments were performed in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, and experimental protocols were approved and supervised by the University of New Orleans Institutional Animal Care and Use Committee. Every effort was made to minimize the suffering of the animals and to reduce the number of animals used.
Drugs
Morphine Sulfate (Paddock Laboratories; Minneapolis, MN) was injected at a dose of either 0.3, 1, 3, or 10 mg/kg, and naloxone hydrochloride (Sigma; St. Louis, MO) was injected at a dose of 3 mg/kg. Drugs were dissolved in 0.9% saline and administered IP at an injection volume of 10 ml/kg.
Apparatus and Procedure
Formalin test
Thirty minutes after morphine administration, animals received a 40 μl SC injection of 5% formalin into the plantar surface of the left hind paw and were immediately placed in an elevated 11 cm × 23 cm Plexiglas® enclosure with a clear glass floor (IITC, Woodland Hills, California, USA). A mirror positioned at 45° under the floor allowed for un-obscured observation of the animals’ behavior. Time spent licking the injected hind paw was recorded over 5 minute intervals for 1 hour.
Tail Flick
Analgesia was tested with the tail flick paradigm using the IITC tail flick testing apparatus. Mice were loosely restrained in a towel, and a 1 cm2 beam of light was focused on the distal 1/3 of the tail. Latencies to withdraw were measured at baseline, at 5 and 15 min post-injection, and at 15 minute intervals thereafter until 2 hours post drug administration. A maximum withdrawal latency of 12 seconds was imposed to prevent tissue damage.
Opioid tolerance was measured in animals receiving single daily injections of 3 or 10 mg/kg morphine for 5 consecutive days. The percent maximum possible effect in the tail flick test was calculated 30 minutes after morphine on days 1 and 5 of drug administration by using the following formula: Percent Maximum Possible Effect (%MPE) = 100 × [(test latency − basal latency) / (cut-off time − basal latency)]. Opioid withdrawal was measured using a naloxone challenge. On day 5 of repeated morphine administration, animals were given IP injections of naloxone hydrochloride (3 mg/kg) 30 min post-administration of morphine and immediately placed in clear Plexiglas® cylinders approximately 15 cm in diameter and 30 cm high. A variable for global withdrawal score was computed by weighting number of jumps by 1, wet dog shakes by 5, and fecal boli by 5.
Statistical Analysis
For the formalin test, results are presented as time spent licking group means ± SEM for each dose by time. For statistical analysis, data were divided into early (1st 5 minutes) and late (remaining 55 minutes) phases, and two-factor (genotype × phase) repeated measures ANOVAs were performed for each dose. Tail flick results are presented as time courses for each genotype, which were analyzed by two-factor (dose×time) repeated measures ANOVA for each genotype. Nonlinear regression was used to fit dose-response data from the 45 minute time point and to calculate ED50 values and 95% confidence limits. Tolerance is presented as tail flick latency %MPE group mean ± SEM for acute and repeated administration of morphine sulfate. These data were analyzed by a two-factor (genotype × day) repeated measures ANOVA for each dose. Results of withdrawal are presented as global withdrawal score group mean ± SEM, which were compared by genotype using independent samples t-test. Results were considered significant at p < 0.05, and follow-up planned multiple comparisons were made subject to a modified Bonferroni correction.
