Abstract
Our previous work indicated that apolipoprotein (apo) E4 assumes a more expanded conformation in the postprandial period. The postprandial state is characterized by increased VLDL lipolysis. In this article, we tested the hypothesis that VLDL lipolysis products increase VLDL particle fluidity, which mediates expansion of apoE4 on the VLDL particle. Plasma from healthy subjects was collected before and after a moderately high-fat meal and incubated with nitroxyl-spin labeled apoE. ApoE conformation was examined by electron paramagnetic resonance spectroscopy using targeted spin probes on cysteines introduced in the N-terminal (S76C) and C-terminal (A241C) domains. Further, we synthesized a novel nitroxyl spin-labeled cholesterol analog, which gave insight into lipoprotein particle fluidity. Our data revealed that the order of lipoprotein fluidity was HDL∼LDL<VLDL<VLDL+lipoprotein lipase. Moreover, the conformation of apoE4 depended on the lipoprotein fraction: VLDL-associated apoE4 had a more linear conformation than apoE4 associated with LDL or HDL. Further, by changing VLDL fluidity, VLDL lipolysis products significantly altered apoE4 into a more expanded conformation. Our studies indicate that after every meal, VLDL fluidity is increased causing apoE4 associated with VLDL to assume a more expanded conformation, potentially enhancing the pathogenicity of apoE4 in vascular tissue.
Keywords: lipid fluidity, postprandial state, structural conformation, very low density lipoprotein
Apolipoprotein E (apoE), a 34 kDa protein that is important in lipid metabolism and cholesterol transport, has three common alleles (ϵ2, ϵ3, and ϵ4). ApoE polymorphisms influence the risk of atherosclerotic cardiovascular disease and neurodegenerative disorders (1). ApoE3 binds preferentially to HDL and apoE4 to VLDL (2). ApoE contains a 22 kDa N-terminal domain (residues 1–191) and a 10 kDa C-terminal domain (residues 222–299) separated by a protease-sensitive loop (3). ApoE4 shows a more pronounced domain interaction or closed conformation than the other apoE isoforms because it has Arg-112, which enables Arg-61 in the N-terminal domain to interact with Glu-255 in the C-terminal domain, a feature responsible for the preferential association of apoE4 with VLDL (4, 5).
Upon binding to lipid, apolipoproteins undergo conformational rearrangements (6, 7) that affect their function. The association of apoE isoform–dependant postprandial lipoprotein metabolism with vascular disease is not well understood. Previously, we reported that lipolytic products of VLDL reduce the intermolecular interaction of apoE4, i.e., the self-association of apoE4 via its C terminus (8); however, the mechanism for such effect was unknown. In this study, we used electron paramagnetic resonance (EPR) spectroscopy to investigate intramolecular interaction, i.e., domain interaction in apoE4 affected by VLDL lipolysis products. Our findings show that the conformation of apoE4 is modulated postprandially and is mediated by VLDL particle fluidity. These conformational changes may be important in lipoprotein-vascular cell binding and possibly in vascular injury.
MATERIALS AND METHODS
Materials for this work are as follows: streptokinase, ICN Pharmaceuticals (Costa Mesa, CA); vacutainers and venipuncture supplies, Fisher Scientific International (Hampton, NH); glass capillaries, VitroCom (Mountain Lakes, NJ); flat cell (catalog no. ES-LC11), JEOL (Tokyo, Japan); lipoprotein lipase, 5-doxy and 16-doxylstearic acid spin probes, and guanidine thiocyanate, Sigma-Aldrich (St. Louis, MO); (1-oxyl-2,2,5,5-tetramethyl-3-pyrroline-3-methyl) methanethiosulfonate and 12-doxylstearic acid, Toronto Research Chemicals (Toronto, Canada); titan gel electrophoresis supplies, Helena Laboratories (Beaumont, TX); dimyristoylphosphatidylcholine (DMPC), egg yolk phosphatidylcholine, extruder were from Avanti Polar Lipids (Alabaster, AL); and trioelin, cholesteryl oleate, cholesterol, and free fatty acids were from Nu-Chek-Prep (Elysian, MN). α-Thrombin was from Hematologic Technologies (Essex Junction, VT), and Slide-A-Lyzer dialysis cassettes (MWCO 10,000) were from Pierce (Rockford, IL).
Expression and site-directed spin-labeling of apoE
Recombinant plasmids containing thioredoxin-his tagged apoE sequences in pET32a-NT were expressed in BL21 (DE3) Escherichi coli cells as described previously but with modifications (9, 10). The protein was purified with a Ni-affinity column, complexed with DMPC vesicles, and extruded through 100-nm polycarbonate membrane filters. The thioredoxin-his tag was cleaved from apoE with human α-thrombin. The apoE/DMPC complex was lyophilized, dispersed in 50 ml of methanol, and centrifuged at 6,000 g for 20 min at 4°C to remove the DMPC. The pelleted protein was dissolved in denaturation buffer (6 M guanidine and 2× TBS), purified by a second Ni-affinity column step to remove the N-terminal tag, labeled with a methanethiosulfonate spin label, renatured, and assayed for protein content as described (10). The purity and integrity of the protein was tested by SDS-PAGE. The apoE mutants used for this study were apoE4 with two cysteine moieties substituted at amino acid positions 76 and 241 (76C-241C) and apoE3-like protein (R61T-76C-241C).
Human plasma samples
Healthy human subjects were recruited from the University of California, Davis. The study was approved by the Human Subjects Research Committee of the University of California, Davis. Written informed consent was obtained from each participant. The volunteers were fed a moderately high-fat meal (40% calories from fat), and blood was obtained by venipuncture into Vacutainer tubes containing streptokinase (1,500 units) or EDTA, before (fasting, 0 h) and 3.5 and 6 h after ingestion of the test meal (8).
Lipoprotein isolation by NaCl density gradient centrifugation
Blood samples were centrifuged at 1,750 g for 10 min at 4°C to separate plasma from cellular blood constituents. Lipoproteins were isolated by sequential flotation with minor modifications (11). Plasma was transferred into ultracentrifuge tubes (Beckman-Coulter), and 0.01% (w/v) NaN3 was added as a preservative. Plasma was diluted 1:2 in 196 mM NaCl and 0.25 mM EDTA (ρ = 1.0063) and centrifuged at 63,000 g for 30 min at 14°C to allow chylomicron flotation. The remaining plasma was spun at 285,000 g for 18 h at 14°C to isolate VLDL. The density of the remaining plasma was adjusted to 1.063 to isolate LDL and to 1.21 to isolate HDL and spun at 285,000 g for 18 h at 14°C for each lipoprotein fraction. Lipid fractions were dialyzed against 150 mM NaCl + 0.25 mM EDTA overnight at 4°C. Plasma and lipid fractions were stored at 4°C and used within 3 days after isolation. Triglyceride rich lipoprotein (TGRL) containing both chylomicrons and VLDL were isolated by centrifuging the plasma samples at 285,000 g for 18 h at 14°C.
Lipoproteins were lipolyzed by incubation with bovine milk lipoprotein lipase (LpL) (3 U/ml) at 37°C for 30 min and assayed for nonesterified fatty acids. LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides (TGs), and nonesterified fatty acids were quantified with an autoanalyzer.
Separation of lipoproteins by Titan gel electrophoresis
Equal amounts of spin-labeled apoE4 or apoE3-like protein (0.2 mg/ml) were incubated with fasting plasma, postprandial VLDL, or LpL-treated postprandial VLDL at 37°C for 1 h and applied (2 µl/lane) to Titan-agarose precasted gels. To assay the association of apoE with each lipoprotein class, lipoprotein bands on the gel were visualized by staining and destaining (8). HDL, LDL, and VLDL bands were excised from the gel, solubilized in 4.5 mol/l guanidine isothiocyanate at 65°C, and analyzed by EPR spectroscopy, and the quantity of spin-labeled apoE associated with the VLDL fraction was determined (8).
