Abstract
Autism Spectrum Disorders (ASD) have been gaining attention, partly as an example of unusual developmental trajectories related to early neurobiological differences. The present investigation addressed the process of learning new words in order to explore mechanisms of language delay and impairment. The sample included 21 typically developing toddlers matched on expressive vocabulary with 21 young children with ASD. Two tasks were administered to teach children a new word and were supplemented by cognitive and diagnostic measures. In most analyses, there were no group differences in performance. Children with ASD did not consistently make mapping errors, even in word learning situations which required the use of social information. These findings indicate that some children with ASD, in developmentally appropriate tasks, are able to use information from social interactions to guide word-object mappings. This result has important implications for our understanding of how children with ASD learn language.
Keywords: autism spectrum disorder, word learning, language development
Over the past several decades, there has been a significant increase in research focusing on children with Autism Spectrum Disorders (ASD). ASD is an umbrella term for developmental disorders that are qualitatively similar; it includes autism, Pervasive Developmental Disorder – Not Otherwise Specified (PDD-NOS, a milder or atypical variant of autism) and Asperger syndrome. Language delay is a common feature of individuals with ASD (the exception is Asperger syndrome) and is often the first recognized symptom (DeGiacomo & Fombonne, 1998). Indeed, some theorists formerly speculated that autism was primarily a language disorder (Rutter & Bartak, 1971). While previous observations of language development suggested that approximately half of individuals with ASD remained non-verbal into adulthood (Bailey, Phillips, & Rutter, 1996), recent longitudinal studies of children who were impaired early in life indicate that by late childhood, 40 percent of children were verbally fluent and another 45 percent had functional (though not completely intact) language (Lord, Risi, & Pickles, 2004). Another study of nearly 1,000 individuals on the spectrum recently reported that approximately one-half of the sample showed delays in language acquisition (41% had delayed words and 51% had delayed phrases, according to parent report), but only nine percent remained completely nonverbal (Hus, Pickles, Cook, Risi, & Lord, 2007).
More recently, epidemiological investigations and studies of preschool children with ASD demonstrate that children who are currently receiving diagnoses may experience less language impairment than earlier cohorts (Chakrabarti & Fombonne, 2001; Charman, Drew, Baird, & Baird, 2003). There are children with ASD who indisputably have severe impairments in the acquisition and fluency of speech. However, such a marked impairment no longer appears to be a defining feature of ASD. As such, it is important to consider the processes by which some children with ASD master considerable language skills. Researchers have recently begun to consider the usefulness of invoking word learning theories in explaining this observed variability.
One theory of word learning highlights the importance of social cues (such as gaze, posture, facial expression and gesture) in the process of mapping a new word (Baldwin & Moses, 2001; Bloom, 2000; Tomasello, 2001). Several classic word learning studies from this approach were conducted by Baldwin (1991; 1993) and focused on typically developing children. These tasks require that both the examiner and the child each hold a simple toy or object, and that the child attend to the toy in his/her own hand. Baldwin compared “follow-in labeling,” where the investigator followed the child's attention to label the object in the child's possession, to “discrepant labeling,” where the investigator labeled the object that was in her own hand and not the focus of the child's attention. In both conditions, the child heard the novel label four times and then was asked to select the target object. The assumption is that if children are not using social information in mapping a new label, they would simply select the object that is the focus of their own attention, regardless of the examiner's focus during labeling. On the other hand, if they are able to understand the significance of the speaker's focus, they should select their own toy less often when the label is presented in the Discrepant condition than when it is introduced in the Follow-in condition.
These word learning paradigms are of particular interest in the ASD population because behavioral features associated with ASD often appear within infancy and toddlerhood. Deficits in social communication skills such as looking at other people, social affective response and responding to name (Osterling, Dawson, & Munson, 2002; Zwaigenbaum et al., 2005) have all been noted within the first year of life. By age 2, there are even clearer difficulties for children later diagnosed with ASD in skills such as sharing enjoyment and/or interest and attending to the voices of other people (Charman et al., 1997; Wetherby et al., 2004).
The appearance of these impairments early in life has suggested to some theorists that the core impairment of ASD is a difficulty orienting and attending to social stimuli (Mundy & Neal, 2001; Sigman, Dijamco, Gratier, & Rozga, 2004). These deficits have been used to support a social motivation hypothesis (Dawson et al., 2004; Dawson et al., 2005) which proposes that early impairments disturb children's ability to extrapolate information from social cues and hence limit normal social experience. One particularly important aspect of social attention is joint attention, the triadic interaction which involves shared attention between two individuals, directed towards a third party, object or event. Joint attention is one of the earliest indications of children's acknowledgement that another individual may be attending to or thinking about something different than the focus of their own attention. As such, it is believed to form the foundation for a number of later achievements, including word learning (Baldwin, 1995; Tomasello & Farrar, 1986; Woodward & Markman, 1998). Indeed, joint attention is a strong concurrent predictor of language development for typically developing children (Morales et al., 2000; Watt, Wetherby, & Shumway, 2006). In samples of children with ASD, joint attention has been shown to predict language concurrently (Charman, Baron-Cohen et al., 2003; Dawson et al., 2004; Landry & Loveland, 1988) and longitudinally (Mundy, Sigman, & Kasari, 1990; Rosenthal-Rollins & Snow, 1998; Sigman & McGovern, 2005).
Young children with ASD have impaired joint attention skills (Charman et al., 1998; Dawson et al., 2004; Landry & Loveland, 1988; Leekam, Lopez, & Moore, 2000; Mundy & Sigman, 1989). If children with ASD manage to develop these skills, their development is typically delayed by years (Leekam, Hunnisett, & Moore, 1998). Although, in general, children with ASD are impaired in joint attention, some children do become proficient in joint attention during the preschool years, particularly in responding to joint attention (Mundy et al., 1990; Naber et al., 2008; Sigman & Ruskin, 1999). Children with ASD and stronger cognitive skills may experience greater success in negotiating joint attention than their more impaired peers (Leekam et al., 1998).
Some researchers have employed the methods described above – using “discrepant” and “follow-in” conditions – with samples of children with ASD. Baron-Cohen, Baldwin and Crowson (1997) found that profoundly language-impaired children with autism (mean age of 9 years, 2 months with a verbal age equivalents of slightly over 2 years) were generally unsuccessful in using speaker gaze to determine the referent of a novel label. The children could, however, map the words onto objects that were the focus of their own attention. Preissler and Carey (2005) recently replicated this finding, also employing a sample with substantial global impairments. In addition to studying children with marked delays, these two investigations also abided by fairly stringent methods (e.g., repeating the novel label only twice, using novel but minimally interesting materials).
If conclusions were drawn based only on the studies described above, one might infer that children with ASD generally use an immature approach to word learning and rarely become proficient in employing detailed social information to guide their interpretation of language (Baron-Cohen et al., 1997; Carpenter, Pennington, & Rogers, 2002; Golinkoff & Hirsh-Pasek, 2006; McDuffie, Yoder, & Stone, 2006; Preissler & Carey, 2005). Many researchers have assumed that these impairments are down-river disturbances of early social impairments: “the lack of the SDG [Speaker Direction of Gaze, or using the speaker gaze to guide mapping] strategy in young children with autism is ultimately part of a joint-attention deficit” (Baron-Cohen et al., 1997, p. 55). Others have made a stronger case for core mechanistic impairments, suggesting that the process of language learning may be fundamentally different in ASD. The most common proposal is that language learning in ASD is based primarily on imitative or associationist learning (Carpenter, Pennington, & Rogers, 2002; McDuffie, Yoder, & Stone, 2006b; Preissler & Carey, 2005).
