Abstract
n-3 polyunsaturated fatty acids (PUFAs) modify T-cell activation, in part by remodeling lipid composition; however, the relationship between n-3 PUFA and B-cell activation is unknown. Here we tested this relationship in vitro and ex vivo by measuring upregulation of B-cell surface molecules, the percentage of cells activated, and cytokine secreted in response to lipopolysaccharide (LPS) activation. In vitro, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) improved the membrane n-6/n-3 PUFA ratio, and DHA lowered interleukin (IL)-6 secretion; overall, n-3 PUFAs did not suppress B-cell activation compared with BSA, oleate, or elaidate treatment. Palmitate treatment suppressed the percentage of B cells activated through lipoapoptosis, which was differentially prevented by cosupplementing cells with MUFAs and PUFAs. Ex vivo, we tested the hypothesis with mice fed a control or high-fat saturated, hydrogenated, MUFA or n-3 PUFA diets. n-3 PUFAs had no effect on the percentage of B cells activated. Unexpectedly, the n-3 PUFA diet increased B-cell CD69 surface expression, IL-6 and IFNγ secretion, and it significantly increased body weight gain. Overall, we propose that changes in lipid composition with n-3 PUFA and suppression of lymphocyte activation is not universal. The study highlights that high-fat n-3 PUFA diets can promote pro-inflammatory responses, at least from one cell type.
Keywords: membrane organization, lymphocyte activation, polyunsaturated fatty acid
n-3 polyunsaturated fatty acids (PUFAs) are increasingly recognized to have therapeutic value as immunosuppressants for the treatment of autoimmunity or inflammation-associated disorders such as rheumatoid arthritis, colitis, and cardiovascular disease (1–4). There are also data to suggest that consumption of n-3 PUFAs can increase susceptibility to infection (5, 6). For example, a recent study by Schwerbrock et al. showed that mice fed diets rich in fish oil had increased susceptibility to influenza infection relative to controls (7). To enhance the use of n-3 PUFAs for the treatment of inflammatory disease symptoms and to minimize their potential drawback of increased infection, it is essential to identify the targets and mechanisms of these fatty acids.
One major cellular target of n-3 PUFAs is naïve lymphocyte activation. Several studies have established that n-3 PUFAs suppress the activation of naïve CD4+ T cells in response to various antigens (8–12). Naïve T cells isolated from fat-1 transgenic or fish oil fed mice do not activate and proliferate as efficiently as controls (13). Similar observations have been made with in vitro studies with T cells (12, 14). A few laboratories have also reported that n-3 PUFAs suppress the activation of dendritic cells (DC) in response to stimulation with lipopolysaccharide (LPS). The Stulnig laboratory showed that the n-3 PUFA eicosapentaenoic acid (EPA) suppressed the ability of human monocytes to differentiate to DCs after pulsing with LPS, which in turn diminished T-cell activation (15). This correlated with a change in the lipid composition of the DC plasma membrane. Another study reported that n-3 PUFAs suppress LPS-mediated activation of naïve DCs by suppressing p38 phosphorylation; however, the relationship between lipid composition and DC activation was not studied (16). From a mechanistic perspective, the inhibition in activation of CD4+ T cells, and possibly DCs, has been linked, in part, to an improvement in the membrane n-6/n-3 ratio. In the case of CD4+ T cells, n-3 PUFAs suppressed cellular activation, in part by modifying the organization of sphingolipid-cholesterol–rich lipid rafts by accumulating n-3 PUFAs into lipid rafts (13, 17). This disrupted protein lateral organization and inhibited subsequent signaling necessary for lymphocyte activation.
While there are several studies on n-3 PUFAs and activation of CD4+ T cells and DCs, the effects of n-3 PUFAs on B lymphocyte activation have not been addressed. In the present study, we tested the hypothesis that an improvement in the n-6/n-3 PUFA ratio would contribute to a suppression of B-lymphocyte activation. Our in vitro data show that although n-3 PUFAs improve the n-6/n-3 PUFA ratio, they generally do not inhibit B-cell activation. We do find that palmitate suppresses B-cell activation through lipoapoptosis, and MUFAs and n-3 PUFAs differentially prevent the effects of palmitate. Ex vivo, contrary to expectations, the data show that n-3 PUFAs promoted the secretion of B-cell pro-inflammatory cytokines and increased body weight gain, relative to controls.
MATERIALS AND METHODS
Cells and fatty acid treatment
Primary B220+ B cells were purified from the spleens of male C57BL/6 mice (age 4–8 weeks) using negative selection (Miltenyi Biotech). All of the experiments with mice fulfilled the guidelines established by the East Carolina University Brody School of Medicine for euthanasia and humane treatment. Naive B220+ B cells were treated with 25–50 µM FFA complexed to BSA at a ratio of 1.5/1, as described previously (18). The fatty acids used in this study were palmitic acid (PA), oleic acid (OA), elaidic acid (ELA), EPA, and docosahexaenoic acid (DHA). Cells were activated with 100 ng/ml LPS (Sigma) in RPMI 1640 1× and treated with fatty acids in the presence of 5% FBS, 2 mM glutamine, and 1% penicillin/streptomyocin at 37°C in a 5% CO2 incubator for up to 48 h.
Diets and mice
C57BL/6 male mice (age 4–8 weeks) were placed on one of five experimental diets for 1, 17, or 98 days and fed ad libitum. The rationale for selecting these time points was the following: 1 day to measure the effects of very short-term feeding; 17 days to essentially match existing short-term feeding studies on n-3 PUFAs (19); and 98 days to model long-term feeding. The diets provided 5.0% or 20% fat by weight, respectively, for the normal diet (ND) or high-fat diets (Harlan-Teklad). High-fat diets were enriched with either saturated (SFA), hydrogenated (HFA), monounsaturated (MUFA), or n-3 polyunsaturated (PUFA) fatty acids. The composition of the diets is presented in Table 1. For ND, ∼13% of the total kcal is from fat (Harlan-Teklad). Of the 13%, the distribution is 2% SFA, 3% MUFA, 8% mixture of PUFAs. For the high fat diets, approximately 41% of the total kcal is from fat (Harlan-Teklad). The distribution of total kcal of fat in the high-fat diets is: 30% SFA, 8% MUFA, and 3% PUFA for the SFA-rich diet; 11% SFA, 23% MUFA, and 7% PUFA for the HFA-rich diet; 7% SFA, 28% MUFA, and 6% PUFA for the MUFA-rich diet; 8% SFA, 9% MUFA and 24% PUFA for the n-3 PUFA–rich diet. Of the 24% PUFAs, about 2% of the total kcal is from DHA, 3% from EPA, 11% from 18:3 n-3 PUFAs, and the remaining n-6 PUFAs.
TABLE 1.
Composition of experimental diets
| Ingredients | ND | SFA-rich Diet | HFA-rich Diet | MUFA-rich Diet | n-3 PUFA-rich Diet |
|---|---|---|---|---|---|
| Casein | 220.0 | 220.0 | 220.0 | 220.0 | 220.0 |
| L-Cystine | 2.5 | 3.0 | 3.0 | 3.0 | 3.0 |
| Corn starch | 370.0 | 173.9 | 173.9 | 173.9 | 173.9 |
| Malodextrin | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 |
| Sucrose | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 |
| Cellulose (fiber) | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Flaxseed oil | 0.0 | 0.0 | 0.0 | 0.0 | 92.5 |
| Fish oil (Menhaden) | 0.0 | 0.0 | 0.0 | 0.0 | 92.5 |
| Vegetable shortening | 0.0 | 0.0 | 185.0 | 0.0 | 0.0 |
| Anhydrous milkfat | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| Coconut oil | 0.0 | 85.0 | 0.0 | 0.0 | 0.0 |
| Soybean oil | 50.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Olive oil | 0.0 | 0.0 | 0.0 | 185.0 | 0.0 |
| Mineral mix, AIN-93M-MX | 35.0 | 42.0 | 42.0 | 42.0 | 42.0 |
| Vitamin mix, AIN-93-VX | 15.0 | 18.0 | 18.0 | 18.0 | 18.0 |
| Choline chloride | 2.5 | 3.0 | 3.0 | 3.0 | 3.0 |
| TBHQ | 0.02 | 0.06 | 0.06 | 0.06 | 0.06 |
C57BL/6 mice were fed a normal diet (ND) or high-fat diets enriched in either saturated (SFA), hydrogenated (HFA), monounsaturated (MUFA), or n-3 polyunsaturated (PUFA) fatty acids. Values are g/kg. TBHQ, tert-Butylhydroquinone.