Results
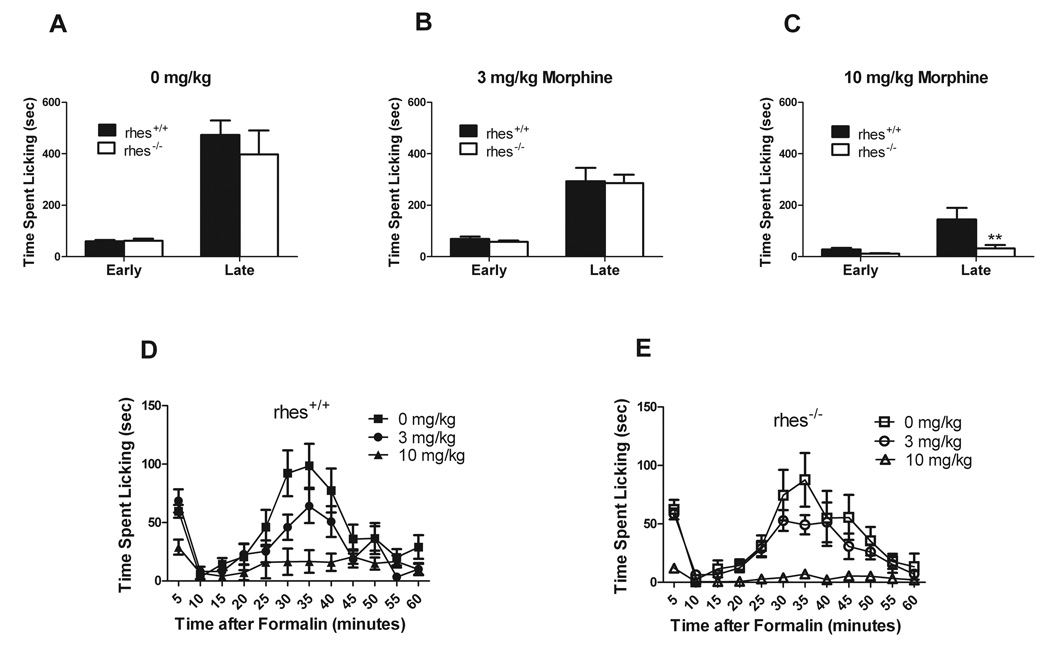
In the formalin test, rhes−/− and rhes+/+ mice did not show any differences in licking in the early or late phase when administered vehicle [F(1,18)=0.424, p > 0.05] or morphine at a dose of 3 mg/kg [F(1,17)=0.155, p >0.05] (Figure 1a and b). However, when administered a dose of 10 mg/kg morphine, rhes−/− mice showed significantly less licking in the late phase [F(1,18)=6.587, p <0.05] compared with rhes+/+ mice, suggesting increased analgesia (Figure 1c). This effect cannot be attributed to a lack of effect in rhes+/+ mice, as they showed significant analgesia at this dose (F = 10.46, p = 0.0005). Full time courses for each genotype are shown in Figure 1d and e.
Fig 1.
Analgesia assessed with the formalin test. (A-C) Time spent licking (group mean ± SEM) in the early (1st five minutes) or late (remaining 55 minutes) phases after 0 mg/kg (A), 3 mg/kg (B), or 10 mg/kg (C) morphine. (D,E) The data are shown as a time course over 60 minutes with group mean ± SEM for each 5 minute interval for rhes+/+ (D) and rhes−/− (E) mice. ** p < 0.01 compared with rhes+/+ mice at the same phase. n = 9–10.
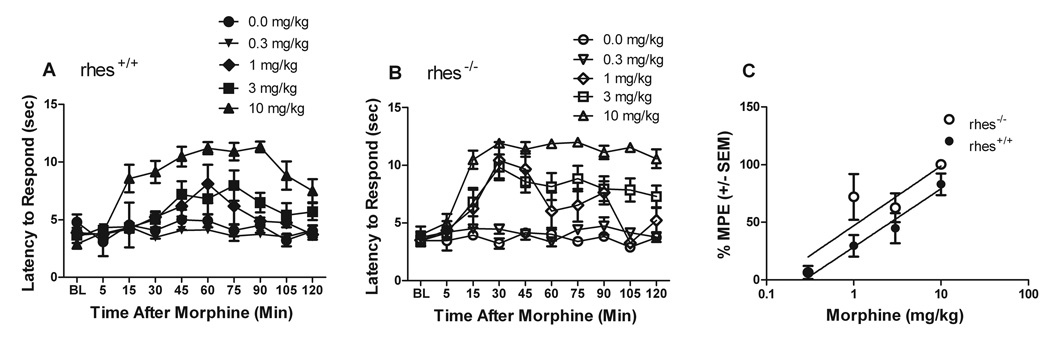
In the tail flick test, rhes−/− mice showed an increased sensitivity to morphine. Two-factor ANOVA on each genotype showed highly significant main effects of time and dose, and a time × dose interaction (time: rhes+/+ [F(9,270)=9.02, p < 0.0001], rhes−/− [F(9,270)=16.0, p < 0.0001]; dose: rhes+/+ [F(4,30)=17.6, p < 0.0001], rhes−/− [F(4,30)=27.1, p < 0.0001]; time × dose: rhes+/+ [F(36,270)=3.04, p < 0.0001], rhes−/− [F(36,270)=4.1, p < 0.0001]) (Figure 2a and b). Follow up comparisons indicate that for rhes+/+ mice, only the 3 and 10 mg/kg doses differed significantly from vehicle, whereas for rhes−/− mice, 1, 3, and 10 mg/kg doses all differed significantly from vehicle. Figure 2c illustrates the %MPE for each genotype at each dose for the 45 minute time point. In rhes+/+ mice, the ED50 value was 2.9 mg/kg (1.6–5.1 mg/kg), whereas in rhes−/−, it was 0.98 mg/kg (0.48–1.9 mg/kg), representing a threefold leftward shift. Overall, these results suggest that rhes−/− mice display enhanced morphine-induced analgesia relative to rhes+/+ mice in two separate models of nociception.