However, both of the previous studies reported that some of the children with ASD were able to use social information to map a word (SDG strategy) rather than resorting to a more immature “Listener Direction of Gaze” (LDG) strategy (mapping the word to the object upon which the child himself is focused). Baron-Cohen and colleagues conceded that “We use the phrase ‘lack of the SDG strategy’ while acknowledging that this description of our results goes a little beyond the evidence presented. Strictly speaking, what we have found is the domination of the LDG over the SDG strategy (italics added)” (p. 54). Nevertheless, there was very little information provided about the group of children who used the latter strategy, which was the strategy demonstrated by the typically developing comparison group. Rather, the group of interest in the study by Baron-Cohen and colleagues (as in other investigations) was the children who showed impairments, while the ones who had intact skills were largely overlooked. In the end, general conclusions were made about ASD and the effects of presumed joint attention deficits on word learning, but the samples and conclusions were biased towards very handicapped children with ASD.
The tendency to ignore the children who show some ability in the area of joint engagement is problematic for two important reasons. First, without also exploring these relatively skillful children, it is difficult to conclude whether word learning is inherently disturbed in ASD or if observed impairments are secondary to joint attention deficits. Second, these observations are not clinically representative of the complex picture of ASD that has recently emerged. Some children with ASD are relatively more skilled in interpreting nonverbal social cues and these skills are associated with concurrent and future language ability (Dawson et al., 2004; Sigman & Ruskin, 1999), which – for a substantial proportion of individuals with ASD – is no longer delayed by late childhood (Eisenmajer et al., 1998; Szatmari, Archer, Fisman, & Streiner, 1995). In sum, whereas there is clear evidence that if children with ASD do not have joint attention, they are generally unable to negotiate these word learning situations, there is little to no evidence that, if they do have joint attention, they are able to learn words by using social information. There is no reason to assume that this would necessarily be the case. For instance, one could argue that, even if a child with ASD had some basic joint attention abilities, he might not know to use these skills in a word learning situation. Clarifying this issue is important for understanding language development in ASD.
The present study takes a new look at word learning in ASD in the interest of building upon previous research, which has examined the performance of children with substantial delays in particularly challenging tasks. The current investigation attempts, instead, to explore performance under optimal conditions, thus identifying children with ASD who are able to negotiate a series of word learning situations. A profile of phenotypic characteristics (in terms of cognition, social engagement and early language) of the children who succeed and do not succeed in different situations will allow preliminary theoretical conclusions about the potential integrity of word learning abilities in ASD.
Two specific approaches were taken in this design. First, the sample is a young and less impaired group of children with ASD who are matched to a sample of typically developing children based on expressive vocabulary level. Expressive vocabulary was selected as the matching variable because it is much more reliably measured than receptive vocabulary (Luyster et al., 2008) and highly correlated with it (Fenson et al., 1994). In addition, using early vocabulary size on the CDI poses less of a risk of attaining floor effects and may be a more representative measure of children's meaningful use of words than some standardized measures. Second, a number of contextual supports were considered necessary in order to make these tasks developmentally appropriate for very young children with ASD, who may have concurrent difficulties in a number of cognitive realms. More specifically, these supports included additional repetitions of the novel word, the use of highly interesting objects and increasing the salience of facial direction, as well as eye gaze, as a source of social cues.
Overall, in tasks that do not require using social information (i.e., Novel Labeling Task and Follow-in condition of the Pragmatics Task), no significant differences were expected across diagnostic groups (i.e., ASD and typically developing) in accuracy of performance, when children were matched for expressive vocabulary size. On the basis of previous word learning studies (Baron-Cohen et al., 1997; Preissler & Carey, 2005) diagnostic group differences were expected in a task that required social information (Discrepant condition of the Pragmatics Task). However, given recent findings on the heterogeneity of joint attention skills in ASD (Charman, Baron-Cohen et al., 2003; Naber et al., 2008), more variability in performance was anticipated for this young, diverse population than has been previously reported.
Method
Participants
Participants were recruited through ongoing longitudinal studies focusing on young children at risk for ASD, which were conducted at the University of Michigan Autism and Communication Disorders Center (UMACC). A consecutive recruitment strategy (as opposed to targeted recruitment) ensured that all children – regardless of skill level – were given the entry task (described below), thus permitting a wide range of children to enter into the study. Recruitment was also conducted by: (1) contacting local daycares and posting informational flyers in order to recruit additional children for the typically developing (TD) group; and (2) screening the client database of UMACC's clinic for children who were appropriate for participation. Children for whom English was not the primary language used at home and children with disabilities that precluded standard administration of the assessments (i.e., severe visual impairment or moderate to severe motor impairments) were excluded. For the ASD sample, no distinction was made between autism and PDD-NOS because of the instability of these specific diagnoses for very young children (Lord et al., 2006; Turner, Stone, Pozdol, & Coonrod, 2006), although decisions about whether a child is on the autism spectrum or not are quite stable starting for children around 2 years of age (Lord et al., 2006).
A total of 38 typically developing children and 29 children with ASD were recruited; however, any child who did not pass a basic “entry-task” (see the “Familiar Object Trial,” described below) was excluded from the remainder of the tasks (in general, the children who did not pass the entry-task were developmentally younger and had lower IQs than those who did). Therefore, due the exclusions based on the entry-task, the final sample included two groups of children: 21 children with ASD and 21 TD children (see Table 1).
Table 1. Sample demographics.
| ASD N=21 |
TD N=21 |
|
|---|---|---|
| Male* | 20 (95.24%) |
13 (61.90%) |
| Female | 1 (4.76%) |
8 (38.10%) |
| Race | ||
| Caucasian | 18 (85.70%) |
20 (95.24%) |
| African-American | 1 (4.76%) |
-- |
| Bi-racial/Other | 2 (9.54%) |
1 (4.76%) |
| Maternal Education | ||
| At least a 4 year college degree | 18 (85.72%) |
20 (90.48%) |
| Some college/associate's degree | 2 (9.52%) |
1 (9.52%) |
| High-school diploma/G.E.D. or less | 1 (4.76%) |
-- |
Note. ASD = autism spectrum disorder; TD = typically developing.
p < .05
All participants had a minimum of 10 object-names reported as comprehended on the MacArthur-Bates Communicative Development Inventory (Fenson et al., 1993). All children in the ASD sample were individually matched to a child in the TD sample based on expressive vocabulary size (as reported on the CDI). Eighteen pairs of children were matched within 30 words out of 396 total words on the CDI, and 3 pairs were matched within 50 words.
Measures
All measures administered in the present investigation have been shown to have adequate test-retest and internal consistency and included the following:
The Mullen Scales of Early Learning (Mullen, 1995) is a measure of cognitive functioning for children from birth to 5 years, 8 months of age that yields an overall IQ score, as well as subtest scores and age equivalents for gross and fine motor skills, visual reception, and receptive and expressive language. In order to obtain a nonverbal mental age, the age equivalent scores for the visual reception and fine motor scales were averaged. Age equivalents for the receptive and expressive language subtests were averaged to yield a verbal mental age. In the standardization sample (Mullen, 1995), the 1- to 2- week test-retest reliability in a sample of 1- to 24- month old children was sufficient for each subscale, ranging in value from .82 to .96. Test-retest reliability for a sample of children 25-56 months ranged in value from .71 to .79.
