Abstract
Excessive production of Aβ (amyloid β-peptide) has been shown to play an important role in the pathogenesis of AD (Alzheimer's disease). Although not yet well understood, aggregation of Aβ is known to cause toxicity to neurons. Our recent study demonstrated the ability for oligomeric Aβ to stimulate the production of ROS (reactive oxygen species) in neurons through an NMDA (N-methyl-d-aspartate)-dependent pathway. However, whether prolonged exposure of neurons to aggregated Aβ is associated with impairment of NMDA receptor function has not been extensively investigated. In the present study, we show that prolonged exposure of primary cortical neurons to Aβ oligomers caused mitochondrial dysfunction, an attenuation of NMDA receptor-mediated Ca2+ influx and inhibition of NMDA-induced AA (arachidonic acid) release. Mitochondrial dysfunction and the decrease in NMDA receptor activity due to oligomeric Aβ are associated with an increase in ROS production. Gp91ds-tat, a specific peptide inhibitor of NADPH oxidase, and Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride, an ROS scavenger, effectively abrogated Aβ-induced ROS production. Furthermore, Aβ-induced mitochondrial dysfunction, impairment of NMDA Ca2+ influx and ROS production were prevented by pre-treatment of neurons with EGCG [(−)-epigallocatechin-3-gallate], a major polyphenolic component of green tea. Taken together, these results support a role for NADPH oxidase-mediated ROS production in the cytotoxic effects of Aβ, and demonstrate the therapeutic potential of EGCG and other dietary polyphenols in delaying onset or retarding the progression of AD.
Keywords: arachidonic acid (AA) release, Ca2+ influx, (−)-epigallocatechin-3-gallate (EGCG), NADPH oxidase, N-methyl-d-aspartate (NMDA) receptor, oligomeric Aβ, reactive oxygen species (ROS)
Abbreviations: AA, arachidonic acid; Aβ, amyloid β-peptide; ACSF, artificial cerebrospinal fluid; AD, Alzheimer's disease; BCS, bovine calf serum; cPLA2, cytosolic phospholipase A2; DHE, dihydroethidium; DMEM, Dulbecco's modified Eagle's medium; EGCG, (−)-epigallocatechin-3-gallate; ERK1/2, extracellular-signal-regulated kinase 1/2; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MnTBAP, Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; NMDA, N-methyl-d-aspartate; ROS, reactive oxygen species; TBS-T, Tris-buffered saline, pH 7.4, with 0.5% Tween 20
INTRODUCTION
AD (Alzheimer's disease) is the most prevalent form of dementia affecting over 5.3 million people in the U.S.A. (Alzheimer's Association 2010), and 35 million worldwide (World Health Organization). With the disproportional increase in the ageing population in the next decade, these numbers are likely to double by 2050. Although the progressive deterioration in memory and cognitive functions in AD is marked by an increase in neuronal loss and synaptic impairment in specific regions of the brain (Selkoe, 2002), the molecular mechanisms leading to the pathogenesis of the disease are still not well understood (Querfurth and LaFerla, 2010). Recent studies have recognized the ability of Aβ (amyloid β-peptide) to aggregate into oligomeric form and induce toxicity in neurons (Shankar et al., 2007; Walsh and Selkoe, 2007; Palop and Mucke, 2010). However, understanding the mechanisms whereby Aβ oligomers impair synaptic function remains elusive.
High oxygen consumption in the brain is associated with oxidative stress, and overproduction of ROS (reactive oxygen species) is known to play an important role in the cytotoxic effects of Aβ (Butterfield et al., 2001; Sultana and Butterfield, 2010). Recent studies have demonstrated NADPH oxidase, the superoxide producing enzyme, as an important source of ROS production in brain cells (Sorce and Krause, 2009). Activation of NADPH oxidase is linked to excitotoxity mediated by the ionotropic glutamate receptors (Kishida et al., 2005; Brennan et al., 2009). Our studies further show that Aβ can also enhance ROS production in neurons via the NMDA (N-methyl-d-aspartate) receptor-dependent NADPH oxidase pathway. In turn, this receptor activation and ROS production can lead to activation of downstream signalling pathway for MAPKs (mitogen-activated protein kinases) and cPLA2 (cytosolic phospholipase A2) (Shelat et al., 2008). Although the involvement in Aβ-induced neuronal dysfunction that underlies the progression of AD has not been fully investigated, there is evidence for involvement of NADPH oxidase activity in oxidative and inflammatory responses in a number of neurodegenerative diseases (Sun et al., 2007).
Despite the increasing recognition of a link between oligomeric Aβ and NMDA receptor function, the extent of neuronal damage due to prolonged exposure of neurons to this toxic form of Aβ has not been investigated in detail. In the present study, we investigated the effects of oligomeric Aβ on rat primary cortical neurons and determined that prolonged exposure of neurons to oligomeric Aβ led to accumulation of NADPH oxidase-dependent ROS production, and in turn mitochondrial dysfunction, NMDA receptor-mediated Ca2+ influx and AA (arachidonic acid) release. We further demonstrated that EGCG [(−)-epigallocatechin-3-gallate], a major polyphenol from green tea, can protect neurons from the cytotoxic effects of oligomeric Aβ.
MATERIALS AND METHODS
Materials
DMEM (Dulbecco's modified Eagle's medium), Neurobasal medium, Pen/Strep, B27-AO (B27 without antioxidants), l-glutamine, Hepes, Ham's F-12 medium, 10×Hanks buffer, Fluo-4 AM, AlarmaBlue™ and trypsin-EDTA were obtained from Invitrogen (Carlsbad, CA, U.S.A.). BCS (bovine calf serum) was purchased from Hyclone (Thermo Fisher Scientific, South Logan, UT, U.S.A.). DHE (dihydroethidium), NMDA, resveratrol, EGCG, poly-l-lysine, BSA and nimopridine were purchased from Sigma–Aldrich (St. Louis, MO, U.S.A.). [1-14C]AA (specific activity 50 mCi/mmol) was obtained from NEN (Boston, MA, U.S.A.). Aβ (Aβ1-42 and Aβ 42-1) was purchased from American Peptide Co. (Sunnyvale, CA, U.S.A.). MnTBAP [Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride] was obtained from EMD Chemicals Inc. (Darmstadt, Germany). The NADPH oxidase inhibitor, gp91ds-tat peptide that specifically targets the gp91 docking site, was originally designed by the Pagano group (Rey et al., 2001) and was synthesized by Anaspec (San Jose, CA, U.S.A.).