Fig 2.
Analgesia assessed with the tail flick test. (A,B) Time course of latency to respond presented as group means ± SEM for rhes+/+ (A) and rhes−/− (B) mice. (C) Dose-response curves for analgesia assessed at 45 minutes post-morphine injection, showing a threefold leftward shift in the ED50 value of rhes+/+ mice relative to rhes−/− mice. n = 5 for 0, 0.3, and 1 mg/kg, n= 9–11 for 3 and 10 mg/kg.
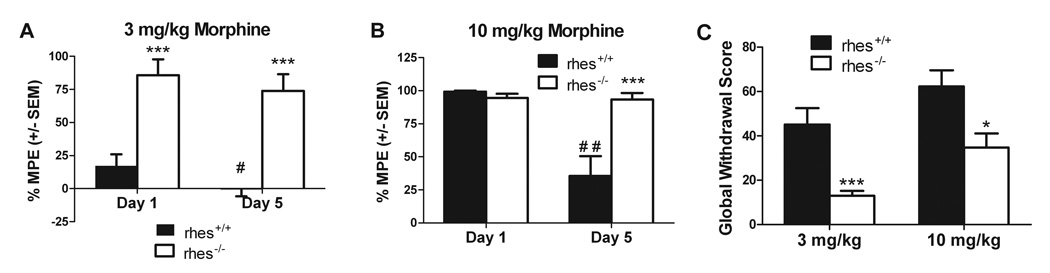
To test for effects of Rhes on adaptation to repeated morphine treatment, mice were given repeated injections (5 days) of morphine (3 or 10 mg/kg) and tested for tolerance to the analgesic effects 30 minutes post-drug administration. At the 3 mg/kg dose, there were significant effects for genotype [F(1,15)=33.10, p < 0.0001] and time [F(1,19)=6.354, p <0.05]. Rhes−/− mice showed significantly increased %MPE after both the acute [t(16)=4.395, p <0.001] and repeated morphine administration [t(16)=6.191, p <0.001] compared with rhes+/+ mice. Rhes+/+ mice showed significantly reduced %MPE after repeated versus acute administrations [t(9)=−2.303, p <0.05], whereas rhes−/− showed no significant difference between administrations [t(9)=−.362, p >0.05], indicating induction of tolerance in rhes+/+ but not rhes−/− mice (Figure 3a). At the 10 mg/kg dose, there were significant effects for genotype [F(1,16)=11.13, p < 0.01] and time [F(1,16)=15.98, p <0.01], and a significant genotype × time interaction [F(1,16)=14.85, p < 0.01]. Rhes−/− mice showed significantly increased %MPE after repeated morphine [t(16)=5.09, p <0.001], relative to rhes+/+ mice. Furthermore, Rhes+/+ mice again showed significantly reduced %MPE between the acute and repeated administrations [t(8)=−4.267, p <0.01], whereas rhes−/− showed no significant difference between administrations [t(8)=−.185, p >0.05], indicating induction of tolerance in rhes+/+ but not rhes−/− mice (Figure 3b). Global withdrawal scores for rhes−/− mice were significantly decreased as compared with rhes+/+ mice at the 3 mg/kg [t(19)=−4.241, p<0.001] and 10 mg/kg [t(16)=−2.827, p < 0.05] doses (Figure 3c).