Samples with spin-labeled lipids
The 5 doxyl-, 12 doxyl-, and 16-doxyl stearic acid (DSA) spin probes solubilized in ethanol were added to lipoproteins and mixed extensively by pipetting to ensure uniform equilibration of the probe with the sample. The final concentration of the 5-, 12-, and 16-DSA probes in the lipoprotein samples was 2 µmol/l. Spin-labeled probe/total cholesterol ratios were maintained the at 1:650.
Synthesis of spin-labeled cholestanol
The synthesis of SL-cholesterol 3-[17-(1,5dimethyl-hexyl)-3- hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenantren- 2-ylidenethyl]2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl radical cholestanol (Fig. 1A) is described as follows. To a solution of 5α-cholestan-3-one (3.86 g, 0.01 mol) in methanol (30 ml), 10% NaOH solution (2 ml), and aldehyde (1.68 g, 0.01 mol) were added. The mixture was stirred at room temperature for 3 h, acidified with 5% H2SO4, and extracted with CHCl3 (3 × 20 ml). The organic phase was washed with brine (2 × 20 ml), dried (MgSO4), and evaporated. The residue was purified by flash chromatography with hexane/ethanol to give the ketone 17-(1,5-dimethyl-hexyl)- 2-(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-ylmethylene)-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenantren-3-one radical. To a solution of ketone (2.69 g, 5.0 mmol) in absolute ethanol (20 ml), NaBH4 (0.19 g, 5 mmol) was added at 0°C. The reaction mixture was allowed to warm up to room temperature. After 1 h at room temperature, the mixture was quenched with water (10 ml), the alcohol was evaporated, and the aqueous phase was extracted with CHCl3 (3 × 20 ml). The organic phase was dried (MgSO4), evaporated, and purified by flash chromatography to give the final product. The yield was 1.93 g (72%); mp 187–188°C; Rf 0.60 (CHCl3/Et2O 2:1); MS, m/z (%) = 538 (M+, 7), 508 (7), 465 (10), and 43 (100). Elemental analysis calculated for C36H60NO2 (538.88), C 80.24, H 11.22, N 2.60% found C 80.20, H 11.25, and N 2.58%.
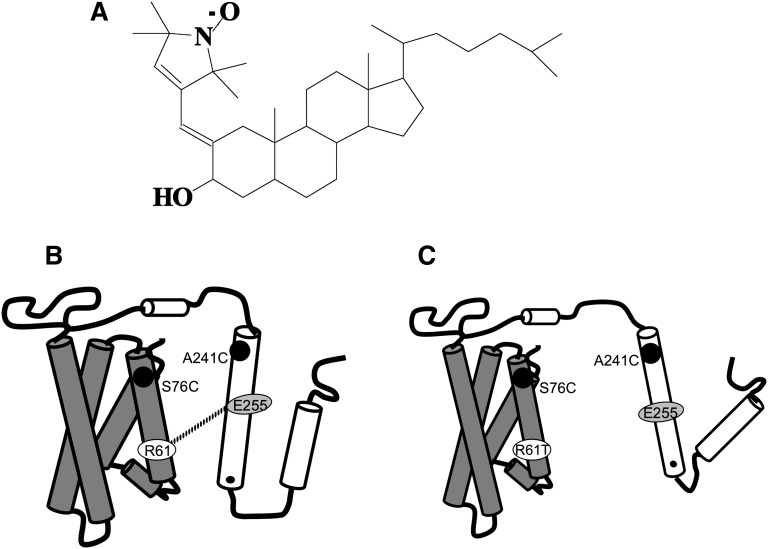
Fig. 1.
A: Structure of spin-labeled cholestanol-3-[17-(1,5dimethyl-hexyl)-3-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenantren-2-ylidenethyl]2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl radical. Its calculated molecular weight based on the elemental analysis (C36H60NO2) is 538.88. Schematic representation of apoE4 (B) and apoE3-like (C) protein structure. In both apoE4 and apoE3, Ala-76 and Ser-241 were mutated to cysteines and labeled with nitroxyl spin label. The dotted line in represents a salt bridge between Arg-61 (R61) and Glu-255 (E255) of apoE4 showing domain interaction. In C, the salt bridge is not shown since Arg-61 is mutated to Thr (R61T), which prevents the domain interaction and is a model for apoE3.
To label lipoproteins, the spin-labeled cholestanol probe of 8 mmol/l was prepared in dimethyl formamide and slowly stirred into the lipoprotein sample to a final concentration of 20 μmol/l in a flat-bottom glass vial with continuous moderate stirring at 37°C for 2 h before the EPR study. In this case, the spin-labeled probe/total cholesterol ratio was 1:65. Significant signal-to-noise issues arise if the spin-labeled probe/total cholesterol ratio is <1:65. The final concentration of the vehicle in the sample did not exceed 0.5% and was kept constant across the samples.
Preparation of synthetic TG-rich emulsions
The TG-rich emulsions were prepared as previously described (12). Briefly, lipids composed of 69% triolein, 22% egg yolk phosphatidylcholine, 6% cholesteryl oleate, and varied concentrations of cholesterol (2–8%) were mixed together and then dried under a stream of nitrogen. The dried mixtures were resuspended in a small aliquot of 10 mM Tris-HCl buffer (pH 8.0) containing 0.1 M KCl and 1 mM EDTA and then emulsified in a bath sonicator at 50°C for an hour or until the homogenous emulsion was formed. Then, the emulsions were extruded using an extruder to form particles by passing 15 to 20 times through polycarbonate membrane of 100 nm pore size. After the extrusion, particles were assayed for their total triglyceride and cholesterol content. Further, the extruded particles were used for apoE binding assay on the day of preparation.
EPR spectroscopy
EPR measurements of apoE4 were performed with a JEOL X-band spectrometer fitted with a loop-gap resonator (8). Spin-labeled apoE in TBS (10 mM Tris, pH 7.4, 150 mM sodium chloride, and 0.005% sodium azide) to a final concentration of 0.2 mg/ml protein was added to plasma or lipoprotein fractions or VLDL spiked, as described in Ref. 8, with 0.5 mM of fatty acids [stearic (18:0), oleic (18:1), linoleic (18:2), or linolenic (18:3) acids], and the ratio between fatty acid and cholesterol content of lipoprotein was maintained at 1:2.6. The samples were loaded into one-sided sealed glass capillaries, incubated at 37°C for 1 h, and scanned by EPR. For all the samples, vehicle controls were used. The spectra were obtained by an average of three scans (2 min each) over 100 G at a microwave power of 2 mW and a modulation amplitude of 1 G at room temperature (20–22°C) or at 37°C. To assess the signal content in guanidine-extracted gel samples, a quartz flat cell was used, and the signal-averaged spectra from six 20-G scans (20 s each) over the central (mI = 0) line were recorded.
Order parameters for the spectra of samples containing spin-labeled lipids were calculated as described (13). For samples containing the 5-DSA label, the order parameter S was calculated as S = [(T‖ – T⊥ − C)/(T‖ + 2T⊥ + 2C)]*1.723, where C = 1.4 – 0.053 (T‖ – T⊥). For the unresolved T‖ in the 12- and 16-DSA spectra, T‖ was estimated from T‖ = 44.5 – 2T⊥.
Statistics
All statistical analyses were performed using ANOVA as guided by SigmaStat software, with pairwise comparisons made using the Holm-Sidak method. Where applicable, data are reported as mean ± SD. Statistical significance was reported for P < 0.001, as indicated.