At the end of the feeding period, splenocytes or B cells were isolated from the spleens and activated with 100 ng/ml LPS in RPMI with 2 mM glutamine, 1% penicillin/streptomyocin at 37°C in a 5% CO2 incubator for up to 48 h. The cells were activated with LPS in either serum-free conditions or with 5% FBS, as indicated in the text. After activation, the majority of the cells were B220+. We did not use homologous serum because we observed that some batches of serum did not activate lymphocytes irrespective of the type of diet. This was likely due to red blood cell contamination.
Antibodies and flow cytometry
All multicolor flow cytometry measurements were made on a Becton Dickinson LSRII or a Becton Dickinson FACSCalibur (San Jose, CA). Prior to staining with fluorophore conjugated antibodies, primary cells were blocked with FcR blocking reagent (Miltenyi Biotech) to eliminate nonspecific binding. To measure surface expression of B-cell activation markers, cells were stained with saturating levels of anti-MHC class II M5/114.15 (BioXCell), anti-CD69 H1.2F3 (BD Pharmingen), and anti-CD80 16-10A1 (BioXcell). Cells were gated on the B220+ population using anti-B220+ antibody (Miltenyi Biotec). Isotype controls (BioXCell), rat IgG2a, rat IgG2b, and hamster IgG2 were routinely used to ensure specificity. For all experiments, cell numbers between different samples were equalized to 1 × 106 cells per sample. Data were acquired on 1–2 × 104 gated live cells. Dead cells were excluded with Sytox Blue (Invitrogen) staining. For all experiments, background autofluorescence was measured on unstained cells.
Antibodies for flow cytometry were conjugated to FITC, Cy3 or Cy5 fluorophores (GE Healthcare) using kits as previously described (18). Median fluorescence intensity (MFI) values from flow cytometry experiments were normalized to the control. This was required because the ratio of fluorophore to antibody varied from batch to batch, which resulted in a variation in the MFI values for a given treatment. Raw MFI values are presented from a single experiment, representative of several measurements.
Inflammatory cytokine profile
The cytokine profile of activated B cells was determined using the manufacturer's protocol for a Multi-Analyte ELISArray kit (SABiosciences). The cytokines and chemokines measured were: interleukin (IL)-1A, IL-1B, IL-2, IL-4, IL-6, IL-17A, IFNγ, tumor necrosis factor (TNF)-α, G-CSF, and GM-CSF. The kit included positive and negative controls, all capture/detection antibodies and calorimetric detection reagents. The profile was determined from the supernatants of activated cells (20). The kit did not allow for quantification of the cytokines but provided relative changes, as determined by measuring the optical density at 450 nm using a Molecular Devices spectrophotometer. The data are plotted as the fold increase in cytokine levels over background to provide information on relative differences in secretion between cytokines for a given sample.
Triglyceride accumulation
The abundance of triglyceride-rich lipid droplets was measured with Nile Red (Invitrogen) staining using flow cytometry. Cells were stained with 250 µl of a 2.5 µg/ml stock of Nile Red in PBS for 30 min on ice, followed by two washes in PBS. Intracellular triglyceride levels were also measured using an enzymatic triglyceride assay kit (BioAssay Systems).
Gas chromatography (GC)
Total lipids were extracted from 4–8 × 106 cells using the Folch method (21). Fatty acids were then methylated using boron trifluoride in methanol (Sigma) for 90 min at 100°C; methyl esters were extracted into hexane and separated by a capillary GC (Shimadzu 2010; Shimadzu Scientific Instruments, Columbia, MD) with a Restek RT-2560 column. Peaks were identified by their retention times relative to standards (Restek). Areas of identified peaks were summed, and each peak area is expressed as the percentage of total peak area for a given treatment.
Column chromatography
Cells were treated with fatty acids spiked with 0.5 µCi of [14C]EPA or [14C]DHA (American Radiolabeled Chemicals) and extracted using the Folch method (21). Lipids were then separated into polar lipid (PL), free fatty acid, and neutral lipid (NL) classes as previously described (22). Briefly, 100 mg of glass aminopropyl beads (Sigma) were placed in a Pasteur pipette plugged with fiberglass. The column was eluted in succession with 3 ml of chloroform/isopropanol (2:1, v/v), 3 ml of diethyl ether/acetic acid (98:2, v/v), and 4 ml of methanol. All organic solvents (Fisher) were HPLC grade. Aliquots (100 µl) of the eluted fractions were counted using a Beckman LS6500 scintillation counter.
Lipoapoptosis measurements
Lipoapoptosis was measured with flow cytometry in terms of Annexin V-Cy5 (BD Pharmingen) and Sytox Blue staining. Measurements were performed as described previously and according to the manufacturer's protocol (BD Biosciences Pharmingen, San Diego, CA) (23).
Analysis
Data are presented as mean ± SEM. For ex vivo studies, 3–6 mice per dietary group are presented. Statistical significance was established using a one-way repeated measures ANOVA followed by a Dunnett's t-test. P values < 0.05 were considered significant.
RESULTS
Treatment of naiumlve B cells with EPA and DHA at 48 h significantly improved the n-6/n-3 PUFA ratio
We first determined if treatment with 50 µM PA, OA, ELA, EPA, and DHA modified the acyl chain composition of naïve B cells after 48 h of activation, relative to the BSA control. The addition of PA, OA, ELA, EPA, and DHA, respectively, increased the levels of 16:0, 18:1c, 18:1t, 20:5, and 22:6 and also had effects on the levels of other fatty acids (Table 2). Overall, PA treatment had no effect on the total levels of SFA, although 16:0 levels were increased, and the total levels of MUFA were lowered. Treatment with OA and ELA significantly increased total MUFA and lowered total SFA. EPA and DHA treatment increased total PUFA and lowered total MUFA. EPA and DHA improved the n-6/n-3 ratio from 2.2 for the BSA control to ∼0.3–0.5 with n-3 PUFAs.
TABLE 2.