Primary cortical neuron cultures
Primary cortical neurons were prepared from fetal brain of Sprague–Dawley rats at E17 (embryonic day 17) using the instructions described previously (Shelat et al., 2008) with slight modifications. In brief, cerebral cortices were dissected in Hanks buffer followed by incubation with 0.05% trypsin (GIBCO, Grand Island, NY, U.S.A.) at 37°C for 45 min. The cortices were mechanically dispersed with a pasture pipette. The cell pellet was re-suspended in D10C medium (DMEM including 10% BCS, 10% Ham's F-12 medium, 2 mM l-glutamine, 2.5% Hepes and 0.25% Pen/Strep). Cells (1.3×105/cm2) were seeded into 50 mg/l poly-l-lysine-coated plates or dishes in D10C medium. After 4–5 h, the D10C medium was replaced with Neurobasal medium containing 2% B27-AO, 2 mM l-glutamine and 1% Pen/Strep. Cells were cultured at 37°C in a humidified incubator with 5% CO2 for at least 8 days before experiments, and half of the medium was replaced with fresh medium every 3–4 days. Immunostaining for specific markers of astrocytes [GFAP (glial fibrillary acidic protein)], microglia (OX-42) and neurons [MAP-2 (microtubule-associated protein-2)] indicated that the 8-day-old neuronal cultures contained only 3–4% astrocytes and 3% microglia (Shelat et al., 2008). Neuronal morphology was routinely visualized using an inverted microscope from Olympus (Center Valley, PA, U.S.A.) with a ×20 objective. Experiments were carried out according to the guidelines set forth by the NIH Guide for the Care and Use of Laboratory Animals.
Preparation and characterization of Aβ
Preparation of oligomeric Aβ
Oligomeric Aβ, referred to as Aβ1-42, was prepared as described (Dahlgren et al., 2002). Briefly, purified Aβs (America Peptide) were dissolved in HFIIP (1,1,1,3,3,3-hexafluoro-2-propanol) to allow full conversion into the monomeric form. The monomeric Aβ was divided into aliquots into Eppendorf tubes and the organic solvent was evaporated using a speed vacuum apparatus (Savant, Fisher Scientific, St. Louis, MO, U.S.A.). The dried Aβ was stored at −80°C until used. To obtain oligomeric Aβ, samples in the Eppendorf tubes were dissolved in DMSO to make a concentration of 5 mM, followed by sonication for 30 s. The suspension was diluted with PBS (pH 7.4) to 0.1 mM Aβ, and the samples were then incubated at 4°C for 24 h. As a control, Aβ42-1 was treated similarly to Aβ1-42.
Analysis of oligomeric Aβ species
Samples were mixed with 2×Tris-tricine buffer (Bio-Rad, Hercules, CA, U.S.A.) and resolved in a 16% Tris-tricine gel. After electrophoresis, samples were transferred to PVDF membrane with a 200 mA current at 4°C for 2 h. The membrane was incubated with 5% non-fat milk in TBS-T (Tris-buffered saline, pH 7.4, with 0.5% Tween 20) at room temperature (25°C) for 1 h. The blot was then reacted with antibodies for Aβ, 6E10 (1:1000; Covance, CA, U.S.A.), overnight at 4°C. After washing three times with TBS-T, the membrane was incubated with goat anti-mouse IgG-horseradish peroxidase (1:5000; Sigma–Aldrich) for 1 h at room temperature, and further washed three times with TBS-T. Immunolabelling was detected by chemiluminescence (SuperSignal West Pico, Pierce, Rockford, IL, U.S.A.).
Methods for assessing neuronal viability
Assessment of mitochondrial function
Mitochondrial dysfunction is an initial step in apoptotic pathways that lead to neuronal cell death. In the present study, mitochondrial function was assessed by the ability of reductase in neurons to reduce MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma–Aldrich]. In brief, primary cortical neuron cultures in 24-well plates were treated with Aβ and/or inhibitors at 37°C for different times. After treatment, the culture medium was replaced by 500 μl of neurobasal medium with 0.5 mg/ml MTT, and further incubated at 37°C for 3 h. After removing the MTT solution, the remaining formazan crystals were dissolved in 500 μl of DMSO. After shaking the plates at room temperature for 5 min, absorbance was read at 540 nm using a Syn4 Plate Reader (BioTek Instruments, Inc, Fisher Scientific, St. Louis, MO, U.S.A.).
Assessment of neuronal membrane integrity
The release of the soluble enzyme, LDH (lactate dehydrogenase), from cells into the culture medium is an indication of loss of membrane integrity. In this assay, neurons were cultured in 24-well plates and treated with 1 μM Aβ at 37°C for 24 h. After treatment, an aliquot of the culture medium was collected and LDH activity was measured using the LDH kit (Roche, Indianapolis, IN, U.S.A.).
AlamarBlue assay
AlamarBlue™ (Invitrogen) is a cell permeable non-fluorescent dye, which can be converted into to red fluorescence via reductive reactions within live cells. This assay is used to determine the extent of cell viability in cultures after various treatments. Briefly, neurons were cultured in 24-well plates for 9 days and then treated with or without 1 μM Aβ at 37°C for 24 h. After treatments, 100 μl of AlamarBlue™ was added to each well and neurons were further incubated at 37°C for 3–4 h. Absorbance was read at 570 nm with measurement at 600 nm as a reference.
Determination of NMDA-induced Ca2+ influx
NMDA-induced Ca2+ influx in neurons was determined using the calcium fluorescent dye, Fluo-4 AM, and performed according to the instructions described previously (Ding et al., 2009). Briefly, neurons were cultured on 12 mm coverslips for 9–10 days. Before stimulation with NMDA, neurons were treated with or without 1 μM Aβ and/or 10 μM EGCG or 1 μM gp91ds-tat for 30 min or 16 h. After pre-treatment, cells were incubated at room temperature (in the dark) with 1 μM Fluo-4 AM for 30 min in ACSF (artificial cerebrospinal fluid) medium (125 mM NaCl, 10 mM Hepes, 5 mM KCl, 3.1 mM CaCl2, 1.3 mM MgCl2 and 10 mM glucose, pH 7.4). Cells were then transferred to a flow through a chamber fitted within the microscope. Time-lapse Ca2+ imaging was performed using an upright wide-field epi-fluorescence microscope (FN1 system, Nikon) with a ×40/0.8 water immersion objective. Excitation was generated with an X-Ford metal halide lamp filtered with a fluo-4 filter cube. Emission was detected by a CoolSNAP-EZ CCD-camera (Photometrics, AZ, U.S.A.). After perfusion to obtain the base-line results, 50 μM NMDA was applied in ACSF medium without MgCl2 but with 1 μM nimopridine, a selective antagonist of the L-voltage-sensitive calcium channel (Ho et al., 2001; Ueda et al., 1997). The perfusion system was equipped with a pinch valve that controls the duration of the application. After exposing neurons to NMDA for 30 s, cells were perfused again with the ACSF medium with MgCl2 (1.3 mM). During perfusion, images of neurons were acquired using the Metamorph software (Molecular Devices, Sunnyvale, CA, U.S.A.) at 2 s intervals for 2 min (a total of 61 frames were obtained). Each treatment condition was performed in triplicate and three random fields were recorded. Results were exported using the Metamorph software to generate background-subtracted images. The magnitude of the Ca2+ signal was defined as the average of ΔF/F0 from each cell and the area under the curve for NMDA stimulation and ACSF washout was determined using the GraphPad Prism Software (GraphPad Prism Software Inc., San Diego, CA, U.S.A.). At least 100 cells were selected to obtain the average area for each group.