RESULTS
Selection of apoE4 (76C-241C) to study conformational changes of apoE
Mutagenesis studies (14, 15) have demonstrated that apoE self-associates via a hydrophobic face along the C-terminal domain helix. This intermolecular interface is largely disrupted when apoE4 binds lipids, as measured by the loss dipolar interaction of spin labels placed at position 264 (8, 10, 16). The reduced intermolecular interaction as measured by spin-labeled apoE4(264C) proved to be a useful marker for apoE4 binding to plasma lipids collected pre- and postprandially (8). To explore the influence of lipolysis on the conformation and distribution of apoE4, we used a previously developed intramolecular domain interaction indicator construct (76C-241C) to monitor conformational differences between apoE3 and apoE4 that are predicted to be important in mediating their functional differences (10). In artificial lipid systems [DMPC, emulsions, and dipalmitoylphosphatidylcholine (DPPC)], where elevated concentrations facilitate spin dilution, no evidence of intermolecular spin interaction (arising from labels within 2.0 nm of one another) is observed from either position 76 or position 241 (10, 16).
In the lipid-free state, apoE4 is distinguished by domain interaction between the N-terminal bundle and the C-terminal helix, which is responsible for its binding preference for VLDL (10, 16). Lipid-bound apoE has an extensive helical structure but adopts alternate conformations depending on lipid composition and particle size. For example, lipid-bound apoE3 with extended helical segments has been found on the edge of phospholipid (DMPC or 1-palmitoyl-2-oleoylphosphatidylcholine) particles (17, 18). A similar conformation of apoE4 has been found on TG-rich emulsions (10). However, as shown by X-ray crystallography (19, 20), apoE4 on DPPC particles has a double-helix structure with a hairpin near the midpoint of the protein sequence. EPR measurements showed that proximity of amino acids 76 and 241, indicating domain interaction in the lipid-free protein, is maintained in the DPPC-bound state (16). Thus, the 76-241 pair is useful for assessing domain interaction in the lipid-free protein and for distinguishing between a hairpin or extended helical conformation in the lipid-bound state.
The indicator constructs, which contain cysteines at amino acids 76 and 241, detect the differences in the distance between the two regions of apoE when these sites are covalently modified with spin labels and assessed by EPR spectroscopy (10). Because apoE3 has an endogenous cysteine that would react with a spin label, we used an apoE3 indicator construct with an R61T mutation (10). This construct behaves structurally and functionally like apoE3 because the mutation abolishes domain interaction (21). Specific labeling of apoE4 (which has no cysteines) is achieved by reacting the sulfhydryl-specific nitroxide label (MTS-SL) with apoE4 containing the substitutions S76C and A241C (Fig. 1B). Based on the level of dipolar interaction evident in the EPR spectrum in apoE4 containing these two spin-labeled side chains, the relative distance between the N- and C-terminal domains is evident. Because apoE3 has Cys-112, we used an apoE3-like protein (Fig. 1C) to determine the isoform-dependence of apoE's response to lipolysis (4, 10).
ApoE4 undergoes a greater spectral change upon incubation with postprandial plasma
The dipolar interaction between the spin labels at two sites can be qualitatively determined by the amount of spectral broadening. Therefore, we examined the double-spin-labeled apoE4 and apoE3-like proteins after incubation in postprandial plasma (Fig. 2A). The spectra in Fig. 2A are plotted so that each represents the same number of spins. The R61T mutation increased the distance between the domains in the lipid-free protein, resulting in less spectral broadening, as shown previously (10). Postprandial plasma decreased the dipolar broadening of apoE4. The effect was greatest at 3.5 h, but the broadening was less than that in the apoE3-like sample. This suggests that this population of apoE4 retains some of the conformational features of its lipid-free state in plasma. In contrast, the apoE3-like protein maintained a more open conformation both in the lipid-free state and in plasma. This difference allows a systematic investigation of apoE isoform-specific behavior in the postprandial state. Fasting and postprandial serum lipid values of six of the healthy volunteers who participated in this study are listed in Table 1.
Fig. 2.
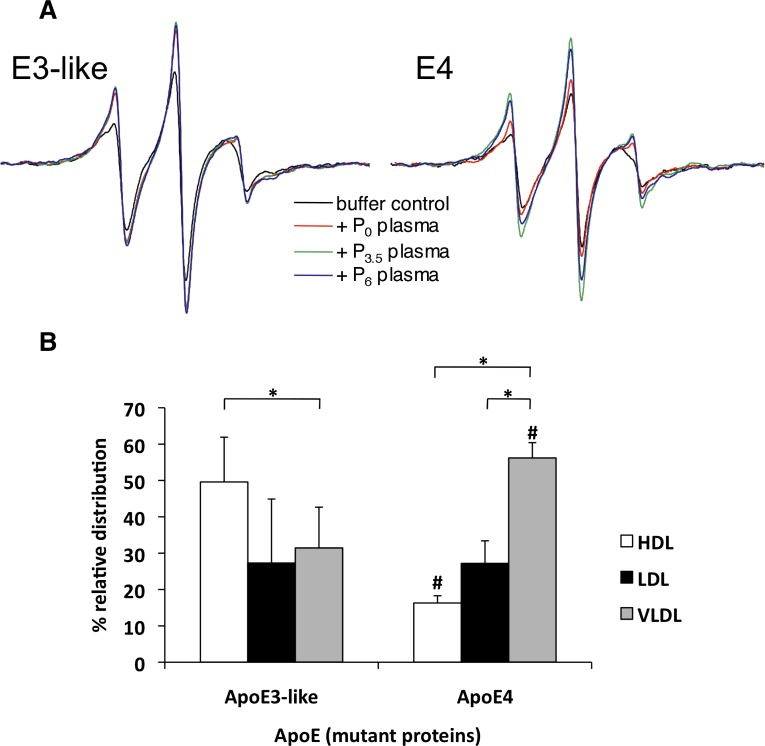
A: Effect of fasting and postprandial plasma (from volunteer 7135) on the EPR spectra of spin- labeled apoE3-like protein and apoE4. ApoE3-like or apoE4 were incubated with pre- or postprandial plasma at 37°C for 1 h before scanning. Each spectrum represents the same number of spins from a spin-labeled apoE3 or apoE4 at a concentration of 0.2 mg/ml. B: Distribution of spin-labeled apoE3-like or apoE4 associated with plasma lipoproteins. The protein samples were incubated at 37°C for 1 h with fasting plasma samples from healthy human subjects. The lipoproteins were separated by gel electrophoresis and stained with Fat Red 7b. The VLDL, LDL, and HDL bands were excised and solubilized into 4.5 mol/l guanidine isocyanate by incubating at 65°C for 3 min. The gel extracts were subjected to EPR spectroscopy. Data represent the average of six independent measurements. Error bars represent SD. *P < 0.001 for significantly different treatments within each apoE (E3/E4) protein group. #P < 0.001 for significantly different treatments within each lipoprotein class.
TABLE 1.