Fig 3.
Attenuation of tolerance and withdrawal in rhes−/− mice. (A,B) %MPE group means ± SEM of 3 mg/kg (A) or 10 mg/kg (B) morphine in rhes+/+ and rhes−/− mice on Day 1 and Day 5 of repeated drug administration. Percent MPE was significantly lower in rhes+/+ mice on day five versus day one after 3 mg/kg and 10 mg/kg morphine, thus indicating tolerance, whereas rhes−/− mice showed no such difference. n = 8–9. ***p<0.001 versus rhes+/+ mice on the same day; # p<0.05, # # p<0.01 versus day 1 in the same genotype. (C) In naloxone-precipitated withdrawal, rhes −/− mice show significantly reduced global withdrawal scores as compared with rhes+/+ mice. n = 8–11. *p<0.05, *** p<0.001 compared with rhes+/+ mice. All bars represent group mean ± SEM.
Discussion
We have demonstrated here that Rhes participates in the acute analgesic response to morphine in mice and, perhaps more importantly, in the adaptation to repeated morphine administration. The effect of increased acute analgesia in rhes−/− mice was surprising in light of previous findings that these mice show increased signaling through AC pathways and decreased ability to activate Gi/o [5], which would predict a decrease in acute analgesia. However, because morphine is now known to induce its effects by activation of multiple signaling pathways, including AC and phospholipase C (PLC) [22], the results may be best interpreted in light of activation of multiple intracellular pathways. Activation of PLC and protein kinase C (PKC) through mu opioid receptors provides inhibition of analgesia (see below). Therefore, Rhes may normally affect this pathway to inhibit analgesia, and loss of Rhes protein would therefore result in increased analgesia.
As a striatally-enriched protein, Rhes function has mainly been investigated in the context of this structure. For example, it has recently been shown that Rhes accounts for the striatal-specific cell death in Huntington’s Disease [25, 26]. Also, a growing body of evidence shows an association of Rhes with behavior and signaling mediated by dopamine. However, as opioid receptors are also enriched in striatum, a possible mechanism for Rhes to affect morphine analgesia is through interaction with opioid receptors themselves. Previous studies with dopamine receptors indicate that it is likely that a loss of Rhes protein would result in a net activation of AC [5, 31], which would not be conducive to an increase in opioid analgesia. However, that effect on AC signaling was through dopamine receptors, and Rhes effects on opioid receptor signaling have not yet been investigated. Given the direction of the effect of the loss of Rhes on analgesia and the previous finding that Rhes promotes a PLC-mediated effect (grooming [19]), it may be that Rhes exerts its effects on analgesia through the PLC pathway. Several lines of evidence indicate the importance of this pathway for mu receptor signaling: (1) Mu opioid receptors increase inositol (1,4,5)triphosphate formation in a pertussis toxin-reversible manner [21]; (2) genetic deletion of PLC β3 [32] or PKCε [17] in mice enhances morphine analgesia; and (3) a small molecule inhibitor of Gβγ-dependent PLC increases morphine analgesia [3, 15]. Furthermore, the hyperalgesia induced by ultra-low dose morphine is mediated by the PLC-PKC pathway [2, 6, 8]. Thus, a pro-nociceptive system is activated through mu opioid receptors and PLC-PKC pathways. If, analogous to dopamine D1 receptor-mediated grooming, Rhes promotes PLC pathway activation by mu opioid receptors, then a loss of Rhes would decrease activation of this pathway and relieve its pro-nociception.