Measurement of ROS production
ROS production in primary cortical neurons was assayed using DHE. Oxidation of DHE by superoxide anions produces fluorescent ethidium, which is intercalated into DNA (Chapman et al., 2005). Therefore ROS production can be quantified by measuring the fluorescence intensity of ethidium in cells. In brief, neurons were cultured (to 70% confluence) on 35 mm dishes coated with 50 mg/l poly-l-lysine. Neurons were then treated with Aβ, EGCG, gp91ds-tat or MnTBAP for 30 min or 16 h in neurobasal medium without Phenol Red but with 0.5 mg/ml BSA. At 30 min before image acquisition, cells were loaded with 10 μM DHE and incubated at 37°C. Fluorescence images were acquired using a Nikon TE-2000 U inverted microscope with a ×20 NA 0.95 objective and a cooled CCD camera controlled with a computer running the MetaView imaging software (Universal Imaging, West Chester, PA, U.S.A.). The fluorescence excitation source was controlled with a Uni-Blitz mechanical shutter. For image acquisition, a short exposure time (200 ms) and low-intensity excitation light were applied to minimize photo-bleaching. Digital images were analysed using the MetaView software with automatic background subtraction. Threshold fluorescence was obtained for each image before the quantification. For each field, the total fluorescence was measured and expressed as average fluorescence versus total number of cells (recorded as bright field image). For each treatment group, three random images from the same field were taken and analysed, and each treatment was repeated three times independently for statistical analysis.
Measurement of AA release
Measurement of AA release was performed essentially as previously described (Xu et al., 2002; Shelat et al., 2008) with minor modifications. Briefly, primary cortical neurons in a 35-mm dish were treated with or without 1 μM Aβ for different times (0, 4, 12 and 24 h) at 37°C and 0.1 μCi of [1-14C]AA was added for the final 4 h. This time is sufficient for more than 75% of the [1-14C]AA to be incorporated into membrane phospholipids (Xu et al., 2002). After incubation, excess free [1-14C]AA in the cells and medium was removed by three successive washes with buffer A containing 145 mM NaCl, 5.5 mM KCl, 1.1 mM MgC12, 1.1 mM CaC12, 5.5 mM glucose, 20 mM Hepes, pH 7.4 and BSA (0.5 mg/ml). The phospholipid-labelled neurons were stimulated with 100 μM NMDA at 37°C for 30 min, and the release of [1-14C]AA into the culture medium was determined with a Beckman LS 5800 liquid scintillation counter (Fullerton, CA, U.S.A.). Radioactivity remaining in the cells was also determined by adding 2 ml of methanol to the dish and harvesting the cells using a scraper. Net radioactivity released into the culture medium in response to NMDA was obtained by subtracting the background radioactivity released from cells incubated in the absence of NMDA. Labelled AA released from neurons treated with Aβ for different time periods and subsequently subjected to a 30-min stimulation with NMDA were expressed as percentages of the total radioactivity in labelled cells, i.e. radioactivity released into the medium over 30 min plus radioactivity remaining in the cells.
Statistical analysis
The results were analysed either by one-way or two-way ANOVA followed by Bonferroni's multiple comparison tests (V4.00, GraphPad Prism Software, Inc, San Diego, CA, U.S.A.). Values of P<0.05 were considered significant.
RESULTS
Prolonged exposure of neurons to oligomeric Aβ induces mitochondrial dysfunction but not neuronal cell death
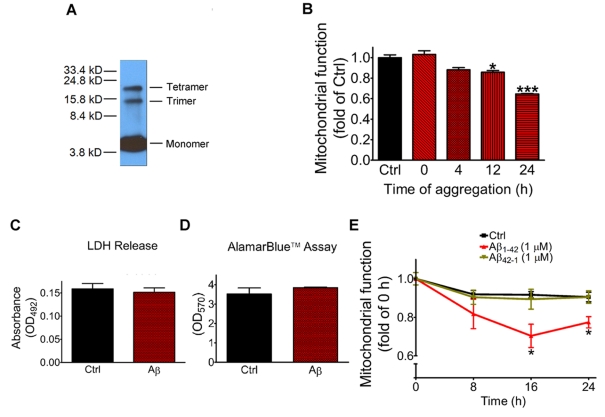
In the present study, we prepared oligomeric Aβ according to the instructions described by LaDu's group (Dahlgren et al., 2002). Western-blot analysis of the preparation showed the presence of monomers, trimers and tetramers, but no obvious dimers or higher-molecular mass oligomers (Figure 1A). To test whether the time of Aβ aggregation affects neuronal toxicity, Aβ was aggregated at 4°C for 0, 4, 12 or 24 h before exposure to rat primary cortical neurons and further incubation at 37°C for 24 h. Results in Figure 1(B) show a time-dependent decrease in mitochondrial function (i.e. MTT reduction) with increase in time of Aβ aggregation. Significant decrease in MTT was observed after exposing neurons to Aβ (1 μM) that was aggregated for 12 (P<0.05) and 24 h (P<0.001).
Figure 1. Neuronal toxicity of oligomeric Aβ.
Oligomeric Aβ was prepared as indicated in the Materials and methods section. (A) The sample from a typical preparation was analysed by Western blot showing the presence of trimers and tetramers as well as monomers after aggregation in PBS at 4°C for 24 h. Positions of molecular mass markers are shown on the left in kDa. (B) Aβ was aggregated for different time periods (0, 4, 12 and 24 h) at 4°C and then 1 μM was incubated with rat primary cortical neurons for 24 h. Results depict mitochondrial function using the MTT assay. (C) Neurons were treated with or without Aβ (1 μM) for 24 h followed by assay of LDH release as an indication of a loss in membrane integrity. (D) Neurons were treated with or without oligomeric Aβ (1 μM) for 24 h and neuronal viability was determined using the AlarmaBlue™ assay. (E) Rat primary cortical neurons were incubated with or without oligomeric Aβ1-42 (1 μM) or Aβ42-1 (1 μM) for 0, 8, 16 or 24 h, and mitochondrial function was measured using the MTT assay. Results are mean±S.E.M. values for three independent experiments and are analysed by one-way ANOVA followed by Bonferroni's multiple comparison tests.*P<0.05 and ***P<0.001, as compared with control.