Treatment of naïve B cells with n-3 PUFA for 48 h improves the n-6/n-3 PUFA ratio
| Fatty Acid | BSA | PA | OA | ELA | EPA | DHA | PA+OA | PA+ELA | PA+EPA | PA+DHA |
|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 1.9 ± 0.3 | 0.9 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.4 | 2.2 ± 0.4 | 0.8 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| 16:0 | 32.1 ± 0.6 | 37.8 ± 0.5a | 23.9 ± 0.4b | 16.0 ± 1.4c | 29.2 ± 3.9 | 39.2 ± 2.5a | 33.4 ± 0.5 | 28.7 ± 0.7 | 42.2 ± 0.5c | 41.4 ± 0.2c |
| 16:1 | 13.0 ± 0.4 | 11.3 ± 0.6 | 1.4 ± 0.2c | 5.8 ± 0.7c | 1.1 ± 0.4c | 2.4 ± 0.3c | 6.7 ± 0.5c | 13.5 ± 0.8 | 3.3 ± 0.2c | 4.2 ± 0.2c |
| 18:0 | 11.5 ± 0.6 | 10.2 ± 0.6 | 7.7 ± 0.4 | 4.7 ± 0.3 | 12.4 ± 2.5 | 9.9 ± 0.5 | 7.9 ± 0.4 | 5.5 ± 0.5 | 8.3 ± 0.4 | 9.0 ± 0.8 |
| 18:1t | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 43.4 ± 3.2c | 0.5 ± 0.2 | 0.1 ± 0.0 | 0.3 ± 0.1 | 23.6 ± 0.8c | 0.4 ± 0.2 | 0.0 ± 0.0 |
| 18:1c | 17.8 ± 0.2 | 12.4 ± 1.0 | 45.9 ± 0.8c | 11.4 ± 0.8 | 11.1 ± 3.1 | 5.6 ± 0.3 | 33.9 ± 0.5c | 11.0 ± 0.4 | 3.7 ± 0.3 | 4.7 ± 0.2 |
| 18:2 n-6 | 4.9 ± 0.3 | 8.4 ± 2.5 | 3.0 ± 04 | 4.0 ± 0.3 | 10.4 ± 5.3 | 2.7 ± 0.1 | 2.8 ± 0.2 | 4.5 ± 0.5 | 1.9 ± 0.2 | 2.0 ± 0.1 |
| 18:3 n-6 | 0.5 ± 0.1 | 0.5 ± 0.2 | 2.6 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 18:3 n-3 | 0.1 ± 0.3 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.4 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.2 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| 20:2 n-6 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| 22:0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.8 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| 22:1 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.4 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 20:4 n-6 | 8.6 ± 0.7 | 10.0 ± 1.3 | 5.8 ± 0.4a | 6.6 ± 0.4 | 3.6 ± 0.7c | 5.0 ± 0.2b | 6.3 ± 0.1 | 6.4 ± 0.3 | 3.4 ± 0.2c | 4.1 ± 0.1b |
| 20:5 n-3 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 15.0 ± 1.4c | 1.9 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.1 | 18.1 ± 0.5c | 0.8 ± 0.1 |
| 24:0 | 0.5 ± 0.1 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.0 |
| 24:1 | 0.7 ± 0.1 | 0.3 ± 0.1 | 1.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.9 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.1 |
| 22:5 n-3 | 2.0 ± 0.1 | 1.8 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.1 | 10.4 ± 6.4 | 0.9 ± 0.0 | 1.2 ± 0.1 | 1.2 ± 0.0 | 15.5 ± 0.8c | 0.7 ± 0.1 |
| 22:6 n-3 | 3.8 ± 0.4 | 3.0 ± 0.5 | 2.4 ± 0.2 | 2.6 ± 0.1 | 1.1 ± 0.2 | 28.5 ± 0.5c | 2.4 ± 0.0 | 2.4 ± 0.1 | 0.7 ± 0.1 | 30.5 ± 0.1c |
| ΣSFA | 46.8 ± 1.5 | 50.8 ± 2.3 | 33.7 ± 0.7c | 22.8 ± 1.7c | 44.7 ± 1.5 | 52.0 ± 1.0 | 43.0 ± 0.6 | 35.8 ± 0.8c | 52.2 ± 1.1 | 52.2 ± 1.6 |
| ΣMUFA | 32.3 ± 0.6 | 24.5 ± 1.5c | 49.8 ± 0.6c | 61.1 ± 1.9c | 13.7 ± 2.5c | 8.6 ± 0.3c | 42.1 ± 0.6c | 48.5 ± 1.0c | 7.7 ± 0.3c | 9.4 ± 0.3c |
| ΣPUFA | 20.9 ± 1.5 | 24.7 ± 1.5 | 16.5 ± 0.3 | 16.1 ± 1.0 | 41.6 ± 2.2c | 39.4 ± 3.0c | 14.9 ± 0.3a | 15.7 ± 0.2 | 40.1 ± 0.8c | 38.4 ± 1.0c |
| n-6/n-3 | 2.2 ± 0.1 | 3.4 ± 0.5b | 2.8 ± 0.2 | 2.5 ± 0.1 | 0.5 ± 0.5c | 0.3 ± 0.1c | 2.6 ± 0.1 | 2.7 ± 0.1 | 0.2 ± 0.0c | 0.2 ± 0.0c |
B cells were isolated from the spleens of C57BL/6 mice and activated with LPS and treated with fatty acids (50 µM each). Extracted lipids were then analyzed via GC. Values are average ± SEM, n = 4. Asterisks indicate difference from BSA.
Abbreviations: DHA, docosahexaenoic acid; ELA, elaidic acid; EPA, eicosapentaenoic acid; GC, gas chromatography; LPS, lipopolysaccharide; OA, oleic acid; PA, palmitic acid.
P < 0.05.
P <0.01.
P <0.001.
Differential effects of fatty acids on percentage of cells activated, upregulation of cell surface molecules, and cytokine secretion
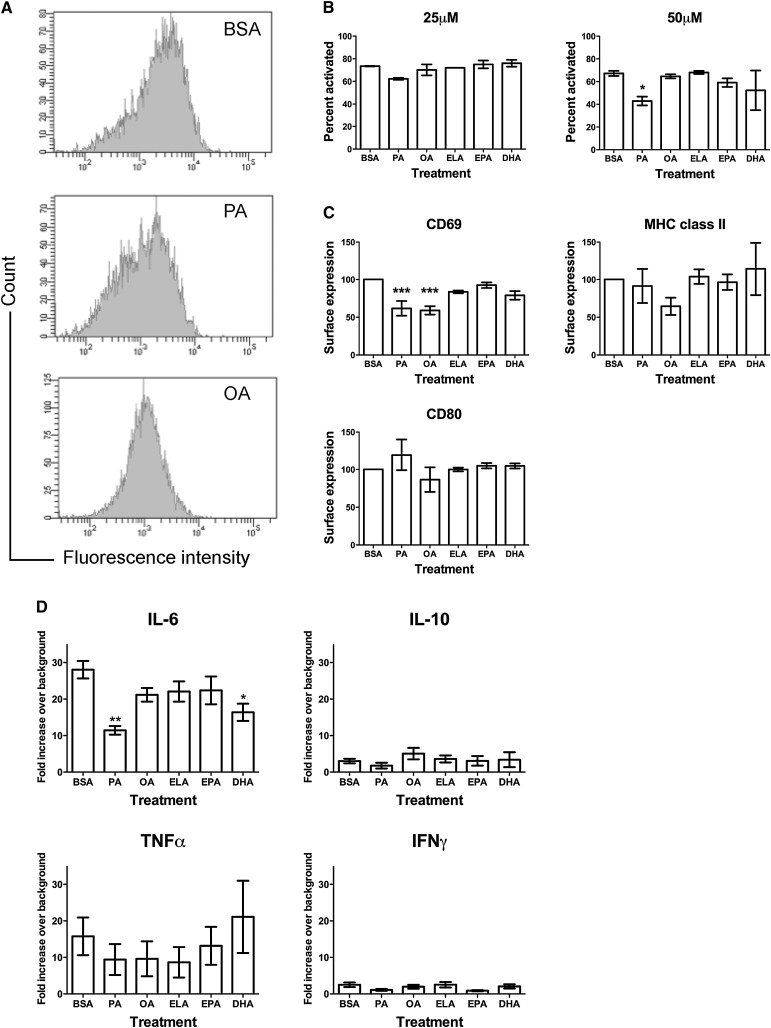
We tested the hypothesis that an improvement in the n-6/n-3 PUFA ratio with EPA and DHA treatment would suppress B-cell activation. The effects of n-3 PUFAs on B-cell activation were compared with the BSA control, PA, OA, and ELA. B-cell activation was measured in terms of the percentage of B cells activated, their upregulation of cell surface molecules, and secretion of cytokines. The percentage of B cells activated after 48 h of treatment with 25 and 50 µM fatty acid was measured with flow cytometry (Fig. 1A). Activated cells were identified based on changes in forward scatter and upregulation of MHC class II, CD80, and CD69 activation markers. Of all the fatty acids tested, only PA treatment had an effect on the percentage of B cells activated. Treatment of cells with 25 µM PA started to lower the percentage of activated B cells at 48 h, although this failed to reach statistical significance (P > 0.05) (Fig. 1B). At 50 µM PA, the percentage of B cells activated was significantly lower relative to BSA (Fig. 1B).
Fig. 1.
Differential effects of fatty acids on the percentage of B cells activated, upregulation of surface molecules, and cytokine secretion. (A) Sample flow cytometry histograms to show staining of the B-cell activation marker CD69. (B) Percentage of B220+ B cells activated with LPS at 48 h after treatment with BSA or 25–50 µM PA, OA, ELA, EPA, and DHA. (C) Surface expression of B cell activation markers CD69, MHC class II, and CD80 after 48 h of treatment with 50 µM fatty acids. (D) Cytokine secretion from B220+ B cells treated with BSA or 50 µM PA, OA, ELA, EPA, and DHA treated for 48 h. Values are mean ± SEM, n = 3–10. Asterisks indicate difference from BSA, *P < 0.05, **P <0.01, ***P < 0.001. DHA, docosahexaenoic acid; ELA, elaidic acid; EPA, eicosapentaenoic acid; LPS, lipopolysaccharide; OA, oleic acid; PA, palmitic acid.
The degree of B-cell activation was measured in terms of changes in the MFI of fluorescently labeled antibodies against MHC class II, CD80, and CD69 (Fig. 1C). After 48 h of treatment, both PA and OA lowered the surface levels of CD69 by ∼40%, compared with the control. A similar trend was observed for MHC class II surface expression with OA treatment, which was not statistically significant (P > 0.05). PA and OA treatment had no effect on the surface levels of CD80 relative to the BSA control. ELA, EPA, and DHA treatment had no effect on the surface levels of CD69, MHC class II, and CD80.