Lipid values of healthy human volunteers recruited for the study (mg/dl)
| Preprandial (0 h) |
Postprandial (3.5 h) |
Postprandial (6 h) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volunteer No. | TG | TC | LDL | HDL | TG | TC | LDL | HDL | TG | TC | LDL | HDL |
| 7125 | 185 | 218 | 140 | 45 | 324 | 234 | 145 | 46 | 217 | 222 | 139 | 41 |
| 9126 | 153 ± 15 | 203 ± 23 | 134 ± 18 | 39 ± 4 | 311 ± 70 | 207 ± 24 | 134 ± 20 | 40 ± 4 | 191 ± 34 | 206 ± 30 | 136 ± 25 | 40 ± 6 |
| 2654 | 143 | 171 | 99 | 57 | 149 | 177 | 98 | 58 | 78 | 170 | 98 | 58 |
| 2020 | 78 | 147 | 75 | 46 | 106 | 150 | 75 | 49 | 58 | 146 | 73 | 47 |
| 2206 | 169 ± 6 | 192 ± 10 | 34.5 ± 0.5 | 124 ± 7 | 290 ± 40 | 191 ± 5 | 127 ± 8 | 35 ± 0 | 208 ± 22 | 198 ± 6 | 133 ± 8 | 34.5 ± 0.5 |
| 6341 | 158 | 216 | 130 | 42 | 376 | 216 | 127 | 38 | 257 | 230 | 138 | 41 |
Volunteers 9126 and 2206 were reinvited for the study; therefore, the data from those volunteers are averages of two independent measurements. The data from volunteers 7125, 2654, 2020, and 6341 are from a single experiment. TG, total TGs; TC, total cholesterol; LDL, direct measurement of LDL-cholesterol; HDL, direct measurement of HDL-cholesterol.
Preferential distribution of spin-labeled apoE3-like protein and apoE4 with plasma lipoproteins
To determine if the apoE3-like and apoE4 mutants have binding preferences similar to those of the native proteins, we incubated fasting plasma from healthy volunteers with spin-labeled apoE3-like protein (61T-76C-241C) or apoE4 (76C-241C) for 1 h at 37°C. The samples were separated by agarose gel electrophoresis. The extent of binding and relative distribution of exogenously added apoE3-like protein or apoE4 with plasma lipoprotein fractions, VLDL, LDL, or HDL, was determined by assaying the amount of spin-labeled proteins by EPR spectroscopy (8). Like the native proteins, apoE3-like protein bound preferentially to HDL (49% vs. 27% to LDL and 31% to VLDL) (Fig. 2B), and apoE4 bound predominantly to VLDL (56% vs. 16% to HDL and 27% to LDL.
Comparison of apoE conformations on lipoprotein particles
To assess the conformation of apoE on lipoprotein fractions, spin-labeled apoE4 and apoE3-like protein were incubated with VLDL, LDL, or HDL at 37°C for 1 h before scanning. Incubation of apoE3-like protein with HDL or VLDL elicited similar reductions in spectral broadening, resulting in spectra with larger amplitudes (Fig. 3A). In contrast, HDL produced only moderate narrowing of the apoE4 spectrum, suggesting that the conformation of the lipid-free protein is largely retained in HDL. Although VLDL generated a greater reduction in broadening than HDL, the broadening did not approach apoE3-like levels (see lipolysis results below).
Fig. 3.
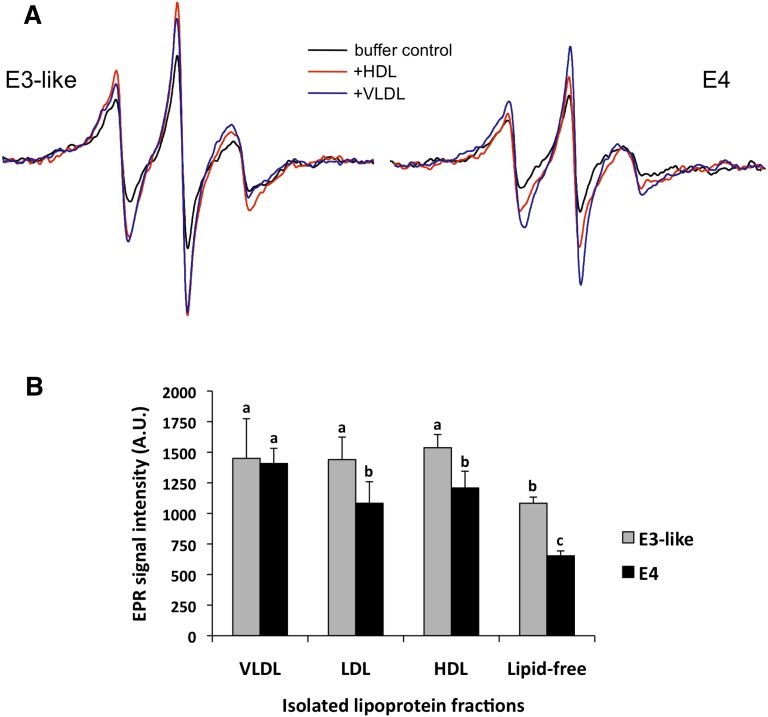
Conformations of apoE3-like protein and apoE4 on VLDL, LDL, or HDL. A: EPR spectra of apoE4 and apoE3-like protein after incubation with VLDL or HDL (from volunteer 7135) or with buffer alone. B: The average change in the central (MI = 0) line width estimated by the peak intensity of the spin-labeled apoE when combined with lipoprotein fractions from three healthy human subjects. Values are the mean ± SD of six independent measurements. Different letters are significantly different from each other (P < 0.001)
To control for individual variations in the properties of lipoproteins from each volunteer, we averaged the change in the center peak (mI = 0) line height amplitude for both apoE4 and the apoE3-like proteins when combined with VLDL, LDL, or HDL from six volunteers (Fig. 3B). For the apoE3-like protein, all the lipoprotein fractions induced an increase of ∼30% over the lipid-free protein. However, for apoE4, the increase in the center peak amplitude was much higher for apoE4 bound to VLDL (2.15-fold) than for apoE4 bound to either HDL (1.8-fold) or LDL (1.7-fold). The proximity of the spin labels in the two domains in apoE4 was most similar to that of apoE3 on VLDL-bound apoE4 (Fig. 3). These data suggest that the conformation of apoE4 is more affected on VLDL than on HDL or LDL. Using synthetic lipids, Hatters et al. (10) found similar apoE4 conformational changes. However, this is the first report to show the differences in apoE4 conformation on native lipoproteins.
Dependence of apoE conformation on postprandial HDL and VLDL
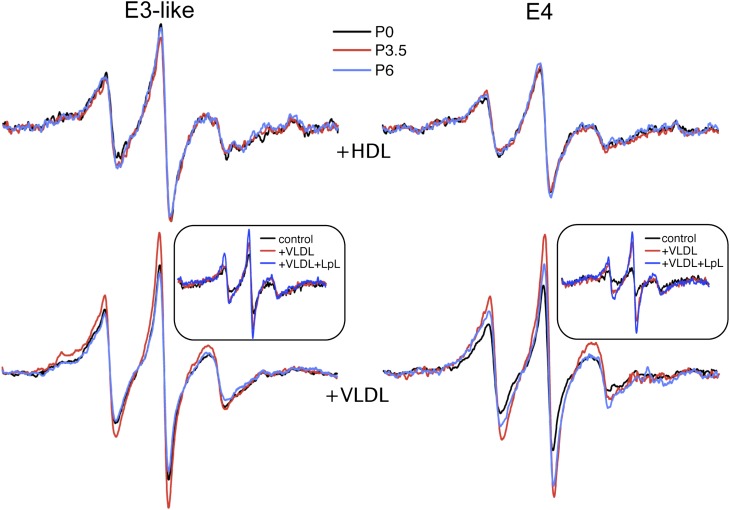
To confirm that the decrease in apoE spin interaction after exposure to postprandial plasma can be attributed to the VLDL-associated protein, we incubated apoE4 and apoE3-like proteins with lipoprotein fractions isolated from fasting plasma (P0) or postprandial plasma obtained at 3.5 (P3.5) or 6 h (P6). P6 plasma served as an internal control to P0 plasma. ApoE4 maintained the proximity of the 76 and 241 probes to a larger extent on HDL (Fig. 4). In contrast with the effect seen when combined with the mixture of lipoproteins in plasma (Fig. 2A), postprandial HDL did not influence the conformation of apoE4, as judged by the spectrum of the doubly labeled protein (Fig. 4). Similarly, the conformation of the apoE3-like protein was not altered by incubation with HDL fractions from the fasting (P0) or postprandial states (P3.5 and P6). However, both spin-labeled apoE4 and the apoE3-like proteins displayed significant spectral narrowing after exposure to P3.5 VLDL (Fig. 4).