Following the study with MTT reduction to access mitochondrial function, we also determined whether exposure of neurons to oligomeric Aβ (1 μM) is associated with an increase in cell death. LDH release was used to determine loss of membrane integrity, and AlamarBlue™ assay was used to determine neuronal viability. As shown in Figures 1(C) and 1(D), incubation of neurons with oligomeric Aβ (1 μM) for 24 h neither caused an increase in LDH release nor changes in live cell numbers. These results indicate that despite mitochondrial dysfunction, Aβ-treated neurons remain viable.
The specificity for Aβ1-42 for mediating mitochondrial dysfunction was tested by comparing with the reverse peptide, Aβ 42-1, which was prepared using the same instruction as Aβ1-42. As shown in Figure 1(E), neurons exposed to Aβ42-1 (1 μM) did not result in mitochondrial dysfunction and MTT levels were comparable with controls without Aβ.
Prolonged exposure of neurons to oligomeric Aβ inhibits NMDA-induced Ca2+ influx
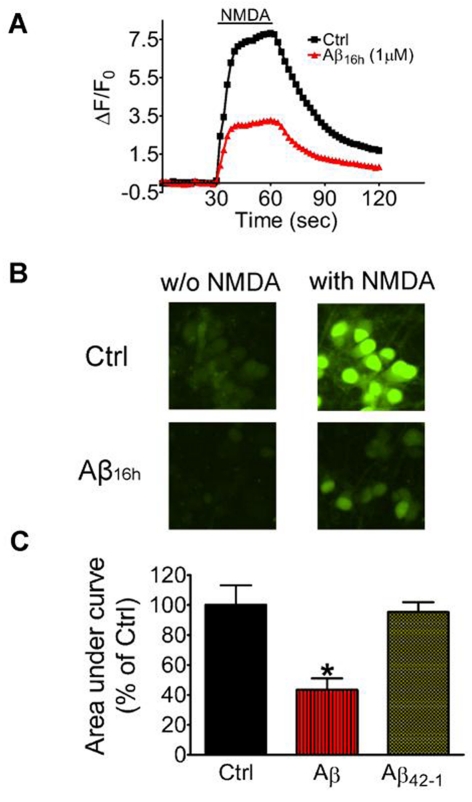
Since prolonged exposure of neurons to oligomeric Aβ resulted in mitochondrial dysfunction but not cell death, we next examined whether this form of Aβ can alter other neuronal functions, such as NMDA receptor-mediated Ca2+ influx. In the present study, primary cortical neurons were loaded with Fluo-4 AM and perfused with NMDA (50 μM) for 30 s. As shown in Figure 2(A), NMDA stimulation elicited a rapid and transient increase in Ca2+ influx. However, when neurons were exposed to oligomeric Aβ (1 μM) for 16 h before stimulation with NMDA, a significant decrease (57%, P<0.05) in Ca2+ influx was observed (Figure 2A). Representative images of neurons taken at the time of NMDA stimulation also showed a decrease in fluorescence between neurons pre-treated with Aβ for 16 h as compared to controls (Figure 2B). As a negative control, neurons treated with Aβ42-1 (1 μM) for 16 h exhibited no significant changes in NMDA-induced Ca2+ influx, as compared with untreated controls (Figure 2C). These results indicate that impairment of NMDA receptor-mediated Ca2+ influx is associated with prolonged exposure of neurons to oligomeric Aβ.
Figure 2. Prolonged exposure of neurons to Aβ impairs NMDA receptor-mediated Ca2+ influx.
Primary cortical neurons were cultured on coverslips and exposed to oligomeric Aβ1-42 (1 μM) or Aβ42-1 (1 μM) for 16 h. Neurons were labelled with Fluo-4-AM for 30 min before stimulation with NMDA (50 μM) for 30 s in ACSF medium containing nimopridine (1 μM) without Mg2+, and then followed by perfusion with ACSF medium with Mg2+. Average fluorescence of Fluo-4 was measured as described in the Materials and methods section. (A) Representative curves depicting NMDA-induced Ca2+ influx with or without oligomeric Aβ for 16 h. (B) Representative fluorescent images of neurons taken before and during (30 s) NMDA stimulation. (C) Bar graphs to depict NMDA-induced Ca2+ influx as determined by calculating the area under the curve using the GraphPad Prism Software 4. Results are means±S.E.M. for three independent experiments and are analysed by one-way ANOVA followed by Bonferroni's multiple comparison tests.* P<0.05, as compared with control.
Prolonged exposure of neurons to oligomeric Aβ decreases NMDA-induced AA release
We previously reported that Aβ causes AA release from neurons through a signalling pathway involving NMDA receptor, NADPH oxidase, ERK1/2 (extracellular-signal-regulated kinase 1/2) and cPLA2 (Shelat et al., 2008). In the present study, we examined the effect of prolonged exposure of neurons to oligomeric Aβ on NMDA-induced AA release. Cortical neurons were pre-treated with or without Aβ (1 μM) for different time periods (0–24 h) and labelled with [1-14C]AA for the final 4 h before stimulation with NMDA (100 μM) for 30 min. As shown in Figure 3, a significant (P<0.05) decrease in the NMDA-induced AA release from prelabelled-phospholipids was observed on exposure of neurons to oligomeric Aβ for 12 or 24 h. As a negative control, no obvious decrease in AA release was observed on incubation of neurons with the reverse peptide (Aβ42-1) (results not shown). These results are consistent with results in Figure 2 showing that prolonged Aβ exposure to neurons inhibited NMDA-stimulated Ca2+ influx.
Figure 3. Prolonged exposure of neurons to Aβ inhibits NMDA receptor-mediated AA release.
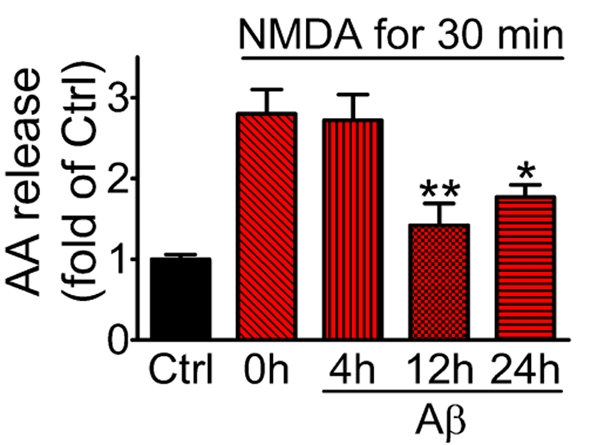
Neurons were treated with Aβ (1 μM) for 0, 4, 12 and 24 h, and subsequently labelled with [1-14C]AA for the final 4 h. After removing free [1-14C]AA, neurons were stimulated with NMDA (100 μM) for 30 min and [1-14C]AA release from phospholipids into the medium was measured after subtracting background release. Results are means±S.E.M. for three independent experiments and are analysed by one-way ANOVA followed by Bonferroni's multiple comparison tests; *P<0.05 and **P<0.01, as compared with control.