Cytokine secretion from B cells was tested in response to treatment with the different fatty acids at 50 µM (Fig. 1D). We tested for the secretion of 10 different cytokines (see “Materials and Methods”) from the supernatants of 48 h activated B cells from the differing treatment conditions. Of these 10, only secretions of IL-6, TNF-α, IFNγ, and IL-10 were detected. IL-6 and TNF-α were secreted more than IFNγ and IL-10. 50 µM PA treatment appeared to lower the secretion of all four cytokines, although only a change in IL-6 was statistically significant (P < 0.05). DHA treatment also significantly suppressed IL-6 levels, although not to the same extent as PA treatment. The other fatty acids did not have an effect on the levels of the four secreted cytokines.
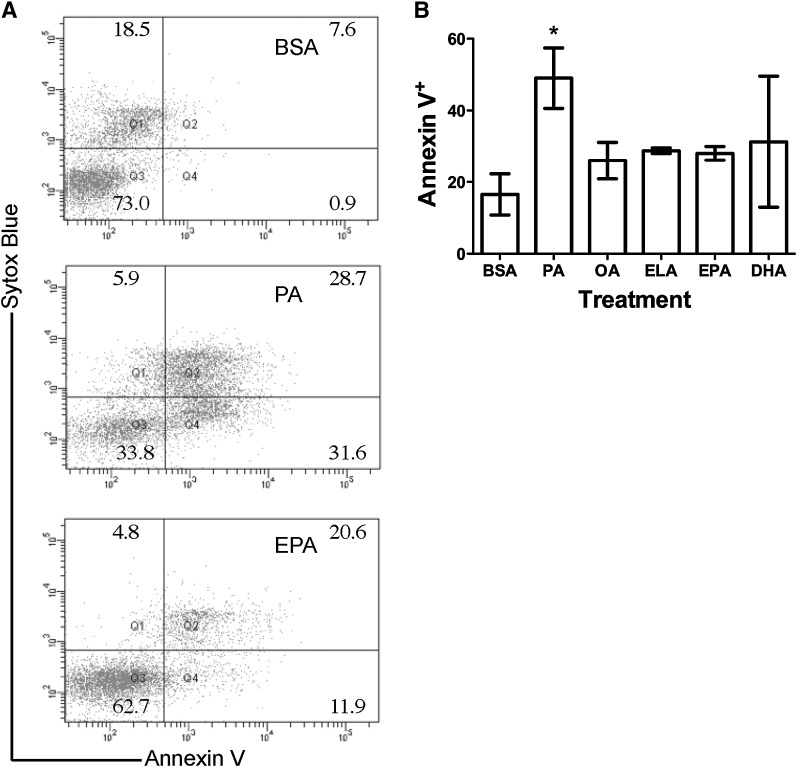
PA treatment suppressed the percentage of activated B cells by promoting lipoapoptosis
Next, the mechanism by which PA treatment suppressed the percentage of activated B cells was addressed. As it is established that PA treatment can induce lipoapoptosis in some cell types, we determined if primary B cells were apoptotic by measuring Annexin V binding with flow cytometry (Fig. 2A). Treatment of B cells with 50 µM PA for 48 h increased the percentage of apoptotic cells by 3-fold relative to the BSA control (Fig. 2B). Within the percentage of cells that were apoptotic, PA-treated cells were generally late apoptotic (Annexin V+ Sytox Blue+). Treatment with OA, ELA, EPA, and DHA had no effect on B-cell apoptosis compared with BSA. The large error observed with DHA treatment was due to a single outlier in the data set.
Fig. 2.
PA treatment promotes B cell lipoapoptosis. (A) Sample dot plots of Annexin V/Sytox Blue staining of B220+ B cells treated with the BSA control, PA, or EPA. (B) Percentage of cells that are Annexin V+ at 48 h of treatment with the BSA control or 50 µM PA, OA, ELA, EPA, or DHA. Values are mean ± SEM, n = 4. Asterisk indicates difference from BSA, *P < 0.05. DHA, docosahexaenoic acid; ELA, elaidic acid; EPA, eicosapentaenoic acid; OA, oleic acid; PA, palmitic acid.
MUFAs and n-3 PUFAs prevented the effects of PA on B cells
Several studies show that OA treatment can prevent PA-induced lipoapoptosis in specific cell types (24–27). Far less is known about the effects of other unsaturated fatty acids in preventing PA-induced lipoapoptosis. We wanted to determine if cosupplementing PA-treated cells with OA, ELA, EPA, or DHA could prevent the PA-induced suppression of B-cell activation by inhibiting apoptosis (Fig. 3). Indeed, at 48 h, treatment of primary B cells with OA, ELA, EPA, and DHA increased the percentage of activated B cells (Fig. 3A). The change in the percentage of activated cells correlated with a change in lipoapoptosis. Annexin V staining showed that cosupplementing PA-treated cells with any of the unsaturated fatty acids had significantly lowered the percentage of apoptotic cells, relative to PA treatment alone (Fig. 3B).
Fig. 3.
Cosupplementing PA-treated cells with n-3 PUFAs prevents the effects of PA on B-cell phenotype and lipoapoptosis by increasing cell size. (A) Percentage of B cells activated and (B) percentage of apoptotic cells at 48 h of treatment with 50 µM fatty acids. (C) IL-6 secretion from B220+ B cells treated with 50 µM PA, and 50 µM PA + 50 µM OA, ELA, EPA, or DHA for 48 h. (D) MFI values for Nile Red staining at 48 h of treatment with 50 µM fatty acid. (E) Median forward scatter values for cells treated with 50 µM fatty acids at 48 h. Values are mean ± SEM, n = 3–5. Asterisks indicate difference from PA (A–C) or BSA (D–E), *P < 0.05, **P < 0.01, ***P < 0.001. DHA, docosahexaenoic acid; ELA, elaidic acid; EPA, eicosapentaenoic acid; MFI, median fluorescence intensity; OA, oleic acid; PA, palmitic acid.
We also investigated if cosupplementing the 50 µM PA-treated cells with 50 µM OA, ELA, EPA, or DHA could increase the levels of IL-6 (Fig. 3C), given that the unsaturated fatty acids were preventing lipoapoptosis. Cosupplementing the cells with OA, ELA, and EPA increased the levels of IL-6 relative to PA treatment alone. DHA treatment in the presence of PA tended to increase IL-6 secretion, but the change was not statistically significant compared with PA alone (P > 0.05).
MUFAs and PUFAs differentially prevented lipoapoptosis
We hypothesized that unsaturated fatty acids prevented palmitate-induced lipoapoptosis by sequestering the fatty acids into lipid droplets, as previously reported (27). Lipid droplet accumulation was measured in terms of Nile Red staining with flow cytometry. The MFI of Nile Red showed no change in the levels of lipid droplets (Fig. 3D), except for OA treatment alone. The same results as Nile Red staining were observed with a triglyceride assay kit (data not shown). This finding suggested two possibilities to explain how OA, ELA, EPA, and DHA prevented PA-induced lipoapoptosis. Either the unsaturated fatty acids were preventing uptake of PA into the cell, or the fatty acids were accumulating into the membrane rather than lipid droplets. To address this, the fatty acid composition of cells treated with 50 μM PA in addition to 50 μM OA, ELA, EPA, or DHA was measured. Differential effects were found with the MUFAs versus the n-3 PUFAs on the lipid composition of the B cells. Cosupplementing the PA-treated cells with either OA or ELA at 48 h (Table 2) prevented cellular uptake of PA. 16:0 levels for PA+OA and PA+ELA were similar to those of BSA. Upon treatment with PA+OA or PA+ELA, the levels of OA and ELA were increased relative to the BSA control; however, they were lower compared with treatment with OA or ELA alone.
Compared with MUFA treatment, addition of EPA and DHA to PA-treated cells resulted in increased accumulation of PA (Table 2). The levels of 16:0 in cells treated with PA+EPA were nearly identical to those cells treated with PA alone but higher than BSA. 16:0 levels in PA+DHA were also similar to PA and DHA treatment alone but higher than BSA. Similarly, the levels of 20:5 or 22:6, respectively, in cells treated with PA+EPA or PA+DHA were similar to those found for cells treated with EPA or DHA alone.