-
The Autism Diagnostic Observational Schedule (Lord et al., 2000) is a semi-structured, standardized assessment of communication, social interaction and play for children who have been referred for possible autism. Codes on the ADOS are scored from ‘0’ to ‘3’, with a higher score being indicative of greater abnormality. This does not necessarily indicate that a child who scores ‘0’ on an item is performing in the manner that a typically developing child would, it means that there was no autism-related abnormality evident during the structured observation. Children in this study were administered either Module 1 (preverbal or single words) or an experimental version intended for children under 30 months of age, the “ADOS – Toddler Module” (Lord, Luyster, Gotham, & Guthrie, in press). The ADOS was used in generating a “Consensus Best Estimate Diagnosis” (below). In addition, the ADOS item “Response to joint attention” was used as a measure of children's ability to follow the examiner's attention in a standardized situation; a code of ‘0’ indicated that the child was able to follow the examiner's gaze, a code of ‘1’ indicated that the child was able to follow the examiner's point, and codes of ‘2’ and ‘3’ indicated that the child was not able to follow the examiner's attention (Lord et al., 1999).
The reliability of the ADOS has been previously established. In the standardization sample, all items in Module 1have good inter-rater reliability (mean exact agreement above .80) (Lord et al., 2000) and all items in the Toddler Module have fair inter-rater reliability (mean exact agreement of at least .71, with the majority exceeding .80) (Luyster et al., under revision). In the current investigation, the assessment was scored by administrators who had previously established consistent item-level inter-rater reliability of at least 80% on protocol and algorithm items (Lord, Rutter, DiLavore, & Risi, 1999).
The Autism Diagnostic Interview – Revised (LeCouteur, Lord, & Rutter, 2003) is a standardized 90-minute caregiver interview that yields scores for socialization, communication and restricted and repetitive behaviors in children referred for autism. Because the ADI-R is not normed for very young children, clinicians took into account information collected during its administration but did not use algorithm scores. All items on the ADI-R have been shown to have fair inter-rater reliability (mean exact agreement ranging from .90 to .93) (Lord, Rutter, & Le Couteur, 1994). All assessments for the current project were scored by administrators who had previously established consistent item-level inter-rater reliability of at least 90% on protocol and algorithm items (Rutter, Le Couteur, & Lord, 2003).
The MacArthur-Bates Communicative Development Inventory – Words and Gestures or Words and Sentences (Fenson, 1989; Fenson et al., 1993) is a parent checklist of early receptive and expressive vocabulary, as well as nonverbal communicative skills. It has been shown to have excellent reliability for the typically developing population (.95 for test-retest at 1.38 months for Words and Sentences form; all scales above .86 for test-retest reliability at 1.35 months for Words and Gestures form) (Fenson et al., 1994) and have good validity when used with children with autism (Charman, Drew et al., 2003; Luyster, Lopez, & Lord, 2007). One of two forms (Words and Sentences, or Words and Gestures) was selected based on the child's age and developmental level.
Consensus Best Estimate Diagnosis. Criteria for Autistic Disorder or PDD-NOS by the DSM-IV (American Psychiatric Association, 1994) and information from the ADI-R and ADOS were used to make a best estimate diagnosis by the authors. Children who were not judged to meet DSM-IV criteria for autism or PDD-NOS were excluded, even if they met cutoffs on the ADI-R and ADOS.
Materials
Pilot studies indicated that children with ASD did not consistently demonstrate high motivation to complete tasks when the endpoint of the activity was ambiguous. As a result, all word learning tasks involved using a small wastebasket with a swinging top (painted to look like a ladybug), into which the child deposited the object. This procedure allowed for clear closure (placing the object in the basket), thus increasing attention and motivation, and it also allowed for procedural uniformity. Materials for specific tasks are described below.
A full set of “familiar objects” was established for the pre-test entry task, or the “Familiar Object Trial”; this set included the following objects: dog, spoon, fork, brush, comb, bucket, cup, shoe, cat, ball, airplane, car, duck, flower, keys, baby and bottle. The word learning tasks required separate sets of materials; two full “kits” were established, both of which contained a complete set of word learning materials. The Novel Labeling Task materials were comprised of a set of three novel objects. The Pragmatics Task included six novel objects, three of which were used in each condition. Objects were randomly assigned to different roles in the activity (see Procedures) prior to administration. All the objects used in the Novel Labeling Task and Pragmatics Task were selected to be interesting (i.e., made a small noise or movement) but not extremely exciting, and included items such as plastic castanets, a “glitter globe” (akin to a snow globe) and a flexible rubber change purse.
Procedure
This project received approval from the University of Michigan Medical School Institutional Review Board. Recruitment of families of children with ASD was contingent upon either (1) parental contact of research staff at UMACC or (2) prior parental indication of interest in future research participation (for those participants who were recruited through UMACC's client database). Upon parental interest, details of the study were provided and consent was obtained. Two appointments were completed for each family. The first appointment required the attendance of either one or both parents and allowed for the completion of the ADI-R. The second appointment required the attendance of either one or both parents, as well as the child participant. During the child's evaluation, cognitive testing was completed first, followed by the word learning tasks (described below). The ADOS was administered last. At least one parent was in the testing room and in close proximity to the child during the administration of all measures. The tests were all administered in a quiet room, with the parent(s) and one examiner present. The entire session was videotaped.
Procedures in the word learning tasks were based on methods which were shown to be effective in very young children by Woodward, Markman and Fitzsimmons (1994): multiple objects were placed in front of the child and then playfully placed in a bucket. Novel labels were randomly assigned to be used in each word learning task from a list of 20 simple, nonsense words, most of which had been previously used in word learning studies (e.g., dipu, blicket, fep, toma, peri, etc.). For all tasks, the placement of different objects in front of the child (from left to right) was randomly assigned prior to administration.
Familiar Object Trial
In order to select items which were familiar to a particular child and for which he/she knew labels, parents were asked during a pre-appointment phone call which of the familiar labels their child best understood. The Familiar Object Trial took approximately one minute. Children were presented with three objects (one familiar and two distracters) and asked a test question (e.g., “Can you put the dog in the bucket?”). Contingent upon successful completion of the Familiar Object Trial, the three word learning tasks were administered. The justification for this was that children who were unable to complete the paradigm with a known label and object would be unlikely to be able to meaningfully complete the activity with a novel label and object. As a result, this activity served as a sort of “entry task” for all participants. Only those children who passed the Familiar Object Trial were administered the word learning tasks. Children who passed the Familiar Object Trial were then administered the Preference Observation, then the Novel Labeling Task, and finally the Pragmatics Task (conditions were counterbalanced). All children received the Preference Observation as the initial task; the order of the Novel Labeling Task and Pragmatics Task was randomly assigned.
Children who did not pass the pre-test activity (ASD sample, n=8; TD sample, n=17) did not receive any further word learning activities and were not included in any analyses reported below. For both samples, children who did not pass the pre-test activity had significantly lower verbal and nonverbal IQs and verbal mental age (ps < .05) than their peers who did pass the activity. In the TD sample, the children who failed were also chronologically younger and had lower nonverbal mental ages (ps <.05), but this was not the case in the ASD sample.
Preference Observation
Prior to administering the Novel Labeling and Pragmatics tasks, the investigator initiated a brief play session, during which each experimental item was introduced to the child, and the child was encouraged to “put it in the bucket”. If the child was excessively interested in or afraid of an item, that toy was removed from the item set and replaced by another pre-determined object.
Novel Labeling Task
Based on the work of Woodward, Markman and Fitzsimmons (1994), the Novel Labeling task was divided into a training phase and a testing phase. It addressed whether children were able to map new labels onto novel objects. Two novel objects were used: one was introduced as the “labeled” object (i.e., the toma) and the second served as the “commented” object.
Training phase
The investigator addressed the child while moving the object in the child's front visual field and said, “That's a toma. See, it's a toma. Look, it's a toma.” This “phrase-triplet” was repeated two more times, resulting in nine consecutive repetitions of toma. In order to draw a similar amount of attention to the non-labeled object, the investigator next commented on the non-labeled object using similar phrase-triplets: “Ooh, look at that. Yeah, see it? Wow, look at that,” for an overall total of nine comments. Each phrase-triplet began when the child's attention was on the toy and was completed regardless of any attention shift on the part of the child. However, a pause was introduced prior to the next phrase triplet to get the child's attention back to the toy (e.g., by moving it, shaking it, bringing it into the child's visual field). When the child's attention was back on the toy, the next phrase-triplet began.