Prolonged exposure of neurons to Aβ increases ROS production
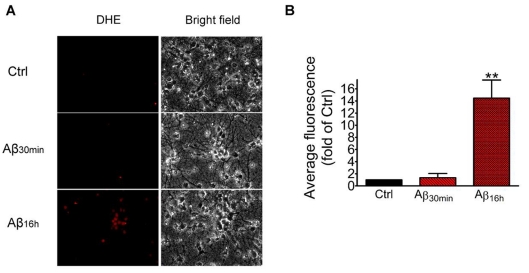
In our earlier study, although NMDA (100 μM) could cause a robust increase in ROS production in neurons, the amount of ROS produced by Aβ (1-5 μM) was very low (Shelat et al., 2008). In this next set of experiments, we determined levels of ROS in neurons after prolonged exposure to Aβ. As shown in Figures 4(A) and 4(B), a significant increase (P<0.01) in ROS production was found in neurons on exposure to oligomeric Aβ (1 μM) for 16 h. Examination of neurons under the bright field microscope indicated that neuronal morphology was preserved after prolonged exposure to Aβ (Figure 4A).
Figure 4. Prolonged exposure to Aβ induces ROS accumulation in neurons.
(A) Neurons were pre-treated with Aβ (1 μM) for 30 min or 16 h, and then loaded with DHE. In each field, DHE fluorescent neurons and the corresponding bright field images were recorded. (B) Average fluorescent intensity of neurons was calculated. Results are means±S.E.M. for three independent experiments and analysed by one-way ANOVA followed by Bonferroni's multiple comparison tests. **P<0.01 as compared with control.
Gp91ds-tat protects neurons from prolonged Aβ-induced ROS production, mitochondrial dysfunction and inhibition of NMDA-mediated Ca2+ influx
Since prolonged exposure of neurons to oligomeric Aβ is associated with ROS accumulation, we subsequently evaluated the source of ROS using the cell permeable NADPH oxidase peptide inhibitor, gp91ds-tat. Results in Figure 5(A) show that ROS accumulation due to prolonged exposure of Aβ (1 μM) was completely inhibited by pre-treatment of neurons with gp91ds-tat (1 μM). These results suggest that NADPH oxidase is the primary source of ROS in neurons induced by Aβ.
Figure 5. NADPH oxidase inhibitor, gp91ds-tat, inhibits ROS production and protects neurons against Aβ-induced cytotoxicity.
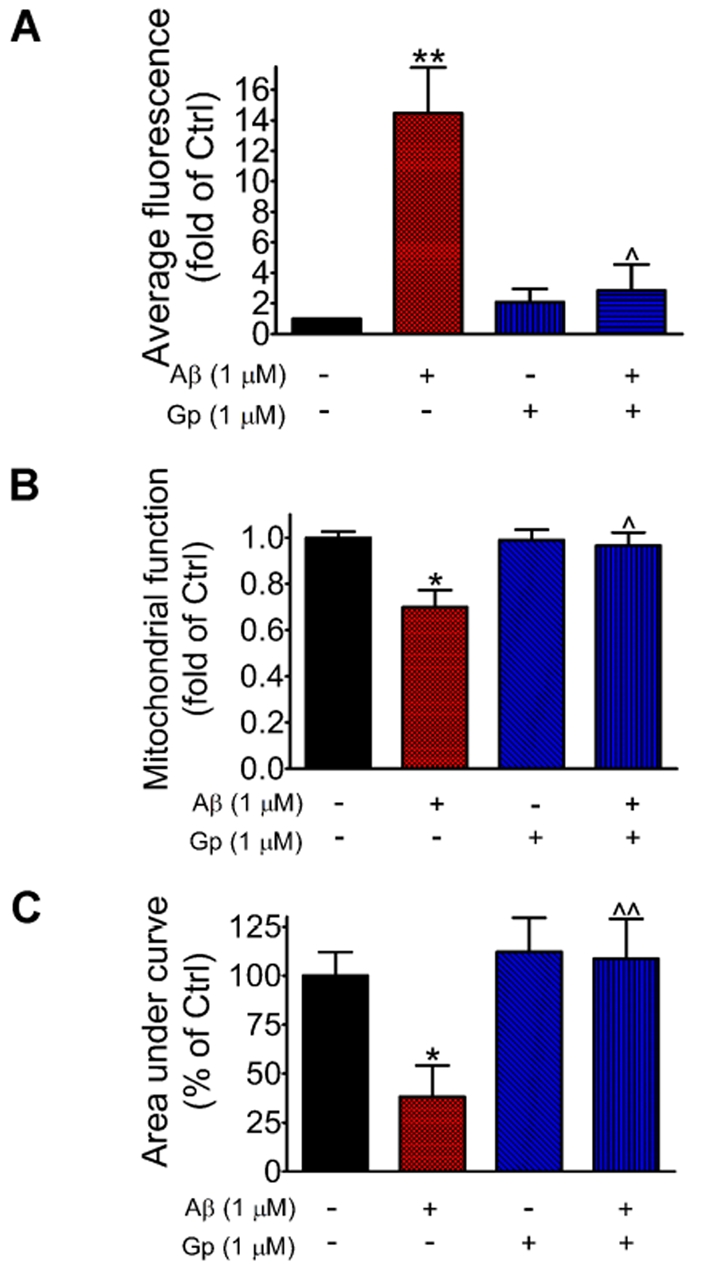
(A) Rat primary cortical neurons were treated with gp91ds-tat (1 μM) for 30 min before exposure to Aβ (1 μM) for 16 h and followed by ROS determination as described for Figure 4. Results represent the average fluorescent intensity of DHE in neurons and are means±S.E.M. for three independent experiments. Two-way ANOVA revealed a significant interaction (P = 0.0072) and significant effects of Aβ (P = 0.0039) and gp91ds-tat (P = 0.0180). Bonferroni post-tests showed a significant difference between control and Aβ (**P<0.01) and between Aβ treatment without versus with gp91ds-tat (∧P<0.05). (B) Neurons were treated with gp91ds-tat (1 μM) for 30 min before exposure to Aβ (1 μM) for 16 h followed by the MTT assay. Results are means±S.E.M. for three independent experiments. Two-way ANOVA revealed a significant interaction (P = 0.0320) and significant effects of Aβ (P = 0.0160) and gp91ds-tat (P = 0.0433). Bonferroni post-tests showed a significant difference between control and Aβ (*P<0.05) and between Aβ treatment without versus with gp91ds-tat (∧P<0.05). (C) Neurons were incubated with gp91ds-tat (1 μM) for 30 min before exposure to Aβ (1 μM) for 16 h, and NMDA (50 μM)-induced Ca2+ influx was determined as described in Figure 2 Results are means±S.E.M. for three independent experiments. Two-way ANOVA revealed a significant interaction (P = 0.0167) and significant effects of Aβ (P = 0.0095) and gp91ds-tat (P = 0.0027). Bonferroni post-tests showed a significant difference between control and Aβ (*P<0.05) and between Aβ treatment without versus with gp91ds-tat (ˆˆP<0.01).