It was verified that addition of 50 µM EPA or DHA in the presence of 50 µM PA resulted in accumulation of these fatty acids into the membrane, as we speculated. This was accomplished by using column chromatography with radiolabeled EPA and DHA to determine their localization in the absence and presence of PA into neutral lipid, free fatty acid, and polar lipid fractions (Table 3). [14C]EPA and [14C]DHA predominately localized to the PL fraction (∼76%) with lesser amounts in NL (∼13%) and FFA (∼11%) upon treatment with 50 µM of either EPA or DHA. Cosupplementing the 50 µM PA-treated cells with either EPA or DHA provided similar results (Table 3). Therefore, EPA and DHA predominately localized to the membrane. The significant accumulation of fatty acids predominately into the membrane upon addition of EPA and DHA to PA-treated cells suggested an increase in cell size. Therefore, cell size was measured in terms of forward scatter with flow cytometry (Fig. 3E). B cells treated with PA+EPA and PA+DHA showed a significant increase in cell size relative to the BSA control. No effect on cell size was found with any of the other unsaturated fatty acid treatments.
TABLE 3.
EPA and DHA are incorporated primarily into polar lipids
| Treatment | NL | FFA | PL |
|---|---|---|---|
| 50 µM EPA | 13.0 ± 2.8 | 11.4 ± 4.9 | 75.6 ± 7.7 |
| 50 µM DHA | 13.3 ± 2.6 | 10.9 ± 4.5 | 75.8 ± 1.9 |
| 50 µM PA + 50 µM EPA | 11.9 ± 1.0 | 18.5 ± 5.6 | 69.6 ± 4.6 |
| 50 µM PA + 50 µM DHA | 14.0 ± 2.6 | 6.0 ± 0.3 | 80.0 ± 2.4 |
B220+ B cells were treated with 50 µM EPA or DHA, spiked respectively with [14C]EPA or [14C]DHA, in the absence or presence of 50 µM PA for 48 h. Total lipids were extracted and separated into neutral lipid, FFA or polar lipid fractions using column chromatography. Incorporation of the radiolabel into different lipid classes was determined by scintillation counting. Values represent the percentage of the total radioactivity per treatment. Data are average ± SD from two independent experiments.
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FFA, free fatty acid; NL, neutral lipid; PA, palmitic acid; PL, polar lipid.
High-fat diet enriched in n-3 PUFAs improved the n-6/n-3 PUFA ratio of splenocytes
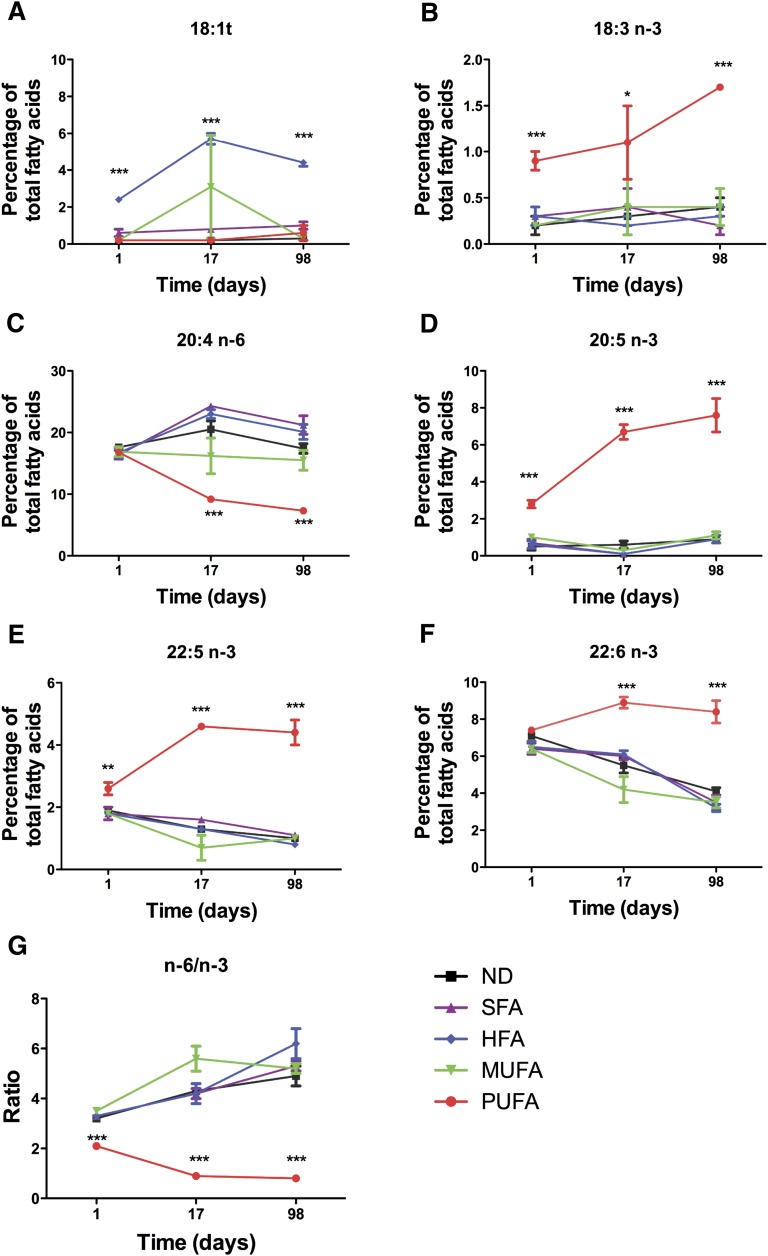
Next we tested the hypothesis that an improvement in the n-6/n-3 ratio of primary B cells isolated from mice fed different diets could suppress B-cell activation. To conduct these studies, we first had to determine how long to feed mice with the diets to remodel the lipid composition of the cell. These studies were essential because the effects of our diets on lipid composition have never been tested before. We determined the rate at which the high-fat diets enriched in SFA, HFA, MUFA, or n-3 PUFA, relative to a low-fat normal diet (ND), modified the lipid composition of splenocytes (Fig. 4). We selected splenocytes, which include B cells, because this provided a complete picture of how the diet modified the kinetics of fatty acid composition. Significant changes in the fatty acid composition of cells isolated specifically from the HFA- and n-3 PUFA–rich diets were observed (Fig. 4). After 24 h of feeding, the HFA diet increased the levels of 18:1t, which further increased after 17 days and leveled off by 98 days of feeding (Fig. 4A). The n-3 PUFA diet, within 24 h of feeding, increased the levels of 18:3n-3, 20:5n-3, and 22:5 n-3, which further increased after 17 days and generally leveled off by 98 days (Fig. 4B,D,E). 22:6 levels did not significantly increase until 17 days of feeding and remained constant up to 98 days, while the levels of 20:4 decreased at 17 days and remained constant up to 98 days with the n-3 PUFA diet (Fig. 4C, F). Overall, the n-3 PUFA diet improved the n-6/n-3 ratio, which emerged at 24 h, whereas the other diets slightly increased the n-6/n-3 ratio with time (Fig. 4G).
Fig. 4.
Changes in fatty acid composition as a function of time for splenocytes isolated from mice fed different diets. Levels of (A) 18:1t (B) 18:3 n-3 (C) 20:4 n-6 (D) 20:5 n-3 (E) 22:5 n-3 (F) 22:6 n-3 and (G) n-6/n-3 PUFA ratio as a function of time after feeding mice ND or high-fat diets enriched in SFA, HFA, MUFA, and n-3 PUFA. (A–F) values (mean ± SEM) are percentage of the total fatty acids, n = 3 for 1 and 17 days, and n = 4 for 98 days. Asterisks indicate difference from ND, *P < 0.05, **P < 0.01, ***P < 0.001. Values on the Y axis vary from plot to plot, and for some values, the error bars are smaller than the data points. For simplicity, only fatty acids that showed differences relative to the control are presented. HFA, hydrogenated fatty acid; MUFA, monounsaturated fatty acid ND, normal diet; PUFA; polyunsaturated fatty acid SFA, saturated fatty acid.