Testing phase
After the training phase, the investigator presented all three objects (target, commented and a distracter), asking “Can you put the toma in the bucket?”
Pragmatics Task
Based on previous research by Baldwin (1991; 1993), this task used two conditions (Discrepant and Follow-in) to assess children's ability to use social understanding to determine the referent of a new label. In each condition, a different set of three unusual objects was used. Videotapes of all Pragmatics Task administrations were reviewed by the authors; all administrations included in the present analyses complied with the following procedures.
Discrepant training
The investigator placed two novel items on the table and demonstrated an interesting action with each toy. While sitting opposite the child, she placed the toy previously designated as the “child's toy” in the child's hand and picked up the “investigator's toy,” resting it in the palm of her hand. The investigator held her object at approximately 60 degrees from her midline, with her face turned towards her own toy (i.e., and away from the child's toy, which was visible only in her peripheral vision). While the child was holding and looking at the “child's toy” and the investigator was holding the “investigator's toy,” she then said “That's a peri. See, it's a peri. Look, it's a peri” while maintaining her gaze towards the toy in her palm. The phrase-triplet was uttered two more times (resulting in nine presentations of the label); at no point was a phrase-triplet introduced when the child was already looking at the investigator's toy (which, if done, would have not been discrepant attention). Each phrase-triplet began when the child's attention was on his/her own toy and was completed regardless of any attention shift on the part of the child. As in the Novel Labeling Task, a pause was introduced prior to the next phrase triplet to get the child's attention back to his/her own toy. When the child's attention was back on his/her own toy, the next phrase-triplet began. After the ninth label, the child was allowed to play with both objects for up to one minute.
Discrepant testing
The investigator placed three objects (two novel objects from the training phase and one distracter) in front of the child and asked, “Can you put the peri in the bucket?”
Follow-in training
Except that a second set of novel toys was used, Follow-in training was similar to Discrepant (i.e., investigator sat opposite the child and placed “child's toy” in the hand of child and placed the “investigator's toy” in her own palm). Furthermore, rather than shifting her object and face to the side, the examiner faced forward, gazing directly towards the child and the child's toy at the time that she vocalized the novel label (e.g., dax). The investigator began each phrase-triplet only when the child was looking at his own toy, and the same procedure was used in the delivery of the phrase-triplets (i.e., pausing between triplets to re-direct the child's attention, if needed).
Follow-in testing
Follow-in testing was identical to that of the Discrepant condition, but a different distracter was used.
Results
Despite matching, the diagnostic groups were not anticipated to be generally equivalent in other areas like developmental and chronological age or IQ scores (see Table 2). However, after matching, they were similar on verbal mental age. It is important that these children were at a similar level of language development, as measured both by productive vocabulary size and developmental level (on a standardized measure). Preliminary analyses indicated no gender differences in performance in the word learning tasks. Due to small sample sizes, non-parametric tests were used for the following analyses. Effect size (Cohen's d) and power analyses are reported where appropriate and were conducted using the G*Power statistical software (Faul, Erdfelder, Lang, & Buchner, 2007). For all binomial tests reported below (against chance, or 33%), assuming a small-to-medium effect size of .4 (Cohen, 1988) and a sample size of 21 per group, the analyses are powered at 97% (1-β).
Table 2. Sample characteristics.
| ASD N=21 |
TD N=21 |
|
|---|---|---|
| Mean Chronological Age (in months)*** | 30.86 (SD = 10.49) |
20.62 (SD = 2.94) |
| Minimum -- Maximum | 17 - 61 | 14 - 24 |
| Mean Nonverbal IQ (NVIQ)*** | 95.17 (SD = 23.04) |
119.95 (SD = 15.75) |
| Minimum -- Maximum | 36 - 135 | 95 - 141 |
| Mean Nonverbal MA (NVMA)** | 28.55 (SD = 9.04) |
24.31 (SD = 4.83) |
| Minimum -- Maximum | 17 - 52 | 16 - 32 |
| Mean Verbal IQ (VIQ)*** | 82.33 (SD = 19.81) |
114.62 (SD = 16.04) |
| Minimum -- Maximum | 37 - 125 | 87 - 141 |
| Mean Verbal MA (VMA) | 25.29 (SD = 8.87) |
23.64 (SD = 5.31) |
| Minimum -- Maximum | 11 - 41 | 14 - 34 |
| Mean Number of Words Said on CDI | 158.15 (SD = 102.01) |
159.71 (SD = 112.72) |
| Minimum -- Maximum | 1 - 299 | 12 - 388 |
| Mean Number of Words Understood on CDI | 245.29 (SD = 108.77) |
278.48 (SD = 83.81) |
| Minimum -- Maximum | 36-393 | 51-396 |
| Mean Score on ADOS ‘Response to joint attention’** | 0.43 | 0.00 |
| Number of children with score of 0 | 13 | 21 |
| Number of children with score of 1 | 7 | 0 |
| Number of children with score of 2/3 | 1 | 0 |
Note. ASD = autism spectrum disorder; TD = typically developing; CDI = MacArthur-Bates Communicative Development Inventory; ADOS = Autism Diagnostic Observation Schedule.
p < .01
p < .001
Novel Labeling Task
Results indicated that 14 of the 21 (66.67%) TD children and 16 of the 21 (76.19%) children with ASD passed the Novel Labeling Task. Using a binomial distribution, results indicated that the rate of passing the Novel Labeling Task was significantly greater than what would be expected by chance (33%) in both groups (p < .01), with no group difference in the rate of passing the Novel Labeling Task (two-sided Fisher's exact test, p = .73).
To our surprise, Mann-Whitney tests (collapsing across diagnostic groups) indicated that there were not significant differences in chronological age (CA), expressive vocabulary size, nonverbal IQ (NVIQ), nonverbal mental age (NVMA), verbal IQ (VIQ) or verbal mental age (VMA) between those children who successfully passed the Novel Labeling Task and those who did not (see Table 3). However, using the data from only those children who passed the task, Mann-Whitney tests indicated a significant diagnostic group difference in chronological age (such that the ASD group was older than the TD group; U = 56.50, p < .05), and in NVIQ and VIQ (with the TD group higher in both, U = 43.00, p < .01 and U = 26.00, p < 0.01, respectively). Effect sizes (1.00, 1.26 and 1.79, respectively) were all large (Cohen, 1988). There were no group differences in mental age or expressive vocabulary size within the groups of children who passed the Novel Labeling Task.