Fig. 4.
Postprandial apoE conformation is modulated by VLDL but not HDL. Spectra of apoE4 or apoE3-like protein containing spin labels at positions 76 and 241 in combination with HDL or VLDL fractions isolated from volunteers at either the fasting P(0) or postprandial time points (3.5 and 6 h). Insets: Lipolysis-induced structural changes in apoE-associated with VLDL. The indicated protein was incubated with VLDL or LpL-treated VLDL at 37°C for 1 h before scanning. An incubation of the protein in buffer alone is also shown as reference. All samples contained 0.2 mg/ml apoE.
VLDL lipolysis affects the conformation of apoE
Because increased lipolytic activity is associated with the postprandial state, we examined the effect of VLDL lipolysis on apoE conformation. Equal amounts of spin-labeled apoE3-like protein or apoE4 were incubated with LpL-treated or untreated postprandial VLDL for 1 h at 37°C before scanning. Compared with lipid-free apoE4 in buffer, apoE4 incubated with postprandial VLDL generated a 2.7-fold greater signal intensity, which increased to 3.5-fold when apoE4 was incubated with LpL-treated VLDL (Fig. 4, inset). ApoE3-like protein showed a 1.4-fold increase in signal intensity when treated with VLDL and a 1.8-fold increase when treated with LpL-treated VLDL.
To determine if lipolyzed VLDL has higher affinity for apoE4, we used agarose gel electrophoresis to examine apoE4 that had been incubated with VLDL or LpL-treated VLDL. The VLDL bands on the gels were visualized by staining, and the gel slices were solubilized in 4.5 mol/l guanidine isothiocyanate by incubating at 65°C for 3 min. We measured the signal from the samples to determine the amount of exogenously added protein associated with VLDL or LpL-treated VLDL. Lipolysis did not significantly alter the association of VLDL with either apoE4 or apoE3-like protein. Variations in association of apoE4 with VLDL or LpL-treated VLDL were negligible. Therefore, the differences in the EPR signal amplitude reflect changes in apoE4 conformation, not the amount of apoE4 bound to the lipoproteins.
Lipid fluidity of lipoproteins
Next, we used DSA probes to investigate whether differences in lipoprotein particle fluidity influence the structure of apoE. To measure particle fluidity, we added 5-DSA, 12-DSA, or 16-DSA (2 µmol/l) to HDL, LDL, VLDL, or LpL-treated VLDL and subjected the lipoproteins to EPR spectroscopy. The spin labels in each probe showed restricted movement upon equilibration with LDL or HDL but relatively free mobility upon equilibration with VLDL (Fig. 5). Increased mobility of the spin label, indicated by a narrower and sharper spectrum, can be quantified by a decrease in the order parameter S (Table 2). These results show that VLDL is more fluid than LDL and HDL.
Fig. 5.
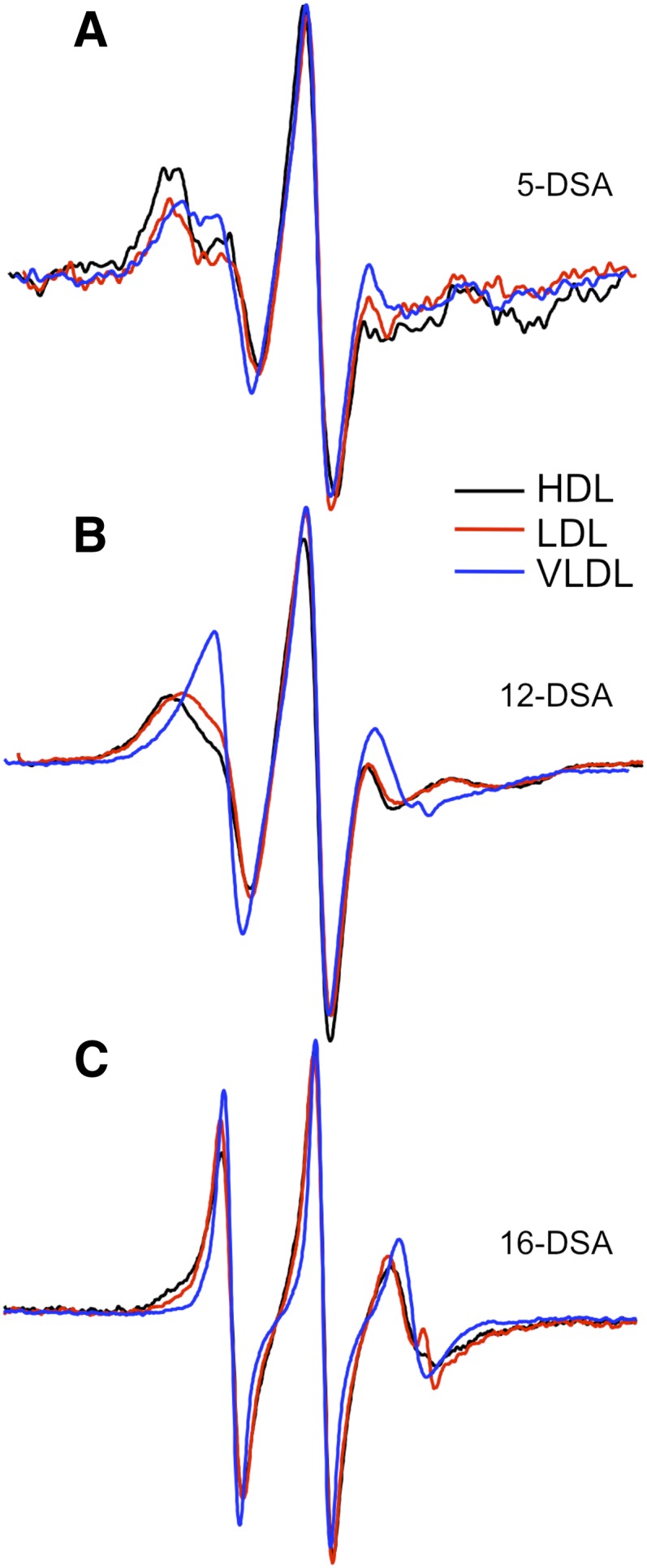
Lipid fluidity of classes of lipoproteins. VLDL, LDL, and HDL were isolated from fasting plasma by density gradient centrifugation, mixed with nitoxyl spin-labeled stearic acid, and subjected to EPR spectroscopy. A, 2 µmol/l 5-DSA; B, 2 µM 12-DSA; and C, 2 µM 16-DSA.
TABLE 2.
Order parameters for spin-labeled fatty acids in isolated plasma lipid fractions
| Fraction | 5-DSA | 12-DSA | 16-DSA |
|---|---|---|---|
| HDL | 0.78 | 0.58 | 0.29 |
| LDL | 0.79 | 0.58 | 0.30 |
| VLDL | 0.59 | 0.44 | 0.21 |
| VLDL + LpL | 0.58 | 0.38 | 0.15 |
Effect of lipolysis on TGRL fluidity
We investigated the role of LpL in the increased fluidity of postprandial lipoproteins. The effect of LpL treatment on the lipid order within VLDL and chylomicrons also was examined using spin-labeled fatty acids (Fig. 6A). Both the 12- and 16-DSA probes displayed clear narrowing of the lines and lowered order parameters (Table 2) with lipolysis treatment, revealing greater mobility, and increased rates of motion, indicating a more fluid environment. Lipolysis had little effect on the 5-DSA probe, suggesting that the headgroup region is less affected by lipolysis.