High-fat diets enriched in n-3 PUFAs did not suppress the percentage of activated B cells but increased CD69 surface expression
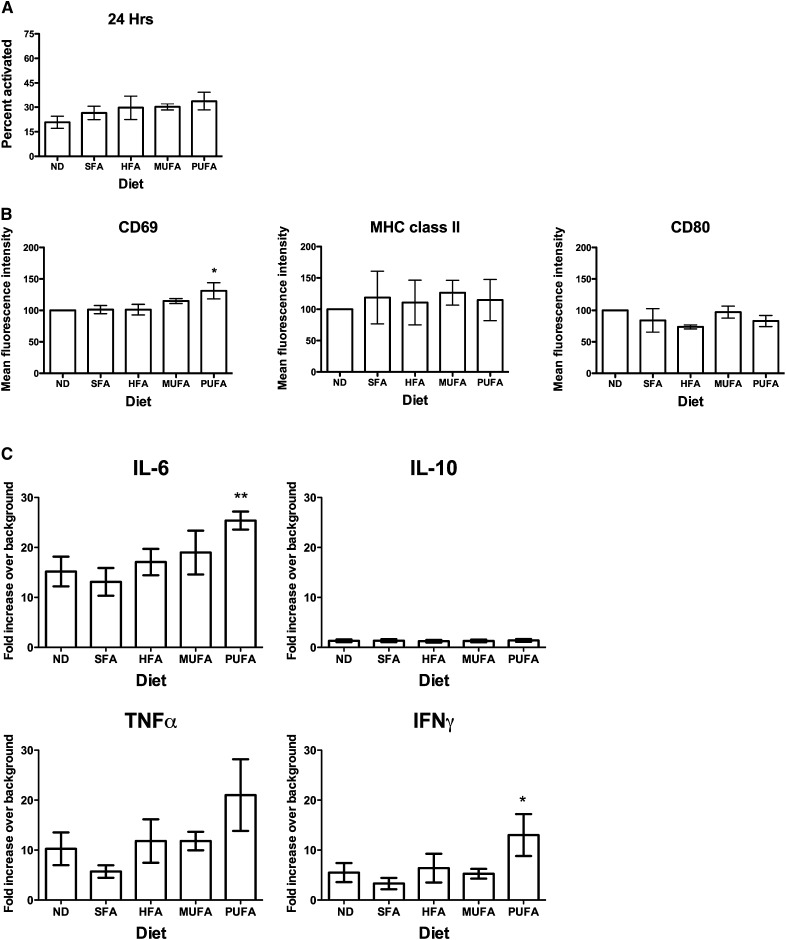
Based on the kinetic measurements, a feeding strategy was pursued with the high-fat diets and the purified control diet for 98 days. We tested if n-3 PUFAs could suppress B-cell activation ex vivo (Fig. 5). Similar to the in vitro studies, the percentage of B cells activated and their degree of activation was determined with flow cytometry. Splenocytes were activated with LPS in cell culture with 5% FBS for 24 h. Experiments were not conducted at 48 h after activation as the effects of the fatty acids from the diet were diminished at this time in the FBS-containing medium; however, the levels of n-3 PUFAs were still evident after 24 h of activation. The n-6/n-3 ratio for splenocytes activated with LPS in the normal diet was ∼3.7 ± 0.2, and for cells from the n-3 PUFA diet, it was ∼1.5 ± 0.4 at 24 h in serum-containing medium.
Fig. 5.
High-fat n-3 PUFA diet does not modify the percentage of activated B cells but increases CD69 surface expression and secretion of IL-6 and IFNγ. (A) Percentage of B cells activated after 24 h of activation and (B) MFI of CD69, MHC class II, and CD80 of B cells isolated from mice fed ND, SFA, HFA, MUFA, and n-3 PUFA diets. (C) Cytokine secretion from B220+ B cells isolated from diet modified mice. Data are n = 3 (A) and n = 4 (B–C) per diet. Asterisks indicate different from ND, *P < 0.05, **P < 0.01. HFA, hydrogenated fatty acid; MUFA; monunsaturated fatty acid ND, normal diet; PUFA; polyunsaturated fatty acid SFA, saturated fatty acid.
No significant change was observed in the percentage of activated cells with any of the high-fat diets (Fig. 5A) (P > 0.05). Given the slight increase, albeit statistically insignificant, in the percentage of B cells activated (Fig. 5A) with the HFA, MUFA, and n-3 PUFA diets, we further verified that B-cell activation was not affected with these diets. Experiments were conducted in serum-free conditions, where the endogenous levels of n-3 PUFAs were maintained at 24 and 48 h ex vivo (data not shown). Although the percentage of cells activated in serum-free conditions was ∼25% less than in serum-containing conditions, no change in activation was measured with diets enriched in SFA, HFA, MUFA, and n-3 PUFA, relative to the ND control (data not shown). In terms of surface molecule expression (Fig. 5B), the HFA, MUFA, and n-3 PUFA diets had no effect on MHC class II or CD80 surface expression. However, the n-3 PUFA diet did increase surface levels of CD69 by 31% compared with the ND control.
n-3 PUFAs increased B-cell secretion of IL-6 and IFNγ
Similar to the in vitro studies, the secretion of 10 different cytokines from the LPS-stimulated splenocytes after 24 h in 5% FBS-containing medium was measured. Consistent with the cell culture experiments, secretion of IL-6, TNFα, IFNγ, and IL-10 was observed (Fig. 5C). Compared with ND, the high-fat n-3 PUFA diet significantly increased IL-6 and IFNγ levels but had no effect on IL-10 levels. TNF-α levels were increased with the n-3 PUFA diet but failed to reach statistical significance (P > 0.05). By normalizing the data to the ND control, the effects of n-3 PUFAs on TNF-α were also statistically significant (P < 0.05); however, we did not report the normalized data (where ND is set to 100%) because it does not provide an accurate assessment of how the different cytokines are differentially secreted. The results with n-3 PUFAs on IL-6 and IFNγ were also verified with purified B cells in both serum and no-serum conditions (data not shown). The SFA, HFA, and MUFA diets had no effect on the secretion of the four cytokines relative to the ND control.
n-3 PUFA diet significantly increased body weight gain and spleen size
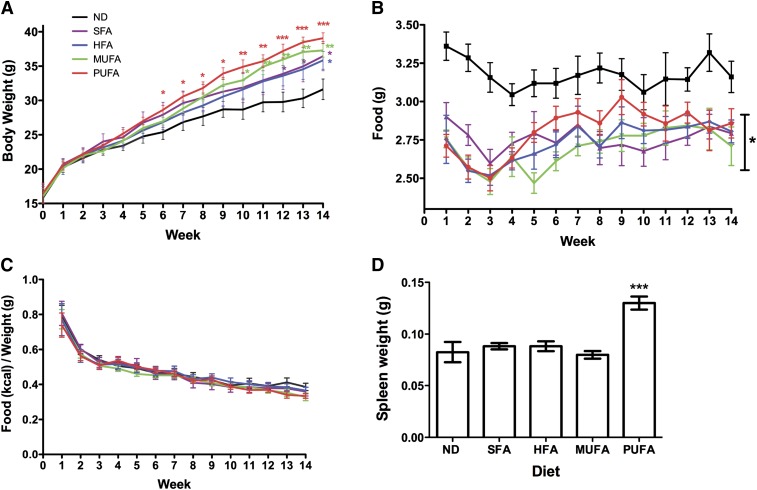
During the course of the animal study, we made an unexpected observation. Mice on the n-3 PUFA–rich diet gained the most weight relative to the ND control (Fig. 6A). The increase in weight gain significantly emerged after 6 weeks of feeding and continued up to 14 weeks. A similar observation was made with the other high-fat diets, although this is accepted in the field (28). It is important to note that animals on the SFA, HFA, and MUFA diets gained weight relative to the ND control at later time points than those on the n-3 PUFA diet. For the most part, the mice consuming any of the high-fat diets consumed less food on a daily basis as a function of time compared with the ND control (P < 0.05) (Fig. 6B). There were specific time points that were an exception, which included week 7 for SFA; week 11 for MUFA; week 11 for HFA; and weeks 7, 9–11 for n-3 PUFA diets. However, the increase in body weight was not due to any changes in the consumption of food over this time period. At specific time points, mice fed the n-3 PUFA diet consumed more calories (data not shown), but when normalized to their body weights, all of the mice on the different diets consumed the same number of kcal (Fig. 6C). The weight of the spleens from the mice fed the n-3 PUFA diet also increased, relative to the control (Fig. 6D).
Fig. 6.