Table 3. Performance in the Novel Labeling Task.
| ASD | TD | ||||
|---|---|---|---|---|---|
| Fail (n=5) | Pass (n=16) | Fail (n=7) | Pass (n=14) | ||
| Chronological Age (in months) | Mean (SD) | 37.00 (14.19) |
28.94a (8.75) |
20.86 (3.39) |
20.50 (2.82) |
| Median (Interquartile range) | 32.00 (22) |
30.00 (16) |
21.00 (4) |
21.00 (4) |
|
| Nonverbal IQ (NVIQ) | Mean (SD) | 85.61 (37.74) |
98.16 (16.97) |
121.86 (15.30) |
119.00b (16.46) |
| Median (Interquartile range) | 87.00 (69.47) |
97.50 (24.75) |
119.00 (21.00) |
123.50 (28.00) |
|
| Nonverbal MA (NVMA) | Mean (SD) | 29.40 (8.09) |
28.29 (9.55) |
25.21 (4.77) |
23.86 (4.97) |
| Median (Interquartile range) | 30.00 (15.00) |
28.00 (14.75) |
27.00 (6.50) |
23.00 (8.13) |
|
| Verbal IQ (VIQ) | Mean (SD) | 75.78 (33.47) |
84.39 (14.34) |
121.71 (12.28) |
111.07b (16.90) |
| Median (Interquartile range) | 80.00 (58.56) |
87.50 (22.25) |
128.00 (22.00) |
110.50 (26.25) |
|
| Verbal MA (VMA) | Mean (SD) | 26.70 (10.21) |
24.85 (8.73) |
24.93 (5.27) |
22.99 (5.40) |
| Median (Interquartile range) | 26.50 (18.50) |
24.25 (16.00) |
26.00 (9.00) |
21.25 (6.59) |
|
| Number of Words Said on CDI | Mean (SD) | 161.85 (126.55) |
157.00 (97.98) |
200.14 (124.58) |
139.50 (105.19) |
| Median (Interquartile range) | 103.00 (242) |
190.00 (196) |
196.00 (194) |
122.50 (189) |
|
Note. ASD = autism spectrum disorder; TD = typically developing; CDI = MacArthur-Bates Communicative Development Inventory
Significantly higher than TD ‘Pass’ group, p < .05
Significantly higher than the ASD ‘Pass’ group, p < .01
Pragmatics Task
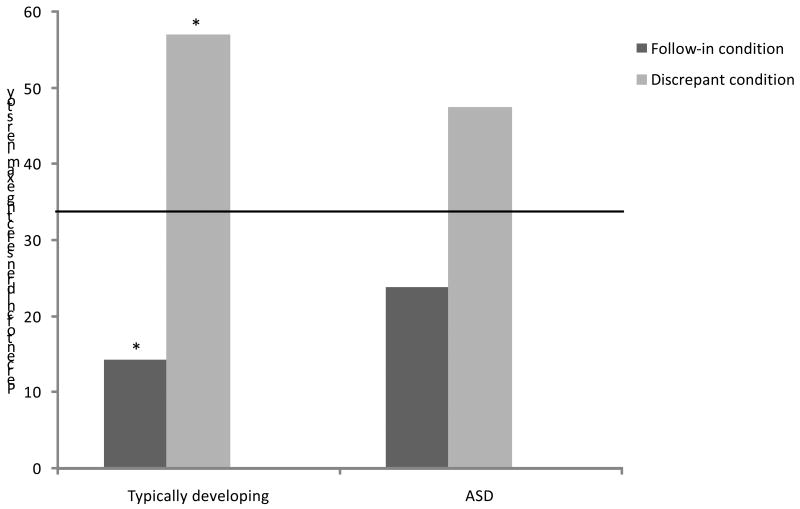
See Figure 1 for the results of the present investigation (chance level indicated with the solid horizontal line). To review, if children picked their own toy more often in the Follow-in condition (examiner labels child's toy) than in the Discrepant condition (examiner labels examiner's toy), that was considered evidence of the use of social information to guide mapping. Note that each bar shows the proportion of children out of the entire group sample (n=21 for each group) who selected the child's own toy in that condition, and that every child received both conditions.
Figure 1. Selection of child's own toy in Pragmatics Task, by diagnostic group and condition.
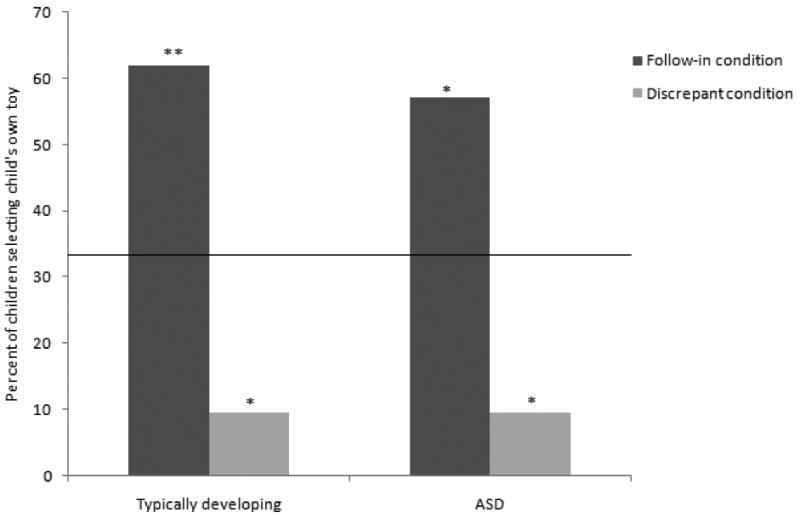
Note: Solid horizontal line indicates chance level.
* significantly different than chance, p<.05
** significantly different than chance, p<.01
Performance was similar across diagnostic groups. The rate of selecting the child's own toy did not differ across the TD children and children with ASD for either the Follow-in (two-sided Fisher's exact test, p = 1.00) and Discrepant (two-sided Fisher's exact test; p = 1.00) conditions.
In the TD sample (see Figure 1), 13 children (61.90%) selected their own toy in the Follow-in condition and 2 children (9.52%) did so in the Discrepant condition; two-tailed McNemar test, p < .01). In a McNemar test, the odds ratio of discordant pairs is used to specify the effect size (Faul et al., 2007). Given an odds ratio of discordant pairs of 0.08 and a sample size of 21, the McNemar test for the TD sample was powered at 93%. There was also an effect of condition for the ASD group (see Figure 1, two-tailed McNemar test, p < .01), such that the child's toy was selected more often in the Follow-in condition (12 children, or 57.14%) than in the Discrepant condition (2 children, or 9.52%). Given an odds ratio of discordant pairs of 0.09 and a sample size of 21, the McNemar test for the ASD sample was also powered at 93%. Thus, for both diagnostic groups, children were significantly more likely to pick their own toy when the examiner had been labeling that toy (i.e., in the Follow-in condition) than when the examiner had not been labeling that toy (i.e., in the Discrepant condition).
In order to determine if children selected the correct toy more or less often than would be expected by chance, binomial tests were used for each diagnostic group. The children in the TD sample selected their own toy at a rate which was significantly greater than chance in the Follow-in condition (61.90%, p < .01; chance = 33%) and significantly less than chance in the Discrepant condition (9.52%, p < .05; chance = 33%). The children in the ASD group selected the child's toy at a rate significantly above chance in the Follow-in condition (57.14%, p < .05; chance = 33%) and selected their own toy significantly below chance in the Discrepant condition (9.52%, p < .05; chance = 33%).
Similar analyses were conducted for the rate of selecting the examiner's toy (see Figure 2), although previous studies did not generally address this question. The rate of selecting the examiner's toy did not differ across the diagnostic groups for either the Follow-in (two-sided Fisher's exact test, p = .70) and Discrepant (two-sided Fisher's exact test; p = .76) conditions.
Figure 2. Selection of examiner's toy in Pragmatics Task, by diagnostic group and condition.
Note: Solid horizontal line indicates chance level.
* significantly different than chance, p<.05
Three children in the TD sample (14.29%) selected the examiner's toy in the Follow-in condition and 12 children (57.14%) did so in the Discrepant condition (two-tailed McNemar test, p < .01). An odds ratio could not be calculated due to an empty cell. In the ASD group, the examiner's toy was selected by 5 children (23.80%) in the Follow-in condition and 10 children (47.62%) in the Discrepant condition (two-tailed McNemar test, p = .30). That is, the typically developing children were significantly more likely to pick the examiner's toy when the examiner had been labeling that toy (i.e., the Discrepant condition) than when she had not (i.e., the Follow-in condition. Although the ASD group showed the same pattern, the difference across conditions was not significant.