We further tested whether gp91ds-tat can protect neurons from the toxic effects of prolonged exposure to oligomeric Aβ. As shown in Figure 5(B), pre-treatment of neurons with gp91ds-tat completely prevented Aβ-induced mitochondrial dysfunction. Pretreatment with gp91ds-tat also protected neurons from Aβ-induced inhibition of NMDA receptor-mediated Ca2+ influx (Figure 5C).
EGCG from green tea inhibits the neurotoxic effects of Aβ
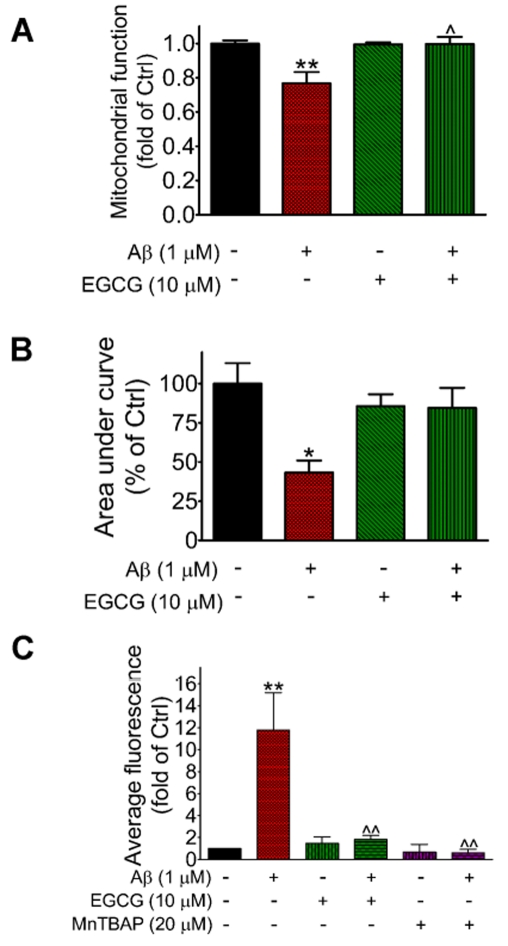
Since inhibitors of NADPH oxidase-mediated ROS production can protect neurons from the toxic effects of Aβ, it is reasonable that other natural antioxidants may also inhibit Aβ-induce neurotoxicity. Therefore our experiment was extended to testing botanical polyphenols known to possess anti-oxidative properties. Results show that exposing neurons to EGCG (10 μM), an antioxidant from green tea, completely prevented mitochondrial dysfunction induced by 1 μM Aβ (Figure 6A). Incubation of neurons for 16 h with EGCG alone (up to 100 μM) had no effect on mitochondrial function (results not shown). Albeit not as effective as EGCG, resveratrol (25–50 μM), another polyphenol enriched in grapes, could also inhibit Aβ-induced mitochondrial dysfunction in neurons (results not shown).
Figure 6. EGCG protects neurons against Aβ-induced cytotoxicity.
(A) Rat primary cortical neurons were treated with EGCG (10 μM) for 30 min before exposure to Aβ (1 μM) for 16 h, and followed by determination of mitochondrial function using the MTT assay. Results are means±S.E.M. for three independent experiments. Two-way ANOVA revealed a significant interaction (P = 0.0139), and significant effects of Aβ (P = 0.0149) and EGCG (P = 0.0172). Bonferroni post-tests showed a significant difference between control and Aβ (**P<0.01) and between Aβ treatment without versus with EGCG (∧P<0.05). (B) Neurons were incubated with EGCG (10 μM) for 30 min before exposure to Aβ (1 μM) for 16 h, followed by determination of NMDA-induced Ca2+ influx as described in Figure 2. Analysis by two-way ANOVA revealed a significant interaction between EGCG and Aβ (P = 0.032), and a significant effect of Aβ (P = 0.027). Bonferroni post tests showed a significant difference between control and Aβ (*P<0.05). (C) Neurons were treated with EGCG (10 μM) or MnTBAP (20 μM) for 30 min before exposure to Aβ (1 μM) for 16 h, followed by determination of ROS as described in Figure 4. Results are means±S.E.M. for three independent experiments. Two-way ANOVA revealed a significant interaction between Aβ and EGCG (P = 0.0109), and significant effects of Aβ (P = 0.0075) and EGCG (P = 0.0184). A significant interaction between Aβ and MnTBAP (P = 0.0183) was also found. Bonferroni post-tests showed a significant difference between control and Aβ (**P<0.01) and between Aβ treatment without versus with EGCG or MnTBAP (ˆˆP<0.01).
Since prolonged exposure of neurons to Aβ resulted in inhibition of NMDA-induced Ca2+ influx (Figure 2), we further tested whether pre-treatment of neurons with EGCG could prevent this deleterious effect. As shown in Figure 6(B), pre-treatment of neurons with 10 μM EGCG completely blocked the inhibition of NMDA-induced Ca2+ influx caused by prolonged exposure to 1 μM Aβ for 16 h. EGCG alone exerted no significant effect on NMDA-induced Ca2+ influx, as compared to untreated control (Figure 6B).
Results in Figure 4 demonstrated that prolonged Aβ exposure caused ROS accumulation in neurons. It was therefore important to determine whether EGCG may play a role in inhibiting Aβ-mediated ROS production. Figure 6(C) showed that pre-treatment with EGCG (10 μM) before exposing neurons to Aβ (1 μM) for 16 h completely prevented Aβ-induced ROS production. As a positive control, MnTBAP (20 μM), a compound widely used as an SOD (superoxide dismutase) mimetic (Carvour et al., 2008) and ROS scavenger (Dietrich et al., 2010), also inhibited ROS production induced by Aβ exposure for 16 h (Figure 6C). On the other hand, neither EGCG nor MnTBAP alone could alter basal ROS levels in neurons (Figure 6C).