Fig. 6.
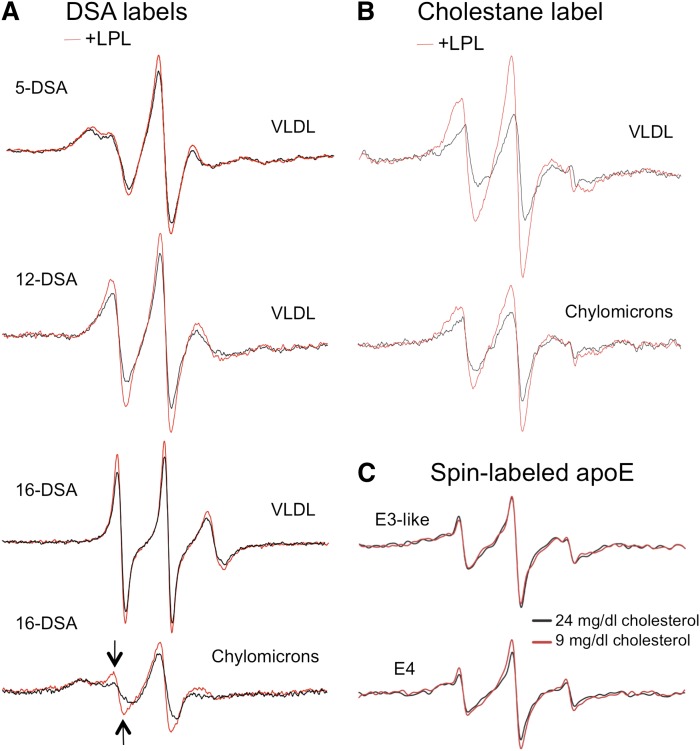
Effect of lypolysis on fluidity of TGRL. A: The effect of lipolysis on the EPR spectra of doxyl-labeled stearic acid in VLDL (top three sets) or chylomicrons (bottom set). In each experiment, an equal amount of TGRL sample (200 mg/dl TG) was pretreated with LpL for 30 min to generate lypolysis products prior to addition of the 16-DSA spin label. The arrows in the chylomicron spectrum highlight the increase in the population of highly mobile lipids when the sample is pretreated with LpL (red tracing) as opposed to without LpL pretreatment (black tracing) indicating an increase in lipid fluidity after LpL treatment. B: Effect of lipolysis spin-labeled cholestanol was added slowly while stirring the lipoprotein sample at 37°C and incubated for 2 h prior to scanning. C: Domain interaction of apoE on synthetic lipid particles containing two different concentrations of cholesterol (9 mg/dl or 24 mg/dl). Particles were prepared from synthetic TG rich emulsions as described in Materials and Methods and then incubated with spin-labeled apoE4 or the E3-like protein for 1 h at 37°C, followed by EPR spectroscopy.
To investigate the actions of lipolysis on TGRL fluidity, we used fatty acid spin probes (doxyl stearic acid) and a cholesterol spin probe (spin-labeled cholestanol) to study lipid fluidity changes. We incubated TGRL, untreated or treated with LpL, with the spin-labeled cholestanol spin probe (20 µmol/l) and performed EPR spectroscopy to assess the mobility of cholesterol-rich domains. Lipolysis increased the fluidity TGRL (both chylomicrons and VLDL) (Fig. 6B). The influence of deceased TGRL fluidity was also explored by preparing triolein-phosphatidylcholine emulsions of differing cholesterol content, which is expected to reduce lipid fluidity at higher levels. As shown in Fig. 6C, apoE4 displays a slightly higher level of domain interaction when incubated with TGLR mimics containing the higher cholesterol level, while no difference was detected for the apoE3-like sample. In summary, the data with various lipid probes indicate that lipolysis increases the fluidity of lipoproteins, and when added to the previous data, suggest that the proximity of positions in apoE indicative of domain interaction provide a marker of lipolysis activity and thereby lipoprotein fluidity.
To further establish the relationship between lipoprotein fluidity and effects on the conformation of apoE4, we spiked VLDL with fatty acids (final concentration, 0.5 mmol/l) of 18-carbon chain with different degrees of unsaturation. Linoleic (18:2) and linolenic (18:3) fatty acids (28% and 35%) were more effective than stearic (18:0) and oleic (18:1) fatty acids (12% and 18%) in reducing domain interaction of apoE4 (data not shown).
Finally, to evaluate the compositional changes in VLDL after lipolysis, we assayed the free fatty acid content of postprandial VLDL (350 mg/dl TG) in the absence of LpL treatment. Lipolysis of VLDL (3 U/ml) increased the levels of NEFAs by 15-fold (from 0.04 to 0.655 mmol/l). Total NEFA content in fasting and postprandial VLDL were not significantly different. However, upon lipolysis fasting, VLDL NEFA content increased about 5- to 7-fold, whereas lipolysis of postprandial VLDL yielded a 12- to 18-fold increase in NEFA content. Changes in total TG content could not be detected by our assay, which could be explained because the TG assay measures glycerol, and glycerol is the surrogate for TG measurement, no change in TG is expected. Postprandial free fatty acid content in human plasma can be as high as 0.35–0.65 mmol/l and depends strongly on diet (22). Thus, NEFA levels may play an important role in altering TGRL fluidity and apoE conformation.
DISCUSSION
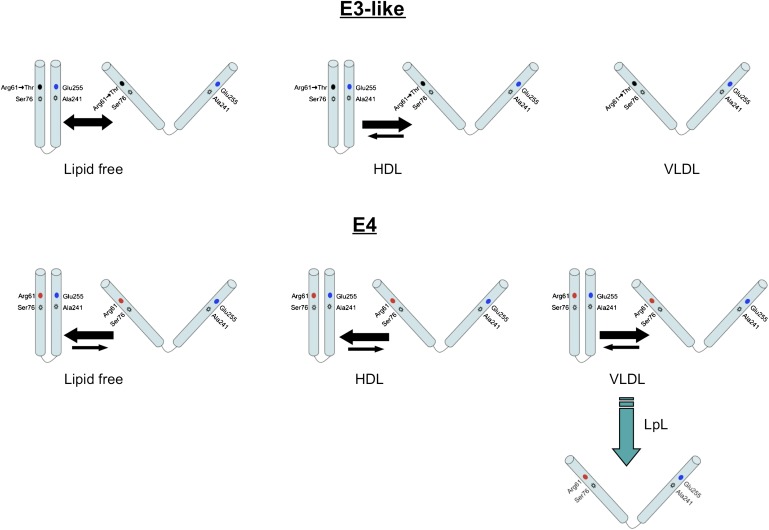
In this study, we used EPR spectroscopy to investigate conformational changes of apoE4 induced by VLDL lipolysis products. Our findings show that the conformation of apoE4 is modulated postprandially and is mediated by VLDL particle fluidity. ApoE4 was more sensitive to surface lipid fluidity than apoE3, and lipid fluidity was inversely related to the proximity of regions in the N- and C-terminal domains of apoE4. VLDL was much more fluid than LDL and HDL, and VLDL-associated apoE4 had a more extended conformation than when associated with LDL or HDL. Our findings suggest a model for apoE association with lipid particles (Fig. 7). These conformational changes may be important in lipoprotein-vascular cell binding and possibly in vascular injury.
Fig. 7.