In order to determine if children selected the examiner's at a rate different than would be expected by chance, binomial tests were used for each diagnostic group. The children in the TD sample selected the examiner's toy at a rate which was significantly less than chance in the Follow-in condition (14.29%, p < .05; chance = 33%) and significantly greater than chance in the Discrepant condition (57.14%, p < .05; chance = 33%). The children in the ASD group selected the examiner's toy at a rate which not different from chance in either the Follow-in condition (23.80%, p = .23; chance = 33%) or the Discrepant condition (47.62%, p =.12; chance = 33%).
Alternative Analyses of the Pragmatics Task Data: ASD sample only
In order to provide an interpretation that is in keeping with previous studies, similar analyses of “Direction of Gaze” strategy were conducted for the current ASD sample, using the more (Preissler & Carey, 2005) and less (Baron-Cohen et al., 1997) stringent criteria. The typical sample was excluded from this set of analyses, since the intent was to compare the performance of the current ASD sample to that reported by previous investigations.
Using the less stringent criteria (Baron-Cohen et al., 1997) to characterize the children's strategy solely during performance in the Discrepant condition, in the present sample, only two (out of 21) children selected their own toy out of three possible objects (the LDG strategy, or using the listener/child gaze to guide mapping, 9.52%); Baron-Cohen and colleagues reported that 71% of their sample used the LDG strategy. In contrast, 10 of 21 (47.62%) children in the present sample used the SDG strategy – that is, using the speaker/examiner gaze to guide mapping – whereas Baron-Cohen and colleagues reported that 29% of their sample employed the SDG strategy.
Characterizing strategy use based on performance in both the Follow-in and Discrepant conditions permits comparison to the Preissler and Carey (2005) results. To do so, each child was coded based on the observed “strategy” of selecting objects. Three strategy characterizations were used: (1) SDG (selecting toys which were the focus of the speaker across both conditions); (2) LDG (selecting toys which were the focus of the listener across both conditions); and (3) all other strategies. The present results indicated that 48% (compared to 28% reported by Preissler and Carey) of the current ASD sample successfully used the speaker gaze strategy to map words in both the Follow-in and Discrepant conditions. In the current investigation, only 5% of children consistently selected the object that was the focus of their own attention (LDG), in contrast to the 39% reported by Preissler and Carey. In sum, when compared to the previously reported results from Baron-Cohen et al. (1997) and Preissler and Carey (2005), the present investigation found fewer children with ASD showing a consistent tendency to pick the toy that was the focus of their own attention and more children with ASD showing a tendency to select the toy that was the focus of the examiner's attention.
Finally, because the samples from Baron-Cohen et al. (1997) and Preissler and Carey (2005) were children with profound language impairment, it was speculated that there might be more overlap in results if the analyses of the current investigation were limited only to children with significant language impairments. Criteria to define substantial language impairment were generated based on scores on the MSEL. In general, standard scores which were greater than two standard deviations below the mean were considered to be indicative of significant impairment. There were two possible cut-offs; a child had to meet only one: either (1) VIQ equal to or less than 70; or (2) expressive language T-score equal to or less than 30. There were only five children in the current ASD sample who met one or both of these criteria. One of these children successfully selected the expected toy in both the Discrepant (examiner's toy) and Follow-in conditions (child's toy). The other four children did not successfully select the expected toy in either task. Contrary to expectations of predominance of the LDG strategy (that is, consistently selecting the child's own toy, which was the focus of the child's attention) based on previous studies, none of these children selected their own toy in the Follow-in condition or in the Discrepant condition.
Discussion
Our knowledge of what constitutes a core language deficit in ASD is changing rapidly, as more and more children with ASD are identified who do not have profound impairments in the structure and fluency of language (although impairments in pragmatics are usually lasting). This is important not only for autism researchers and clinicians but also for developmental scientists, because of the implications for our understanding of the links among social, cognitive and language development. Overall, it appears that some children with ASD may be on a very different developmental trajectory in language acquisition than what we might have expected ten or twenty years ago (Anderson et al., 2007). This may be partly due to the improvement and increasing pervasiveness of early intervention programs, which often include an explicit focus on language development (Rogers, 2006) and are associated with improved outcomes. The apparent difference in developmental trajectories may also be due to a cohort effect, such that the children who are being identified now are generally less profoundly affected than those diagnosed one or two decades ago, and higher-functioning children have been shown to make more pronounced gains over time (Ben-Itzchak & Zachor, 2007; Sigman & McGovern, 2005) than their less-able peers.
The present study re-evaluated whether some children with ASD were able to use social information to guide their word-object mapping. As expected, the typical and ASD groups (matched on expressive vocabulary) in the current sample did not differ in their ability to learn a name of a novel object when the examiner followed the focus of the child's own attention. In contrast to previous research, however, both groups showed similar patterns of response when the examiner's focus of attention was different from that of the child (with the exception of one analysis, discussed below). The children with ASD did not make consistent errors, even when they were required to use social information in a word learning context. Overall, then, the performance of the ASD sample is generally in line with the findings for typical children reported by Baldwin (1991, 1993) and suggests that some children with ASD can use social information to guide their word-object mapping.
Interestingly, there was one analysis that indicated that the performance in the ASD group was not as pronounced as that in the TD group (although the diagnostic groups were not significantly different from each other). This analysis – which examined rates of selecting the examiner's toy – was not commonly used in previous studies, thus making the results difficult to interpret. However, the results may suggest that the ASD group is able to avoid making mapping errors, particularly in the Discrepant condition (as they did in previous studies), but they may not consistently map the word in this complex situation. These findings echo similar observations by Baldwin (1991), who noted that, infants “(a) successfully learned the labels introduced during follow-in labeling, and (b) displayed no tendency to make mapping errors after discrepant labeling. Thus infants…understand that a speaker's nonverbal cues are relevant to the reference of object labels; they already can contribute to the social coordination involved in achieving joint reference” (p. 875). The implication, then, is that children are successfully using social information to prevent making erroneous mappings, but they have not yet mastered the extra step of making a correct mapping. Elsewhere, Baldwin (1993) noted that this profile appeared to be a developmental precursor to making successful mappings across conditions, and the shift occurred somewhere between 16 and 18 months of age in typical development. The ASD sample had developmentally reached this point according to mental age estimates, but researchers have noted that children with ASD may reach milestones (particularly social-cognitive ones) developmentally later than their typically developing peers (Happé, 1995). It is possible, therefore, that the children with ASD may be following a developmentally delayed but qualitatively typical pathway.
The previous investigations which formed the foundation of the current study (as well as countless other studies in the field of ASD research) were focused on the difficulties shown by a group of profoundly impaired children with ASD (Baron-Cohen et al., 1997; Preissler & Carey, 2005). Understanding the impairments of severely delayed individuals with ASD is inarguably important in characterizing the disorder. However, as our understanding of the variability within the autism spectrum grows, it is important that research also address the competencies of more skilled children with ASD. The results of the present investigation, then, are a valuable complement to the research which demonstrated that profound intellectual disability in ASD was associated with difficulty in using social information (Baron-Cohen et al., 1997; Preissler & Carey, 2005). If these investigations are taken as a whole, the collective results suggest that a sub-group of children with ASD are able to use social information to structure their word-object mappings, a finding which strongly refutes the idea that there is something inherently different about the process of learning new words in all children with ASD. That is, by early childhood, not all children with ASD can use social information in a complex word learning situations but some of them can.