DISCUSSION
Despite the fact that AD was discovered over 100 years ago, the pathogenesis of the disease is still not well understood and effective treatment for the disease remains elusive. Although many studies in the past regarded amyloid accumulation to be a pathological landmark of the disease, the ‘amyloid hypothesis’ has been subjected to challenges (Hardy, 2009). A major problem regarding this hypothesis is the inability to link amyloid accumulation to the severity of the disease, and difficulty in demonstrating toxic forms of Aβ in AD brain. Nevertheless, recent studies have provided convincing evidence that Aβ aggregates, especially when present in the oligomeric forms, can alter neuronal circuitry and impair synaptic activity, events that underlie the cognitive decline in AD (Palop and Mucke, 2010). Work by Shankar's group further demonstrated the release of soluble Aβ oligomers (mainly dimers and trimers) from hippocampal slices of AD transgenic mice, and that low concentrations of the Aβ oligomers in the conditioned media can cause neurotoxic effects and inhibit NMDA-induced Ca2+ influx into synaptic spines (Shankar et al., 2007, 2008). With regard to the presence of Aβ oligomers in the AD brain, a recent study provided strong support for the progressive accumulation of these toxic forms of Aβ with increasing degree of synaptic loss and severity of cognitive impairment (Pham et al., 2010). It is obvious that different types of tissue or cell preparations can produce different patterns of Aβ oligomers, leading to different magnitude of toxicity. In fact, different ratios of Aβ1-42 to Aβ1-40 can offer different patterns of aggregation kinetics and elicit different degrees of neurotoxicity (Kuperstein et al., 2010). In the present study, aggregation of synthetic Aβ using the instruction described by LaDu's group was shown to produce trimers and tetramers, with no obvious presence of dimers or higher-molecular-mass oligomers (Dahlgren et al., 2002; Stine et al., 2003). As discussed in a recent review by Stine et al. (2010), Aβ oligomer aggregation pattern may vary depending on acidity and the ionic strength of the solvent used (Stine et al., 2010). In our hands, this instruction for Aβ aggregation has consistently produced oligomers that impair mitochondrial function in neurons, as demonstrated using the MTT assay. However, despite the decrease in mitochondrial function, exposure to 1 μM oligomeric Aβ for 24 h did not result in neuronal cell death or change in membrane integrity (Figure 1). In line with the progressive nature of AD, it is not surprising that Aβ oligomers produce subtle neurotoxic effects that progress to full impairment of synaptic plasticity and cognitive function before cell death (Ronicke et al., 2010).
Several earlier studies have demonstrated the ability of oligomeric Aβ to exert cytotoxic effects on neurons. Aβ was shown to suppress NMDA-induced currents in cortical neurons, and this effect was attributed in part to alteration of signalling pathways leading to endocytosis of NMDA receptors (Snyder et al., 2005). Other studies showed an interaction between oligomeric Aβ and the NMDA receptor trafficking pathway in neurons, and in turn resulting in alterations of intracellular Ca2+ homoeostasis (Walsh and Selkoe, 2007). Aβ oligomers can cause Ca2+ influx due to stimulation of NMDA and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) receptors, and this type of glutamate receptor-induced excitotoxicity can stimulate downstream pathways leading to mitochondrial membrane dysfunction and neuronal cell death (Alberdi et al., 2010). Indeed, dysregulation of Ca2+ homoeostasis is an important consequence of amyloid toxicity (Demuro et al., 2010; Paul and Connor 2010).
Studies in vivo and in vitro have demonstrated that neuronal excitotoxic events, such as NMDA receptor activation, are linked to ROS production through activation of NADPH oxidase (Kishida et al., 2005; Shelat et al., 2008; Brennan et al., 2009). In a study with hippocampal slices, ROS from NADPH oxidase was shown to activate signalling pathways leading to the activation of ERK1/2 (Serrano et al., 2009). Since ERK1/2 is an important kinase for phosphorylation of cPLA2, it is reasonable that oligomeric Aβ can induce activation of cPLA2 and the subsequent release of AA (Shelat et al., 2008). cPLA2 can perturb membrane phospholipids, and besides being a precursor for the synthesis of eicosanoids, AA is an important lipid mediator for regulation of multiple signalling pathways (Bazan, 2003). In the present study, prolonged Aβ exposure to neurons results in the decrease in NMDA receptor activity and AA release (Figure 3). These results lend further support to the ability of oligomeric Aβ to impair a variety of neuronal functions (Shi et al., 2010). The ability of oligomeric Aβ to inhibit NMDA receptor function may also be due to a number of other factors. Besides modulation of receptor endocytosis (Snyder et al., 2005), there is evidence that Aβ may bind directly to the NMDA receptor subunits (De Felice et al., 2007; Ronicke et al., 2010). Although the mechanism whereby prolonged Aβ exposure impairs NMDA receptor response is not yet clearly understood, our results with an gp91ds-tat clearly demonstrate the important role of NADPH oxidase and ROS in mediating the damaging effects elicited by Aβ.
AD pathology is associated with an increase in mitochondrial abnormalities and a decrease in ATP production (Hirai et al., 2001; Moreira et al., 2010). In fact, oxidatively modified proteins are found in mitochondria (Sultana and Butterfield, 2009), and increased levels of oxidative stress marker proteins and lipids, such as protein carbonyls, 3-nitrotyrosine, hydroxynonenal and isoprostanes, are found in MCI (mild cognitive impairment) brains (Keller et al., 2005; Mattson, 2009). Whether subunits of NMDA receptors are particularly susceptible to these oxidative effects remains to be investigated. Furthermore, despite evidence for soluble Aβ to cross the cell membrane and to directly interact with mitochondrial enzymes, the mechanism underlying mitochondrial dysfunction is not yet fully understood (Yan and Stern, 2005; Krafft and Klein, 2010). Studies with neurons and astrocytes have demonstrated activation of cPLA2 by oligomeric Aβ and, subsequently, this leads to alteration of mitochondrial membrane (Kriem et al., 2005; Zhu et al., 2006). ROS are small molecules with important pleiotropic functions. Besides regulating MAPK activity, ROS can perturb membrane lipids and alter membrane proteins. Therefore the increase in ROS production due to prolonged exposure of neurons to Aβ can be an important underlying mechanism for explaining the oxidative-induced impairment in synaptic function in AD (Ronicke et al., 2010).