The PLC-PKC pathway is also involved in the mechanism of opioid tolerance and dependence. PKC phosphorylation increases during acute tolerance [20]. Furthermore, PLC inhibitors attenuate morphine tolerance [15, 23] and dependence [15], and genetic deletion of PKCγ decreases tolerance development [33]. With regard to opioid tolerance and dependence, rhes−/− mice display a phenotype similar to that of mice with pharmacological or genetic deletion of PLC-PKC pathways. As previous behavioral studies suggest that Rhes promotes behaviors mediated by PLC pathways [19], this may be one mechanism by which rhes gene deletion attenuates tolerance and dependence. However, further studies are necessary to establish a definite role for Rhes in tolerance and/or dependence, particularly studies employing continuous infusion of drug to eliminate any role of withdrawal in between daily injections, and direct tests of Rhes effects on PLC signaling. In addition, an effect via other Gi/o-mediated pathways is possible given the complex effects of Rhes on signaling activated by this G protein [28, 31].
The exact locus of Rhes effects on analgesia is presently unknown. A recent investigation in mice lacking the striatally-enriched AC type 5 has shown that striatum is important for a range of morphine-mediated behaviors, including analgesia, tolerance, and dependence [12]. In addition to its enrichment in striatum, rhes mRNA is also found in several other brain areas, including cortex, olfactory bulb, cerebellum, certain thalamic nuclei, and hippocampus, particularly early in development [7, 9, 10, 31]. Our antibody does not detect any Rhes protein in spinal cord (unpublished observations), but it may not be sensitive enough to detect levels lower than that in striatum. A more sensitive method such as RT-PCR may detect lower levels of expression. Alternatively, Rhes effects on tail flick analgesia may be mediated through descending modulatory pathways.
Conclusions
In summary, we have shown that the GTP binding protein Rhes modulates morphine analgesia, and may participate in tolerance and dependence. Although Rhes may exert these effects through interactions with dopamine systems, a more likely mechanism is interaction with opioid receptor signaling. Rhes may promote pro-nociceptive signaling, with a loss of Rhes protein resulting in increased analgesia, and decreased tolerance and dependence.
Acknowledgments
Supported by the National Institutes of Health (P20RR016816), the Louisiana Board of Regents [LEQSF(2009-11)-RD-A-11)], and University of New Orleans New Faculty Start-up. We thank Dr. YouE He for assistance with genotyping and Dr. James Zadina for constructive reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal Gβγ-dependent stimulation of PLCβ3 originating from G inhibitory protein-μ opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J. Neurochem. 2009;111:171–180. doi: 10.1111/j.1471-4159.2009.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gβγ-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 4.Clifford JJ, Tighe O, Croke DT, Kinsella A, Sibley DR, Drago J, Waddington JL. Conservation of behavioral topography to dopamine D1-like receptor agonists in mutant mice lacking the D1A receptor implicates a D1-like receptor not coupled to adenylyl cyclase. Neuroscience. 1999;93:1483–1489. doi: 10.1016/s0306-4522(99)00297-3. [DOI] [PubMed] [Google Scholar]
- 5.Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De Chiara V, Prosperetti C, Spano D, Herve D, Pasqualetti M, Di Lauro R, Fisone G, Usiello A. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol. Cell. Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Esmaeili-Mahini S, Shimokawa N, Javan M, Maghsoudi N, Motamedi F, Koibuchi N, Ahmadiani A. Low-dose morphine induces hyperalgesia through activation of Gαs, protein kinase C, and L-type Ca2+ channels in rats. J. Neurosci. Res. 2008;86:471–479. doi: 10.1002/jnr.21489. [DOI] [PubMed] [Google Scholar]
- 7.Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific Ras homolog related to Dexras1. J. Neurosci. Res. 1999;57:782–788. [PubMed] [Google Scholar]