Schematics of apoE3-like and apoE4 conformations on in a lipid-free environment and associated with HDL and VLDL. The level of spectral broadening in spin-labeled apoE can be explained by the proximity of the C-terminal and N-terminal domains, which differs, depending on the isoform, lipoprotein association, and particle fluidity. The size of the arrows indicates the level of the distribution of species. The predominant species found in each condition is indicated by the larger arrow and vice versa.
Lipid association induces structural rearrangements in apoE. The conformation of apoE4 differs, depending on whether it is complexed to phospholipid alone or TG-rich emulsions (10). Plasma lipoproteins are highly variable in size and composition, and apoE4 and apoE3 have different preferences for plasma lipoproteins. However, no reports examine these lipoproteins for different effects on apoE conformation. ApoE4 showed different conformations on VLDL versus HDL and LDL, showing a more dramatic effect on VLDL compared with HDL or LDL. ApoE3 conformation was relatively unchanged by association with these different lipoprotein classes. The reason for the apoE4 structural conformational change is not known. Studies with model lipoproteins have shown that molecular packing of lipids and the structures of lipids and apolipoproteins are important determinants of the equilibrium binding and kinetics of transfer of apolipoproteins (23–26). Among lipoproteins, surface lipid fluidity increased in the order HDL < LDL < VLDL and varied inversely with their (protein + cholesterol/phospholipid) ratios (27).
We used the EPR mutant clones of apoE [apoE4 (76C-241C) and apoE3-like protein (R61T, 76C, 241C)] to monitor conformational effects. Like the native apoE proteins, spin-labeled apoE4 preferentially associated with VLDL and apoE3-like protein with HDL after a moderately high-fat meal. Thus, the mutations in these proteins did not affect their structural integrity or their preference for plasma lipids.
Our findings are consistent with earlier work by Massey and Pownall (27), who used fluorescent probes to find a similar fluidity for HDL and LDL but a more fluid environment for VLDL. They showed a positive correlation between surface fluidity of lipoprotein particles with reactivity of phospholipase A2. Gorshkova, Menschikowski, and Jaross (28) showed that phospholipase A2 treatment increases order at the headgroup (as detected by 5-DSA). This is consistent with the insignificant change in VLDL fluidity by the 5-DSA probe. Thus, the surface density may be relatively unaffected, while the core becomes much more fluid. Foucher et al. (29) found that PUFAs make lipoproteins more fluid than saturated. Previously, we showed that adding palmitate to VLDL reduced C-terminal (i.e., intermolecular) interaction and promoted the transition of apoE4 from a tetrameric state to a monomeric state (8). To establish the relationship between lipoprotein fluidity and conformational changes in of apoE4, we tested the effect of 18 carbon fatty acids with different degrees of unsaturation. Linoleic (18:2) and linolenic (18:3) fatty acids showed a greater effect than stearic (18:0) and oleic (18:1) fatty acids in reducing the proximity of the N- and C-terminal regions of apoE4.
Our previous article (8) showed a dramatic change in apoE4 conformation 3.5 h after consumption when compared with fasting apoE4 conformation in healthy humans. Our goal was to determine the mechanism of this clinically relevant change in apoE4 conformation in the postprandial state. Previous studies, including our own, suggested that VLDL lipolysis products could mediate this change. Since the postprandial state is a period of heightened lipolysis, we sought to model the postprandial state by treating postprandial VLDL with lipoprotein lipase. Our studies mimic the clinical findings in the postprandial state and provide strong evidence that VLDL lipolysis products mediate the change in particle fluidity that cause apoE4 conformational changes in the postprandial state.
During lipolysis, fatty acids accumulate at the lipoprotein surface because the rate of fatty acid transfer is much slower than lipolysis (30, 31). Accumulation of fatty acids on the lipoprotein surface could mediate changes in lipoprotein fluidity. Therefore, we investigated lipolysis-induced changes in the fluidity of the particle and its association with structural conformation of apoE.
ApoE4 is pro-inflammatory and associated with increased risk of atherosclerosis and Alzheimer's disease; however, the mechanisms are not known with certainty. Our studies indicate that the postprandial state can significantly alter the structural conformation of apoE4. Further research is necessary to determine whether postprandial conformational changes in apoE4 affect its interactions with cells and contribute to atherogenicity. Postprandial hyperlipemia occurs several times daily, and postprandial lipoproteins could injure arterial endothelium and can cause repetitive arterial injury (32, 33). Because postprandial hyperlipemia has been implicated in the development of atherosclerosis via repetitive injury to the arterial endothelium (34), understanding the role of the apoE isoforms and their conformation in modulating pro-inflammatory processes may be very important in atherogenesis.
Our study provides direct evidence that apoE assumes different conformations, depending on its association with lipoprotein species. ApoE4 preferentially binds to VLDL, but the reasons for its preference are not known. We showed that apoE4 assumes a more linear conformation on VLDL particles. The increase in free fatty acid content in VLDL due to lipolysis is accompanied by an increase in lipid fluidity, which affects apoE4 conformation (Fig. 7). From these results, we demonstrate that the conformation of apoE4 is influenced by lipoprotein classes and lipid fluidity. More fluid particles may promote the partition of apoE side chains (favoring lipid-protein vs. protein-protein interactions). Such modulations in apoE4 structure due to changes in lipoprotein fluidity, especially during postprandial state, may regulate lipoprotein binding and uptake by cellular receptors. Additional studies are needed to understand the relationship between lipoprotein particle fluidity and its relevance to cardiovascular and neurological disease.
CONCLUSIONS
Our study shows differential structural conformation of apoE4 bound to VLDL versus LDL and HDL. Furthermore, lipolysis products of VLDL significantly affect the conformation of apoE4 during the postprandial state by increasing VLDL fluidity. Future studies will explore whether the change in structural conformation of apoE4 has effects on its binding and internalization of apoE4 by cellular receptors. Such studies will increase our understanding of the relevance of apoE4 structural changes in the postprandial state to initiation and contribute to vascular inflammation and atherosclerotic cardiovascular disease.