High-fat n-3 PUFA diet increases body weight gain and induces splenomegaly. (A) Body weight as a function of time for mice fed ND or high-fat diets enriched in SFA, HFA, MUFA, and n-3 PUFA. Average daily (B) food and (C) kcal intake of mice fed the different diets as a function of time. The kcal values are normalized to the body weight. (D) Spleen weight of mice fed the different diets after 98 days of feeding. Values are mean ± SEM, n = 6 per diet. Asterisks indicate difference from ND, *P < 0.05, **P < 0.01, ***P < 0.001. HFA, hydrogenated fatty acid; MUFA; monunsaturated fatty acid ND, normal diet; PUFA; polyunsaturated fatty acid SFA, saturated fatty acid.
DISCUSSION
Relationship between n-3 PUFA fatty acid composition and suppression of lymphocyte activation is not universal
In the present study, we tested the hypothesis that an improvement in the n-6/n-3 ratio with n-3 PUFAs, either in cell culture or through diet, would contribute to suppressing B-cell activation. In vitro experiments showed that n-3 PUFAs significantly improved the ratio of n-6 to n-3 fatty acids, as expected, but did not significantly modify LPS-stimulated activation of B cells. The only exception was the reduction in IL-6 levels with DHA, which is consistent with studies in other cell lines (29, 30). Nevertheless, DHA treatment did not modify the percentage of cells activated or surface molecule expression. The findings do not rule out the possibility that n-3 PUFAs could suppress B-cell activation in a robust manner through other receptors. LPS activates through the Toll-like receptors (TLR), namely, the TLR-4/CD14 complex, and perhaps these receptors are not sensitive to the levels of n-3 PUFAs administered in the in vitro experiments. It is conceivable that activation of B cells through the B-cell receptor could be modified in response to n-3 PUFA modulation. It is also possible that n-3 PUFAs could exert their immunomodulatory effects once the cells are activated into antigen-presenting cells or antibody-producing plasma cells. Indeed, several studies have shown that n-3 PUFAs can modify antigen presentation and production of antibodies such as IgG and IgA (22, 31–34).
Ex vivo, we found that the B cells from the n-3 PUFA–fed mice secreted increased levels of IL-6 and IFNγ. This finding is not in agreement with the notion that an improvement in the n-6/n-3 PUFA ratio will suppress secretion of pro-inflammatory cytokines. Our data are consistent with previous reports on macrophages that show that n-3 PUFAs, especially at higher doses, can promote the secretion of TNF-α in response to LPS (35, 36). However, in this study, we ruled out that the increase in pro-inflammatory cytokines was driven by macrophages by purifying B cells. Future studies will address if other cell types from the spleens of the n-3 PUFA–fed mice display increased production of pro-inflammatory cytokines in response to varying antigens or if this effect is unique to this cell type and antigen.
Several important findings emerged from the lipid composition analysis in vitro and ex vivo. First, as expected, addition of n-3 PUFAs in cell culture resulted in a significant increase in levels of EPA or DHA. Ex vivo, addition of n-3 PUFAs to the diet did not increase the levels of n-3 PUFAs to the same degree. Interestingly, the n-6/n-3 ratio in culture upon the addition of EPA or DHA was nearly the same as that observed ex vivo. We speculate that the similarity in the n-6/n-3 ratio between the cell culture studies and the ex vivo experiments is likely a coincidence. To confirm that treatment with cells in cell culture in the 50 µM range is relevant compared with the mouse model, at least in terms of the n-6/n-3 ratio, additional time and dose-response studies would be required in several cell types.
An important finding was that n-3 PUFAs could remodel the fatty acid composition of splenocytes within 24 h and then level off between 17 and 98 days of feeding. While it is accepted that n-3 PUFAs remodel the fatty acid composition of cells, virtually no studies have thoroughly addressed the change in splenocyte lipid composition with n-3 PUFAs as a function of time. The rapid improvement in the n-6/n-3 ratio points to the possibility that n-3 PUFAs could be used as nutraceuticals to modify composition and organization to selectively initiate a change in cellular function with a high dose of PUFAs for a short time period. Based on data presented here and elsewhere, the most plausible splenic targets that respond to a change in membrane composition to suppress cellular function are CD4+ T cells and possibly DCs (13, 15, 37, 38). The data from this study allow us to propose that n-3 PUFA inhibition of lymphocyte activation is not universal.
Differential prevention of lipoapoptosis with MUFAs and n-3 PUFAs
All of the unsaturated fatty acids (OA, ELA, EPA, and DHA) prevented palmitate-induced lipoapoptosis in a primary cell type. This could also explain why the SFA diet had no effect on B-cell activation ex vivo, as this diet also includes MUFA and PUFA. In culture, the majority of studies have only looked at the effects of OA on preventing lipoapoptosis; our data suggest that prevention of lipoapoptosis may be a broader effect with all unsaturated fatty acids, including a trans fatty acid. However, the effects of the MUFAs are not the same as the PUFAs on preventing lipoapoptosis. The MUFAs prevented uptake of palmitate into the cell. Cosupplementing PA-treated cells with either OA or ELA also lowered uptake of OA or ELA into the cells, compared with treatment with OA or ELA alone; therefore, the data suggest competition in uptake between these fatty acids. Previous studies generally showed that OA sequesters palmitate into lipid droplets (26, 27). For instance, we reported in T2 cells that cosupplementing palmitate with OA could prevent early lipoapoptosis through the accumulation of lipid droplets (23). Perhaps this was not observed here because we used primary cells.
EPA and DHA prevented the toxic effects of palmitate by incorporating into the membrane. It is noteworthy that DHA treatment alone increased the percentage of palmitate, which could be due to decreased desaturation and/or increased synthesis of PA. Therefore, we speculate that DHA is not displacing PA from the membrane like the MUFAs; this could explain why cosupplementing PA-treated cells with DHA did not significantly increase IL-6 secretion compared with PA alone. The results on how EPA and DHA exerted their effects are novel, yet still consistent, with the notion that n-3 PUFAs prevent lipotoxicity (39, 40). More work is clearly needed to understand if the differential effects of MUFAs, including trans fatty acids, and n-3 PUFAs apply to cell types highly prone to lipoapoptosis such as hepatocytes and cardiomyocytes. Perhaps accumulation into lipid droplets is less likely for a cell that is rapidly proliferating, such as B cells. Prevention of lipotoxicity, especially with n-3 PUFAs, is consistent with their potential health benefits for metabolic disorders (41). Type II diabetes and obesity are associated with hypertriglyceridemia and n-3 PUFAs are recognized to have clinical benefits toward lowering triglycerides (42).
n-3 PUFAs, body weight gain, and pro-inflammatory cytokines
Although the focus of our mouse experiments was to assess the effects of n-3 PUFAs on B-cell activation, we unexpectedly observed that mice fed the n-3 PUFA diet significantly gained weight. This may also relate to lipid overload and lipotoxicity. We speculate that this particular diet, which attempts to match the n-6/n-3 ratio of Greenland Eskimos, results in a redistribution of fat, which could prevent lipotoxicity. One previous study has shown that n-3 PUFAs can increase weight gain when compared with a diet rich in SFA; however, this study was limited to old mice and only two diets (43). Our future studies will address whether the high-fat n-3 PUFA–fed mouse is a model of the “healthy overweight” (44) and shows an overall suppression in inflammatory cytokine production when several tissues and cell types are taken into account and normal or even improved whole body metabolism. Alternatively, the n-3 PUFA fed mouse may represent the onset of metabolic dysregulation because of weight gain, which would entail pro-inflammatory responses and possibly insulin resistance. We aim to further pursue additional studies to address if high-fat n-3 PUFA diets modify insulin resistance, serum and hepatic triglyceride levels, and accumulation of fat into differing tissues. Our preliminary data show that the n-3 PUFA–fed mice have normal liver triglyceride levels (Rockett, Carraway, and Shaikh, unpublished observations); therefore, it is possible that high-fat n-3 PUFA diets may have a significant role in prevention of lipotoxicity. Further investigation is clearly required to understand the role of n-3 PUFAs in preventing lipotoxicity, especially in relation to modifying immunity.
Footnotes
Abbreviations:
- DHA
- docosahexaenoic acid
- ELA
- elaidic acid
- EPA
- eicosapentaenoic acid
- GC
- gas chromatography
- HFA
- hydrogenated fatty acid
- IL
- interleukin
- LPS
- lipopolysaccharide
- MFI
- median fluorescence intensity
- MHC
- major histocompatibility complex
- ND
- normal diet
- NL
- neutral lipid
- OA
- oleic acid
- PA
- palmitic acid
- PL
- polar lipid
- SFA
- saturated fatty acid
- TNF-α
- tumor necrosis factor-α
This article was supported by start-up funds from the Brody School of Medicine (S.R.S.) and a Brody School of Medicine Shared Resources Equipment Grant (S.R.S).