There are notable characteristics of the group of children who succeeded in using social information: they were generally of average nonverbal intelligence, and they also had some mastery of joint attention, as indicated by scores on the ADOS “Response to joint attention” item (see Table 2). Interestingly, the mean score of the current sample on this item (0.43) is similar to that reported for the ASD (PDD-NOS) group in the original standardization sample (0.35), suggesting that the present results are consistent with the original ADOS reports (Lord et al., 1999). The apparent importance of joint attention for language found here was also noted by Parish and colleagues (2007), who found that a child's understanding of social intention was concurrently related to vocabulary size. These findings are consistent with previous reports that early joint attention is a powerful predictor of future language ability (Charman, Baron-Cohen et al., 2003; Mundy et al., 1990; Sigman & McGovern, 2005). Indeed, the predictive ability of joint attention is tied to the observation that joint attention is highly variable across young children with ASD, with some preschoolers performing well during standard tests of joint attention and others having more difficulty (Lord et al., 1999; Mundy, Sigman, & Kasari, 1994). That is, joint attention could not be a powerful predictor without showing early variability.
Moreover, whereas previous investigations reported that the use of relatively stringent methods was associated with task failure for children with ASD (Baron-Cohen et al., 1997; Preissler & Carey, 2005), our results suggest that the addition of extra contextual supports improves performance. In particular, the presentation of the label nine times (rather than two), and the added salience of facial direction (in addition to gaze), were likely contributors to the success of the ASD group. It is also possible that other components of the procedures affected child performance. The use of carrier phrases (i.e., “That's a ___. See, it's a ___. Look, it's a ___.”) might have provided additional cues for learning, and the increased amount of time spent with each item (to deliver nine phrases rather than two labels) may have enhanced the mapping process.
There are important points to be noted about the “real world” significance of these findings. It is evident that it is a subset of children with ASD who can use social information across these word learning tasks. Certainly, the general design of the study was modified in order to “scaffold” the children to be successful, and it is unclear how children's behavior might change in the absence of these supports. The strategies used in the present study are not new to ASD intervention, which often aids children in learning joint attention skills (Kasari, Freeman, & Paparella, 2006; Whalen, Schreibman, & Ingersoll, 2006) and in mastering words for objects (Barbera & Rasmussen, 2007). Gaining a better understanding of the robustness of word learning skill will be important to understand how this ability may be related to other cognitive and social skills. That is, if (1) delays in joint attention are already slowing the language development process; and (2) children with ASD require more environmental supports than are usually provided, then the combination of these factors could certainly result in a delay in vocabulary development. On a related note, the present investigation did not address whether the joint attention skills shown by the children were spontaneously occurring or the product of a joint engagement-focused intervention. The conclusions about word learning made here would likely not hinge on this distinction (assuming that instruction did not explicitly teach word learning). However, longitudinal investigations within the context of intervention research might illuminate the underlying developmental processes relating joint attention and language development in children with ASD.
It will also be valuable to determine (at an even more specific level) whether, within these sorts of word learning situations, children with ASD are using the same strategies as their typically developing peers. One important aspect will be to address the question of mapping in discrepant situations further to answer the question of when and if children with ASD consistently map new words in these challenging conditions. Moreover, it will be important to explore whether children with ASD who succeed interpreting the adult's behavior based on an understanding of intention, or have they learned to associate gaze/face direction with the referents of new labels? Similarly, which of the speaker's behaviors (gaze, facial orientation, vocalization) are the most influential for child learning? Such inquires are beyond the scope of this project but will be important to pursue in future studies.
There are limitations of the present investigation which should be acknowledged and addressed by future research. First, the sample size was small and we did not include a developmental delay/language delay (DD/LD) comparison group. Fortunately, because the current ASD group was not significantly intellectual disabled, the lack of a DD/LD comparison sample does not substantially limit the present findings. The uneven male-to-female ratio across groups must also be addressed, because of the gender discrepancy that often appears in early language development, such that males often lag behind females (Fenson, 1989). Again, however, because the ASD sample (which included a higher proportion of males than the typically developing group) was not impaired, the results were not meaningfully limited by the gender ratio. Additionally, it would have been valuable to have a more fine-grained measure of joint attention skills than the ADOS, which is based on fairly discrete activities. The best approach might be to capture the child's spontaneous behaviors during naturalistic interactions (Roos, McDuffie, Weismer, & Gernsbacher, 2008) so as to avoid floor effects on standardized measures; however, other comprehensive measures of joint engagement are available and appropriate for young children (Mundy et al., 2003; Wetherby, 2001).
Finally, the generalizability of these findings may be limited, in that (1) it is not possible to conclude that children with ASD are “fast”-mapping, in a strict sense, and (2) the observed word learning skill may be restricted only to children with ASD and average cognitive abilities or less marked ASD symptoms. The relatively high level of functioning of the present sample is not representative of all children on the autism spectrum; therefore, it should not be assumed that the word learning skills demonstrated by the children in this study are present in all children with an ASD diagnosis.
Historically, our understanding of ASD has been largely based on identifying clear group deficits which provide diagnostic boundaries. However, it is becoming increasingly evident that individual differences reveal much about the developmental heterogeneity of ASD. The considerable diversity of language ability in the current cohort of newly diagnosed children with ASD has important implications for autism clinicians and researchers. Clinically, it highlights the potential for learning in response to tailored, developmentally appropriate – but relatively ordinary – opportunities for language learning. From a theoretical perspective, it allows developmental researchers the opportunity to disentangle the primary deficits observed in ASD from the secondary ones, and to learn about the complicated, intertwined components of social communication and language.
Acknowledgments
This work was completed as part of R. Luyster's doctoral dissertation. It was supported by a Graduate Student Research Award from the Organization for Autism Research and NRSA F31MH73210 from the National Institute of Mental Health to R. Luyster. Support was also provided by grants MH57167 and MH066469 from the National Institute of Mental Health and HD 35482-01 from the National Institute of Child Health and Human Development to C. Lord, and by a grant from the Department of Education to Amy Wetherby. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. We thank Somer Bishop, Mia Coffing, Andrea Cohan, Whitney Guthrie, Amy Esler, Jennifer Kleinke, Dorothy Ramos, Jennifer Richler, and Susan Risi for their assistance in data collection and management. Susan Gelman and Henry Wellman provided invaluable advice throughout the planning and completion of this project. Finally, we would like to express our gratitude to the families and children in the Word Learning, First Words and Toddlers studies.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth. Washington, DC: Author; 1994. [Google Scholar]
- Anderson DK, Lord C, Risi S, Shulman C, Welch K, DiLavore PS, et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Baldwin D. Infants' contribution to the achievement of joint reference. Child Development. 1991;62(5):875–890. [PubMed] [Google Scholar]
- Baldwin D. Infants' ability to consult the speaker for clues to word reference. Journal of Child Language. 1993;20:395–418. doi: 10.1017/s0305000900008345. [DOI] [PubMed] [Google Scholar]
- Baldwin D. Understanding the link between joint attention and language. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1995. pp. 131–158. [Google Scholar]
- Baldwin D, Moses L. Links between social understanding and early word learning: Challenges to current accounts. Social Development. 2001;10(3):309–329. [Google Scholar]
- Barbera B, Rasmussen T. The verbal behavior approach: How to teach children with autism and related disorders. Philadelphia, PA: Jessica Kingsley; 2007. [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M. Do children with autism use the speaker's direction of gaze strategy to crack the code of language? Child Development. 1997;68(1):48–57. [PubMed] [Google Scholar]