Acknowledgments
The authors thank Danielle Baute and Kit Ng for their careful work. The authors also would like to acknowledge Theresa Tonjes of the Clinical Nutrition Research Unit at the University of California, Davis, for her assistance in the quantification of lipoproteins and lipolysis products. Supplies and facilities for part of this study were provided by the Western Human Nutrition Research Center, Ragel Facility, and the Clinical Nutrition Research Unit at the University of California, Davis.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- DMPC
- dimyristoylphosphatidylcholine
- DSA
- doxyl stearic acid
- EPR
- electron paramagnetic resonance
- LpL
- lipoprotein lipase
- TG
- triglyceride
The authors acknowledge the support of the Treadwell Innovative Research Grants Program, the Center for Health and Nutrition Research, and National Institutes of Health Grants HL-HL71488, HL-55667, RO1 AG 028793, and R01 AG029246. The authors also acknowledge that part of the work is supported by the Hungarian National Research Fund (OTKA T048334). Part of this investigation was conducted at a facility constructed with support from Research Facilities Improvement Program Grant C06 RR-12088-01 from the National Center for Research Resources, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Davignon J. 2005. Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler. Thromb. Vasc. Biol. 25: 267–269. [DOI] [PubMed] [Google Scholar]
- 2.Weisgraber K. H. 1990. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J. Lipid Res. 31: 1503–1511. [PubMed] [Google Scholar]
- 3.Wetterau J. R., Aggerbeck L. P., Rall S. C., Jr., Weisgraber K. H. 1988. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J. Biol. Chem. 263: 6240–6248. [PubMed] [Google Scholar]
- 4.Dong L. M., Weisgraber K. H. 1996. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem. 271: 19053–19057. [DOI] [PubMed] [Google Scholar]
- 5.Saito H., Dhanasekaran P., Baldwin F., Weisgraber K. H., Phillips M. C., Lund-Katz S. 2003. Effects of polymorphism on the lipid interaction of human apolipoprotein E. J. Biol. Chem. 278: 40723–40729. [DOI] [PubMed] [Google Scholar]
- 6.Saito H., Lund-Katz S., Phillips M. C. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 43: 350–380. [DOI] [PubMed] [Google Scholar]
- 7.Oda M. N., Forte T. M., Ryan R. O., Voss J. C. 2003. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat. Struct. Biol. 10: 455–460. [DOI] [PubMed] [Google Scholar]
- 8.Tetali S. D., Budamagunta M. S., Voss J. C., Rutledge J. C. 2006. C-terminal interactions of apolipoprotein E4 respond to the postprandial state. J. Lipid Res. 47: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 9.Morrow J. A., Arnold K. S., Weisgraber K. H. 1999. Functional characterization of apolipoprotein E isoforms overexpressed in Escherichia coli. Protein Expr. Purif. 16: 224–230. [DOI] [PubMed] [Google Scholar]
- 10.Hatters D. M., Budamagunta M. S., Voss J. C., Weisgraber K. H. 2005. Modulation of apolipoprotein E structure by domain interaction: differences in lipid-bound and lipid-free forms. J. Biol. Chem. 280: 34288–34295. [DOI] [PubMed] [Google Scholar]
- 11.Cohn J. S., Wagner D. A., Cohn S. D., Millar J. S., Schaefer E. J. 1990. Measurement of very low density and low density lipoprotein apolipoprotein (Apo) B-100 and high density lipoprotein Apo A-I production in human subjects using deuterated leucine. Effect of fasting and feeding. J. Clin. Invest. 85: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida K. A., Schreiber R., Amancio R. F., Bydlowski S. P., Debes-Bravo A., Issa J. S., Strunz C. M., Maranhao R. C. 2003. Metabolism of chylomicron-like emulsions in carriers of the S447X lipoprotein lipase polymorphism. Clin. Chim. Acta. 335: 157–163. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T., Hidaka T., Ogura R., Sugiyama M. 1988. Changes of membrane fluidity and Na+,K+-ATPase activity during cellular differentiation in the guinea pig epidermis. Arch. Dermatol. Res. 280: 29–32. [DOI] [PubMed] [Google Scholar]
- 14.Fan D., Li Q., Korando L., Jerome W. G., Wang J. 2004. A monomeric human apolipoprotein E carboxyl-terminal domain. Biochemistry. 43: 5055–5064. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Vasudevan S., Sojitrawala R., Zhao W., Cui C., Xu C., Fan D., Newhouse Y., Balestra R., Jerome W. G., et al. 2007. A monomeric, biologically active, full-length human apolipoprotein E. Biochemistry. 46: 10722–10732. [DOI] [PubMed] [Google Scholar]
- 16.Hatters D. M., Voss J. C., Budamagunta M. S., Newhouse Y. N., Weisgraber K. H. 2009. Insight on the molecular envelope of lipid-bound apolipoprotein E from electron paramagnetic resonance spectroscopy. J. Mol. Biol. 386: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta V., Narayanaswami V., Budamagunta M. S., Yamamato T., Voss J. C., Ryan R. O. 2006. Lipid-induced extension of apolipoprotein E helix 4 correlates with low density lipoprotein receptor binding ability. J. Biol. Chem. 281: 39294–39299. [DOI] [PubMed] [Google Scholar]
- 18.Narayanaswami V., Maiorano J. N., Dhanasekaran P., Ryan R. O., Phillips M. C., Lund-Katz S., Davidson W. S. 2004. Helix orientation of the functional domains in apolipoprotein e in discoidal high density lipoprotein particles. J. Biol. Chem. 279: 14273–14279. [DOI] [PubMed] [Google Scholar]
- 19.Peters-Libeu C. A., Newhouse Y., Hall S. C., Witkowska H. E., Weisgraber K. H. 2007. Apolipoprotein E*dipalmitoylphosphatidylcholine particles are ellipsoidal in solution. J. Lipid Res. 48: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 20.Peters-Libeu C. A., Newhouse Y., Hatters D. M., Weisgraber K. H. 2006. Model of biologically active apolipoprotein E bound to dipalmitoylphosphatidylcholine. J. Biol. Chem. 281: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 21.Dong L. M., Wilson C., Wardell M. R., Simmons T., Mahley R. W., Weisgraber K. H., Agard D. A. 1994. Human apolipoprotein E. Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J. Biol. Chem. 269: 22358–22365. [PubMed] [Google Scholar]
- 22.Cantwell M. M., Flynn M. A., Gibney M. J. 2006. Acute postprandial effect of hydrogenated fish oil, palm oil and lard on plasma cholesterol, triacylglycerol and non-esterified fatty acid metabolism in normocholesterolaemic males. Br. J. Nutr. 95: 787–794. [DOI] [PubMed] [Google Scholar]
- 23.McKeone B. J., Massey J. B., Knapp R. D., Pownall H. J. 1988. Apolipoproteins C-I, C-II, and C-III: kinetics of association with model membranes and intermembrane transfer. Biochemistry. 27: 4500–4505. [DOI] [PubMed] [Google Scholar]
- 24.Derksen A., Small D. M. 1989. Interaction of ApoA-1 and ApoE-3 with triglyceride-phospholipid emulsions containing increasing cholesterol concentrations. Model of triglyceride-rich nascent and remnant lipoproteins. Biochemistry. 28: 900–906. [DOI] [PubMed] [Google Scholar]
- 25.Ibdah J. A., Lund-Katz S., Phillips M. C. 1989. Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry. 28: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 26.Small D. M., Clarke S. B., Tercyak A., Steiner J., Gantz D., Derksen A. 1991. The lipid surface of triglyceride-rich particles can modulate (apo)protein binding and tissue uptake. Adv. Exp. Med. Biol. 285: 281–288. [DOI] [PubMed] [Google Scholar]
- 27.Massey J. B., Pownall H. J. 1998. Surface properties of native human plasma lipoproteins and lipoprotein models. Biophys. J. 74: 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorshkova I. N., Menschikowski M., Jaross W. 1996. Alterations in the physiochemical characteristics of low and high density lipoproteins after lipolysis with phospholipase A2. A spin-label study. Biochim. Biophys. Acta. 1300: 103–113. [DOI] [PubMed] [Google Scholar]
- 29.Foucher C., Lagrost L., Maupoil V., le Meste M., Rochette L., Gambert P. 1996. Alterations of lipoprotein fluidity by non-esterified fatty acids known to affect cholesteryl ester transfer protein activity. An electron spin resonance study. Eur. J. Biochem. 236: 436–442. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F., Kamp F., Hamilton J. A. 1996. Dissociation of long and very long chain fatty acids from phospholipid bilayers. Biochemistry. 35: 16055–16060. [DOI] [PubMed] [Google Scholar]
- 31.Massey J. B., Bick D. H., Pownall H. J. 1997. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys. J. 72: 1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyson D., Rutledge J. C., Berglund L. 2003. Postprandial lipemia and cardiovascular disease. Curr. Atheroscler. Rep. 5: 437–444. [DOI] [PubMed] [Google Scholar]
- 33.Burdge G. C., Calder P. C. 2005. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br. J. Nutr. 93: 3–9. [DOI] [PubMed] [Google Scholar]
- 34.Eiselein L., Wilson D. W., Lame M. W., Rutledge J. C. 2007. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am. J. Physiol. Heart Circ. Physiol. 292: H2745–H2753. [DOI] [PubMed] [Google Scholar]