Another important finding from this study is the ability of botanical antioxidants to protect neurons from the cytotoxic effects of oligomeric Aβ. Our results show that EGCG from green tea is particularly effective in inhibiting ROS, and protects neurons from Aβ-induced inhibition of NMDA-stimulated Ca2+ influx and mitochondrial dysfunction. Indeed, a number of studies have demonstrated EGCG to elicit neuroprotective effects (Choi et al., 2001; Kim et al., 2005; Rezai-Zadeh et al., 2005; Mandel et al., 2006; Kalfon et al., 2007; Li et al., 2009), and to protect neurons against Aβ-induced toxicity (Bastianetto et al., 2006). EGCG is readily soluble and can cross the blood–brain barrier (Mandel et al., 2006). In animal studies, long-term oral administration of 0.05% or 0.1% EGCG in drinking water (for 6 months) to SAMP8 (senescence-accelerated mice prone-8) mice could decrease levels of Aβ in the hippocampus and improve learning and memory (Li et al., 2009). In cultured hippocampal neurons, EGCG increased neuronal survival after a 48-h exposure to Aβ and this effect was associated with a decrease in the level of MDA (malondialdehyde), a marker for lipid peroxidation (Choi et al., 2001). In our study, EGCG up to 100 μM exerted no toxic effects in neurons, and 10 μM of EGCG was already sufficient to protect neurons from Aβ-induced neurotoxicity (Figure 5). Besides inhibition of Aβ-induced toxicity, there is evidence suggesting multiple effects of EGCG, including inhibition of BACE1 (β-secretase) (Jeon et al., 2003) and aggregation or remodelling of Aβ oligomers (Ono et al., 2003; Bastianetto et al., 2006). An in vitro study with 7PA2 cells demonstrated that EGCG can convert Aβ oligomers into non-toxic spherical assemblies (Bieschke et al., 2010). In transgenic mice overexpressing human APPsw, EGCG increased α-secretase activity and enhanced the production of non-amyloidogenic APP-α (amyloid precursor protein-α) as opposed to the cytotoxic Aβ (Rezai-Zadeh et al., 2005). Other studies with endothelial cells and astrocytes have demonstrated that EGCG inhibits NADPH oxidase activity (Steffen et al., 2008; Jensen et al., 2009).
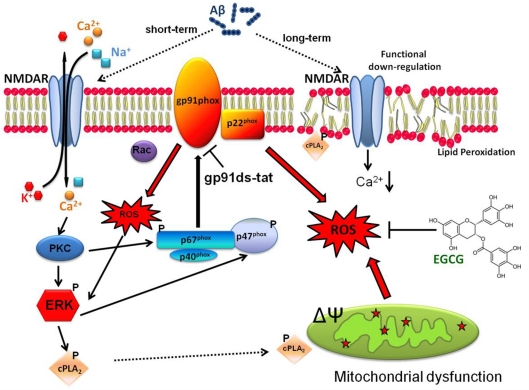
In summary, results from this study demonstrated impairment of neuronal function on prolonged exposure to oligomeric Aβ. As shown in Figure 7, short-term exposure of Aβ to neurons can cause excitatory events including activation of NMDA receptor, increase in Ca2+ influx, ROS production and stimulation of signalling pathways leading to the activation of cPLA2 and AA release (Shelat et al., 2008). In the present study, we show that more ROS is produced on prolonged exposure to Aβ and this event is accompanied by impairment of NMDA-mediated Ca2+ influx and AA release, and mitochondrial dysfunction (Figure 7). Since ROS production on prolonged exposure of neurons to Aβ is inhibited by gp91ds-tat, this study further demonstrates the important role of NADPH oxidase in producing the ROS that mediates the cytotoxic effects of Aβ. Thus besides providing an understanding of the molecular and cellular pathways whereby oligomeric Aβ conveys toxic effects and impairs neuronal function, this study further opens opportunities to target the NADPH oxidase pathway to ameliorate the Aβ effects. In the present study, we demonstrate the ability of EGCG to prevent Aβ-induced ROS accumulation and NMDA receptor dysfunction. These results suggest the possibility for use of other botanical antioxidants as well as dietary polyphenols for delaying the onset or retarding the progression of AD.
Figure 7. A scheme depicting possible mechanisms for neuronal impairment due to prolonged treatment with Aβ and possible sites for the protective effects of NADPH oxidase inhibitor and EGCG.
ACKNOWLEDGEMENTS
Editorial assistance by D. Reith in the preparation of this manuscript is appreciated.
Footnotes
REFERENCES
- Alberdi E, Sanchez-Gomez MV, Cavaliere F, Perez-Samartin A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against β-amyloid-induced toxicity. Eur J Neurosci. 2006;23:55–64. doi: 10.1111/j.1460-9568.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCdelta proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann NY Acad Sci. 2008;1139:197–205. doi: 10.1196/annals.1432.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine Jr WB, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Aβ oligomers induce neuronal oxidative stress through an N-methyl-d-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH, Xiang C, Han BH, Zipfel GJ, Holtzman DM. Soluble amyloid-β, effect on cerebral arteriolar regulation and vascular cells. Mol Neurodegener. 2010;5:15. doi: 10.1186/1750-1326-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia. 2009;57:767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Ortiz D, Shea TB. Amyloid-β promotes calcium influx and neurodegeneration via stimulation of L voltage-sensitive calcium channels rather than NMDA channels in cultured neurons. J Alzheimers Dis. 2001;3:479–483. doi: 10.3233/jad-2001-3507. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochem Int. 2009;55:362–368. doi: 10.1016/j.neuint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SY, Bae K, Seong YH, Song KS. Green tea catechins as a BACE1 (β-secretase) inhibitor. Bioorg Med Chem Lett. 2003;13:3905–3908. doi: 10.1016/j.bmcl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Kalfon L, Youdim MB, Mandel SA. Green tea polyphenol (−) -epigallocatechin-3-gallate promotes the rapid protein kinase C- and proteasome-mediated degradation of Bad: implications for neuroprotection. J Neurochem. 2007;100:992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, Lee SE. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J Agric Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft GA, Klein WL. ADDLs and the signaling web that leads to Alzheimer's disease. Neuropharmacology. 2010;59:230–242. doi: 10.1016/j.neuropharm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Kriem B, Sponne I, Fifre A, Malaplate-Armand C, Lozac'h-Pillot K, Koziel V, Yen-Potin FT, Bihain B, Oster T, Olivier JL, Pillot T. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-β peptide. FASEB J. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D'Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer's disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Li Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Aβ1–42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009;163:741–749. doi: 10.1016/j.neuroscience.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MB. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res. 2006;50:229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010;114:1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-β oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging, in the press. 2010 doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Serrano F, Chang A, Hernandez C, Pautler RG, Sweatt JD, Klann E. NADPH oxidase mediates β-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol Brain. 2009;2:31. doi: 10.1186/1756-6606-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid β peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shi C, Wu F, Xu J. H2O2 and PAF mediate Aβ1-42-induced Ca2+ dyshomeostasis that is blocked by EGb761. Neurochem Int. 2010;56:893–905. doi: 10.1016/j.neuint.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stine Jr WB, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Stine WB, Jungbauer L, Yu C, LaDu MJ. Preparing synthetic Aβ in different aggregation states. Methods Mol Biol. 2010;670:13–32. doi: 10.1007/978-1-60761-744-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer's disease and mild cognitive impairment. . J Bioenerg Biomembr. 2009;41:441–446. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid β protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J Neurochem. 1997;68:265–271. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A β oligomers – a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, Sun GY. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. J Neurochem. 2002;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- Yan SD, Stern DM. Mitochondrial dysfunction and Alzheimer's disease: role of amyloid-β peptide alcohol dehydrogenase (ABAD). Int J Exp Pathol. 2005;86:161–171. doi: 10.1111/j.0959-9673.2005.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-β peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]