REFERENCES
- 1.Duda M. K., O'Shea K. M., Tintinu A., Xu W., Khairallah R. J., Barrows B. R., Chess D. J., Azimzadeh A. M., Harris W. S., Sharov V. G., et al. 2009. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc. Res. 81: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fetterman J. W., Jr., Zdanowicz M. M. 2009. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 66: 1169–1179. [DOI] [PubMed] [Google Scholar]
- 3.Harris W. S., Mozaffarian D., Lefevre M., Toner C. D., Colombo J., Cunnane S. C., Holden J. M., Klurfeld D. M., Morris M. C., Whelan J. 2009. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 139: 804S–19S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder P. C. 2006. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83: 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 5.Anderson M., Fritsche K. L. 2002. (n-3) Fatty acids and infectious disease resistance. J. Nutr. 132: 3566–3576. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh S. R., Edidin M. 2008. Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem. Phys. Lipids. 153: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwerbrock N. M. J., Karlsson E. A., Shi Q., Sheridan P. A., Beck M. A. 2009. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 139: 1588–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P., Kim W., Zhou L., Wang N., Ly L. H., McMurray D. N., Chapkin R. S. 2006. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J. Nutr. 136: 2391–2398. [DOI] [PubMed] [Google Scholar]
- 9.Anderson M. J., Fritsche K. L. 2004. Dietary polyunsaturated fatty acids modulate in vivo, antigen-driven CD4+ T-cell proliferation in mice. J. Nutr. 134: 1978–1983. [DOI] [PubMed] [Google Scholar]
- 10.Chapkin R. S., Arrington J. L., Apanasovich T. V., Carroll R. J., McMurray D. N. 2002. Dietary n-3 PUFA affect TcR-mediated activation of purified murine T cells and accessory cell function in co-cultures. Clin. Exp. Immunol. 130: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pompos L. J., Fritsche K. L. 2002. Antigen-driven murine CD4+ T lymphocyte proliferation and interleukin-2 production are diminished by dietary (n-3) polyunsaturated fatty acids. J. Nutr. 132: 3293–3300. [DOI] [PubMed] [Google Scholar]
- 12.Stulnig T. M., Berger M., Sigmund T., Raederstorff D., Stockinger H., Waldhausl W. 1998. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 143: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim W., Fan Y., Barhoumi R., Smith R., McMurray D. N., Chapkin R. S. 2008. n-3 Polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 181: 6236–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeyda M., Staffler G., Horejsi V., Waldhausl W., Stulnig T. M. 2002. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J. Biol. Chem. 277: 28418–28423. [DOI] [PubMed] [Google Scholar]
- 15.Zeyda M., Saemann M. D., Stuhlmeier K. M., Mascher D. G., Nowotny P. N., Zlabinger G. J., Waldhausl W., Stulnig T. M. 2005. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB activation. J. Biol. Chem. 280: 14293–14301. [DOI] [PubMed] [Google Scholar]
- 16.Wang H., Hao Q., Li Q., Yan X., Ye S., Li Y., Li N., Li J. 2007. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition. 23: 474–482. [DOI] [PubMed] [Google Scholar]
- 17.Chapkin R. S., Wang N., Fan Y., Lupton J. R., Prior I. A. 2008. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta. 1778: 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaikh S. R., Rockett B. D., Salameh M., Carraway K. 2009. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 139: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 19.Switzer K. C., Fan Y., Wang N., McMurray D. N., Chapkin R. S. 2004. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T-cells. J. Lipid Res. 45: 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X., Hiroko O., Kitmura T., Taketo M. M., Oshima M. 2008. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J. Biol. Chem. 283: 19864–19871. [DOI] [PubMed] [Google Scholar]
- 21.Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 22.Shaikh S. R., Edidin M. 2007. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J. Lipid Res. 48: 127–138. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh S. R., Mitchell D., Carroll E., Li M., Schneck J., Edidin M. 2008. Differential effects of a saturated and a monounsaturated fatty acid on MHC class I antigen presentation. Scand. J. Immunol. 68: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy S., Langelier Y., Prentki M. 2000. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 60: 6353–6358. [PubMed] [Google Scholar]
- 25.Miller T. A., LeBrasseur N. K., Cote G. M., Trucillo M. P., Pimentel D. R., Ido Y., Ruderman N. B., Sawyer D. B. 2005. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem. Biophys. Res. Commun. 336: 309–315. [DOI] [PubMed] [Google Scholar]
- 26.Listenberger L. L., Ory D. S., Schaffer J. E. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895. [DOI] [PubMed] [Google Scholar]
- 27.Listenberger L. L., Han X., Lewis S. E., Cases S., Robert V. F., Jr., Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funkat A., Massa C. M., Jovanovska V., Proietto J., Andrikopoulos S. 2004. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J. Nutr. 134: 3264–3269. [DOI] [PubMed] [Google Scholar]
- 29.Moon Y., Pestka J. J. 2003. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J. Nutr. Biochem. 14: 717–726. [DOI] [PubMed] [Google Scholar]
- 30.Ajuwon K. M., Spurlock M. E. 2005. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3–L1 adipocytes. J. Nutr. 135: 1841–1846. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson P., MacPherson G. G., Jenkins C. H., Calder P. C. 1997. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J. Leukoc. Biol. 62: 771–777. [DOI] [PubMed] [Google Scholar]
- 32.Hughes D. A., Pinder A. C. 2000. n-3 Polyunsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am. J. Clin. Nutr. 71: 357S–360S. [DOI] [PubMed] [Google Scholar]
- 33.Pestka J. J., Zhou H., Jia Q., Timmer A. M. 2002. Dietary fish oil suppresses experimental immunoglobulin A nephropathy in mice. J. Nutr. 132: 261–269. [DOI] [PubMed] [Google Scholar]
- 34.Beli E., Li M., Cuff C., Pestka J. J. 2008. Docosahexaenoic acid-enriched fish oil consumption modulates immunoglobulin responses to and clearance of enteric reovirus infection in mice. J. Nutr. 138: 813–819. [DOI] [PubMed] [Google Scholar]
- 35.Blok W. L., de Bruijn M. F., Leenen P. J., Eling W. M., van Rooijen N., Stanley E. R., Buurman W. A., van der Meer J. W. 1996. Dietary n-3 fatty acids increase spleen size and postendotoxin circulating TNF in mice; role of macrophages, macrophage precursors, and colony-stimulating factor-1. J. Immunol. 157: 5569–5573. [PubMed] [Google Scholar]
- 36.Petursdottir D. H., Hardardottir I. 2007. Dietary fish oil increases the number of splenic macrophages secreting TNF-α and IL-10 but decreases the secretion of these cytokines by splenic T cells from mice. J. Nutr. 137: 665–670. [DOI] [PubMed] [Google Scholar]
- 37.Chapkin R. S., Seo J., McMurray D. N., Lupton J. R. 2008. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem. Phys. Lipids. 153: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geyeregger R., Zeyda M., Zlabinger G. J., Waldhausl W., Stulnig T. M. 2005. Polyunsaturated fatty acids interfere with formation of the immunological synapse. J. Leukoc. Biol. 77: 680–688. [DOI] [PubMed] [Google Scholar]
- 39.Dentin R., Denechaud P., Benhamed F., Girard J., Postic C. 2006. Hepatic gene regulation by glucose and polyunsaturated fatty acids: A role for ChREBP. J. Nutr. 136: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 40.Leroy C., Tricot S., Lacour B., Grynberg A. 2008. Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim. Biophys. Acta. 1781: 685–693. [DOI] [PubMed] [Google Scholar]
- 41.Fedor D., Kelley D. S. 2009. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 12: 138–146. [DOI] [PubMed] [Google Scholar]
- 42.Motoyama K. R., Curb J. D., Kadowaki T., El-Saed A., Abbott R. D., Okamura T., Evans R. W., Nakamura Y., Sutton-Tyrrell K., Rodriquez B. L., et al. 2009. Association of serum n-6 and n-3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am. J. Clin. Nutr. 90: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woudstra T. D., Drozdowski L. A., Wild G. E., Clandinin M. T., Agellon L. B., Thomson A. B. R. 2004. An isocaloric PUFA diet enhances lipid uptake and weight gain in aging rats. Lipids. 39: 343–354. [DOI] [PubMed] [Google Scholar]
- 44.Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]