- 8.Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Harrison LM, LaHoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LM, LaHoste GJ, Ruskin DN. Ontogeny and dopaminergic regulation in brain of Ras homolog enriched in striatum (Rhes) Brain Res. 2008;1245:16–25. doi: 10.1016/j.brainres.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel Ras superfamily member-related gene in AtT-20 cells. J. Biol. Chem. 1998;271:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 12.Kim K-S, Lee K-W, Lee K-W, Im J-Y, Yoo JY, Kim S-W, Lee J-K, Nestler EJ, Han P-L. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc. Natl. Acad. USA. 2006;103:3908–3913. doi: 10.1073/pnas.0508812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King MA, Bradshaw S, Chang AH, Pintar JE, Pasternak GW. Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: Evidence for a tonically active anti-opioid system. J. Neurosci. 2001;21:7788–7792. doi: 10.1523/JNEUROSCI.21-19-07788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study, Mudelta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J. Comp. Neurol. 1994;3:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 15.Mathews JL, Smrcka AV, Bidlack JM. A novel Gβγ-subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J. Neurosci. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra AL, Pontani RB, Vadlamani NL. Stereospecific potentiation of opiate analgesia by cocaine: predominant role of noradrenaline. Pain. 1987;28:129–138. doi: 10.1016/0304-3959(87)91066-9. [DOI] [PubMed] [Google Scholar]
- 17.Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 2007;6:29–38. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panchalingam S, Undie AS. SKF83959 exhibits biochemical agonism by stimulating [35S]GTPγS binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmcology. 2001;40:826–837. doi: 10.1016/s0028-3908(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 19.Quintero GC, Spano D, LaHoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla PK, Tang L, Wang ZJ. Phosphorylation of neurogranin, protein kinase C, and Ca2+/calodulain dependent kinase II in opioid tolerance and dependence. Neurosci. Lett. 2006;404:266–269. doi: 10.1016/j.neulet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Smart D, Smith G, Lambert DG. μ-Opioid receptor stimulation of inositol (1,4,5)trisphosphate formation via a pertussis toxin-sensitive G protein. J. Neurochem. 1994;62:1009–1014. doi: 10.1046/j.1471-4159.1994.62031009.x. [DOI] [PubMed] [Google Scholar]
- 22.Smart D, Hirst RA, Hirota K, Grandy DK, Lambert DG. The effects of recombinant rat mu-opioid receptor activation in CHO cells on phospholipase C, [Ca2+]i and adenylyl cyclase. Br. J. Pharmacol. 1997;120:1165–1171. doi: 10.1038/sj.bjp.0701012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith FL, Lohmann AB, Dewey WL. Involvement of phospholipid signal transduction pathways in morphine tolerance in mice. Br. J. Pharmacol. 1999;128:220–226. doi: 10.1038/sj.bjp.0702771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spano D, Branchi I, Rosica A, Pirro MT, Riccio A, Mithbaokar P, Affuso A, Arra C, Campolongo P, Terracciano D, Macchia V, Bernal J, Alleva E, Di Lauro R. Rhes is involved in striatal function. Mol. Cell. Biol. 2004;24:5788–5796. doi: 10.1128/MCB.24.13.5788-5796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-Huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH. Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation among the basic sumoylation enzymes E1 and UBC9. J. Biol. Chem. 2010;285:20428–20432. doi: 10.1074/jbc.C110.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor BK, Joshi C, Uppal H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003;987:135–143. doi: 10.1016/s0006-8993(03)03318-3. [DOI] [PubMed] [Google Scholar]
- 28.Thapliyal A, Bannister RA, Hanks C, Adams BA. The monomeric G proteins AGS1 and Rhes selectively influence Gαi-dependent signaling to modulate N-type (Cav2.2) calcium channels. Am. J. Physiol. Cell Physiol. 2008;295:C1417–C1426. doi: 10.1152/ajpcell.00341.2008. [DOI] [PubMed] [Google Scholar]
- 29.Usui H, Falk JD, Dopazo A, de Lecea L, Erlander MG, Sutcliffe JG. Isolation of clones of rat striatum-specific mRNAs by directional tag PCR subtraction. J. Neurosci. 1994;14:915–4926. doi: 10.1523/JNEUROSCI.14-08-04915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Sutcliffe JG, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in striatum. Mol. Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 31.Vargiu P, de Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- 32.Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinckle PM, Bidlack JM, Gross RA, Jiang H, Wu D. Genetic alteration of phospholipase C β3 expression modulates behavioral and cellular responses to μ opioids. Proc. Natl. Acad. Sci. USA. 1999;96:10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKCγ mutant mice. Pain. 2001;94:245–253. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]