- Ben-Itzchak E, Zachor DA. The effects of intellectual functioning and autism severity on outcome of early behavioral intervention for children with autism. Research in Developmental Disabilities. 2007;28(3):287–303. doi: 10.1016/j.ridd.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bloom P. How children learn the meanings of words. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Carpenter M, Pennington B, Rogers S. Interrelations among social-cognitive skills in young children with autismitalic. Journal of Autism & Developmental Disorders. 2002;32(2):91–106. doi: 10.1023/a:1014836521114. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. Journal of the American Medical Association. 2001;285(24):3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Predicting language outcome in infants with autism and pervasive developmental disorder. International Journal of Language and Communication Disorders. 2003;38(3):265–285. doi: 10.1080/136820310000104830. [DOI] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Cox A, Baird G, Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention and imitation. Developmental Psychology. 1997;33(5):781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Charman T, Drew A, Baird C, Baird G. Measuring early language development in pre-school children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) Journal of Child Language. 2003;30:213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. An experimental investigation of social-cognitive abilities in infants with autism: Clinical implications. Infant Mental Health Journal. 1998;19(2):260–275. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Wijsman E, Schellenberg G, Estes A, Munson J, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- DeGiacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European Journal of Child & Adolescent Psychiatry. 1998;7:131–136. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Eisenmajer R, Prior M, Leekam S, Wing L, Ong B, Gould J, et al. Delayed language onset as a predictor of clinical symptoms in pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1998;28(6):527–533. doi: 10.1023/a:1026004212375. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fenson L. The MacArthur Communicative Development Inventory: Infant and Toddler Versions. San Diego: San Diego State University; 1989. [Google Scholar]
- Fenson L, Dale P, Reznick J, Bates E, Thal D, Pethick S. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59(5):1–173. [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick J, Thal D, Bates E, Hartung J, et al. MacArthur Communicative Development Inventories: User's guide and technical manual. Baltimore, MD: Paul H. Brookes; 1993. [Google Scholar]
- Golinkoff R, Hirsh-Pasek K. Baby wordsmith - From associationist to social sophisticate. Current Directions In Psychological Science. 2006;15(1):30–33. [Google Scholar]
- Happé F. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Development. 1995;66(3):843. [PubMed] [Google Scholar]
- Hus V, Pickles A, Cook EH, Risi S, Lord C. Using the Autism Diagnostic Interview-Revised to increase phenotypic homogeneity in genetic studies of autism. Biological Psychiatry. 2007;61:438–448. doi: 10.1016/j.biopsych.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. Journal of Child Psychology and Psychiatry. 2006;47(6):611. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Landry S, Loveland KA. Communication behaviors in autism and developmental language delay. Journal of Child Psychology & Psychiatry. 1988;29(5):621–634. doi: 10.1111/j.1469-7610.1988.tb01884.x. [DOI] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, Rutter M. The Autism Diagnostic Interview - Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lord C, Luyster R, Gotham K, Guthrie W. ADOS Toddler Module. Los Angeles, CA: Western Psychological Services; in press. [Google Scholar]
- Lord C, Risi S, DiLavore P, Shulman C, Thurm A, Pickles A. Autism from two to nine. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore P, et al. The Autism Diagnostic Observation Schedule -- Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Risi S, Pickles A. Trajectory of language development in Autistic Spectrum Disorders. In: Rice M, Warren S, editors. Developmental language disorders: From phenotypes to etiologies. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. pp. 7–29. [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. The Autism Diagnostic Interview - Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The Autism Diagnostic Observation Schedule -- Toddler Module: A new module of a standardized diagnostic measure for ASD. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Kadlec MB, Bass A, Gower A, Connolly C, Carter A, et al. Language assessment and development in toddlers with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2008;38(8):1426–1438. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Luyster R, Lopez K, Lord C. Characterizing communicative development in children referred for Autism Spectrum Disorder using the MacArthur-Bates Communicative Development Inventory (CDI) Journal of Child Language. 2007;34(3):623–654. doi: 10.1017/s0305000907008094. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Yoder PJ, Stone WL. Labels increase attention to novel objects in children with autism and comprehension-matched children with typical development. Autism. 2006;10(3):288–301. doi: 10.1177/1362361306063287. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Delgado C, Yale M, Neal AR, Schwartz H. Gaze following, temperament and language development in 6-month-olds: A replication and extension. Infant Behavior & Development. 2000;23:231–236. [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc.; 1995. [Google Scholar]
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert J. A manual for the Abridged Early Social Communication Scales (ESCS) University of Miami Psychology Department; 2003. [Google Scholar]
- Mundy P, Neal AR. Neural plasticity, joint attention, and a transactional social-orienting model of autism. In: Glidden LM, editor. International review of research in mental retardation: Autism. Vol. 23. San Diego, CA: Academic Press; 2001. pp. 139–168. [Google Scholar]
- Mundy P, Sigman M. The theoretical implications of joint-attention deficits in autism. Development and Psychopathology. 1989;1:173–184. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism & Developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in autism. Development and Psychopathology. 1994;6(3):389–401. [Google Scholar]
- Naber F, Bakermans-Kranenburg M, van Ijzendoorn M, Dietz C, van Daalen E, Swinkels S, et al. Joint attention development in toddlers with autism. European Child & Adolescent Psychiatry. 2008;17(3):143–152. doi: 10.1007/s00787-007-0648-6. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G, Munson J. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development & Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Preissler M, Carey S. The role of inferences about referential intent in word learning: Evidence from autism. Cognition. 2005;97:B13–B23. doi: 10.1016/j.cognition.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rogers S. Evidence-Based Interventions for Language Development in Young Children with Autism. In: Charman T, Stone W, editors. Social & communication development in autism spectrum disorders: Early identification, diagnosis, & intervention. New York, NY, US: Guilford Press; 2006. pp. 143–179. [Google Scholar]
- Roos EM, McDuffie AS, Weismer SE, Gernsbacher MA. A comparison of contexts for assessing joint attention in toddlers on the autism spectrum. Autism. 2008;12(3):275–291. doi: 10.1177/1362361307089521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal-Rollins P, Snow CE. Shared attention and grammatical development in typical children and children with autism. Journal of Child Language. 1998;25(3):653–673. doi: 10.1017/s0305000998003596. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bartak L. Causes of infantile autism: Some considerations from recent research. Journal of Autism & Childhood Schizophrenia. 1971;1(1):20. doi: 10.1007/BF01537740. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnosic Interview - Revised (ADI-R) Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):221–233. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- Sigman M, McGovern C. Improvement in cognitive and language skills from preschool to adolescence in autism. Journal of Autism and Developmental Disorders. 2005;35(1):15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome and developmental delays. Monographs of the Society for Research in Child Development. 1999;64:1–114. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Archer L, Fisman S, Streiner DL. Asperger's syndrome and autism: Differences in behavior, cognition, and adaptive functioning. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34(12):1662. doi: 10.1097/00004583-199512000-00017. [DOI] [PubMed] [Google Scholar]
- Tomasello M. Perceiving Intentions and learning words in the second year of life. In: Bowerman M, Levinson SC, editors. Language acquisition and conceptual development. New York: Cambridge University Press; 2001. pp. 132–158. [Google Scholar]
- Tomasello M, Farrar MJ. Joint attention and early language. Child Development. 1986;57(6):1454–1463. [PubMed] [Google Scholar]
- Turner L, Stone WL, Pozdol S, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10(3):243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Watt N, Wetherby A, Shumway S. Prelinguistic predictors of language outcome at 3 years of age. Journal of Speech, Language, and Hearing Research. 2006;49(6):1224–1237. doi: 10.1044/1092-4388(2006/088). [DOI] [PubMed] [Google Scholar]
- Wetherby A. Communication and Symbolic Behavior Scales Developmental Profile, Preliminary Normed Edition. Baltimore, MD: Paul H. Brookes Publishing; 2001. [Google Scholar]
- Wetherby A, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2004;34(5):473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Whalen C, Schreibman L, Ingersoll B. The collateral effects of joint attention training on social initiations, positive affect, imitation, and spontaneous speech for young children with autism. Journal of Autism and Developmental Disorders. 2006;36(5):655. doi: 10.1007/s10803-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Woodward A, Markman E. Early word learning. In: Damon W, editor. Handbook of child psychology: Volume 2: Cognition, perception, and language. New York: John Wiley & Sons, Inc.; 1998. p. 371. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]