Abstract
Surfactant accumulates in alveolar macrophages of granulocyte-macrophage colony-stimulating factor (GM-CSF) knockout (KO) mice and pulmonary alveolar proteinosis (PAP) patients with a functional loss of GM-CSF resulting from neutralizing anti–GM-CSF antibody. Alveolar macrophages from PAP patients and GM-CSF KO mice are deficient in peroxisome proliferator-activated receptor-γ (PPARγ) and ATP-binding cassette (ABC) lipid transporter ABCG1. Previous studies have demonstrated that GM-CSF induces PPARγ. We therefore hypothesized that PPARγ promotes surfactant catabolism through regulation of ABCG1. To address this hypothesis, macrophage-specific PPARγ (MacPPARγ) knockout mice were utilized. MacPPARγ KO mice develop foamy, lipid-engorged Oil Red O positive alveolar macrophages. Lipid analyses revealed significant increases in the cholesterol and phospholipid contents of MacPPARγ KO alveolar macrophages and extracellular bronchoalveolar lavage (BAL)–derived fluids. MacPPARγ KO alveolar macrophages showed decreased expression of ABCG1 and a deficiency in ABCG1-mediated cholesterol efflux to HDL. Lipid metabolism may also be regulated by liver X receptor (LXR)–ABCA1 pathways. Interestingly, ABCA1 and LXRβ expression were elevated, indicating that this pathway is not sufficient to prevent surfactant accumulation in alveolar macrophages. These results suggest that PPARγ mediates a critical role in surfactant homeostasis through the regulation of ABCG1.
Keywords: pulmonary alveolar proteinosis, granulocyte-macrophage colony-stimulating factor, peroxisome proliferator-activated receptor-γ
Pulmonary alveolar proteinosis (PAP) is a rare autoimmune lung disease characterized by neutralizing auto-antibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF) (1, 2). This loss of functional GM-CSF results in a filling of the alveolar spaces of the lungs with the lipoproteinaceous material called surfactant. While PAP is a rare lung disorder, surfactant abnormalities are problematic in many lung diseases, including acute respiratory distress syndrome (ARDS), sarcoidosis, and asthma (3).
Pulmonary surfactant is comprised of 90% lipid, 10% protein, and less than 1% carbohydrate. Phospholipids are the major lipid in surfactant and are associated with four surfactant-associated proteins (SP-A, -B, -C, and -D). SP-B and SP-C contribute to the surface tension–lowering properties of surfactant, and SP-A and SP-D are actively involved in the innate immunity of the lung (4). Other lipids associated with surfactant include cholesterol, triglycerides, and free fatty acids. Cholesterol is the major neutral lipid (up to 90%) in pulmonary surfactant (5). Surfactant is produced by type II pneumocytes; two pathways have been described in the clearance of surfactant (6). Type II cells endocytose surfactant lipids and complexes and recycle them into new surfactant. Alveolar macrophages phagocytose and degrade surfactant and are considered to be the primary cell involved in the clearance and catabolism of surfactant (7).
PAP patients produce normal levels of surfactant (8). The accumulation of surfactant in the lungs of PAP patients is due to insufficient surfactant catabolism by alveolar macrophages (8–10). Alveolar macrophages from PAP patients have an activated phenotype resembling foam cells and are engorged with neutral lipid, as evidenced by positive Oil Red O staining (11). The nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) is constitutively expressed in the alveolar macrophages of healthy controls and is upregulated by GM-CSF (12, 13). Our previous studies have shown that the alveolar macrophages of PAP patients and the GM-CSF knockout (GM-CSF KO) mouse model of PAP are deficient in PPARγ (12, 14).
While the role of PPARγ in surfactant catabolism in the lung remains unclear, PPARγ is known to directly and indirectly regulate many genes involved in cholesterol metabolism and transport, including the nuclear transcription factor liver X receptor α (LXRα) and ATP-binding cassette (ABC) lipid transporters ABCG1 and ABCA1 (14–17). Studies have suggested that PPARγ deficiencies result in decreased expression of ABCG1 (14, 16). The deletion of ABCG1 in mice (ABCG1 KO) results in severe pulmonary lipidosis (18). Cholesterol and phospholipid accumulate and foam-cell formation occurs in the macrophages of ABCG1 KO (18–20). Moreover, ABCG1 KO macrophages display reduced capacities to efflux cholesterol and phospholipid (19, 21–23). We therefore hypothesized that PPARγ may promote surfactant catabolism through regulation of the lipid transporter ABCG1. To test this hypothesis, we investigated the alveolar macrophages from macrophage-specific PPARγ knockout (MacPPARγ KO) mice.
MATERIALS AND METHODS
Mice
Animal studies were conducted in conformity with Public Health Service policy on the humane care and use of laboratory animals and were approved by the Institutional Animal Care Committee. C57Bl/6 wild-type (WT) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Macrophage-specific PPARγ knockout (MacPPARγ KO) mice have been previously described (24). Bronchoalveolar lavage (BAL) cells were obtained as described earlier from 8- to 12-week-old MacPPARγ KO mice and age- and gender-matched wild-type C57Bl/6 controls (24). Briefly, the thoracic cavity was opened and the lungs were exposed. After cannulating the trachea, a tube was inserted, and BAL was carried out with warmed (37°C) PBS in 1 ml aliquots × 5. Except where indicated, the sample number (n) refers to sets of BAL cells pooled from 3–5 mice, whereas BAL fluid was analyzed from individual mice. Following previously established guidelines for analysis of acellular components of BAL fluid (25), analysis of BAL fluid lipid used samples with similar volumes recovered [range, 4.25–5.0 ml for wild type; 4.1–4.8 ml for MacPPARγ KO]. Cell viability was measured by trypan blue exclusion. BAL cell differentials from all animals used in the experiments were stained with a Wright-Giemsa stain and revealed >90% macrophages. Cytospins of BAL cells were stained with Oil Red O to detect intracellular neutral lipids. BAL cells were fixed in 4% paraformaldehyde, stained with Gill's hematoxylin (Sigma, St. Louis, MI), and incubated in Oil Red O solution (Rowley Biochemical Inc., Danvers, MA) overnight. BAL cells were washed in 85% propylene glycol and mounted in Mount-Quick aqueous mounting medium (Daido Sangyo Co., Tokyo, Japan). Oil Red O positivity was quantified by counting 100 cells on each cytospin slide from C57Bl/6 and MacPPARγ KO mice.
RNA purification and analysis
Total RNA was extracted from BAL cells by the RNeasy protocol (Qiagen, Valencia, CA). Expression of mRNA was determined by real-time RT-PCR analysis using the ABI Prism 7300 Detection System (TaqMan; Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. RNA specimens were analyzed in duplicate using primer sets for mouse LXRα (Mm00443454), LXRβ (Mm00437262), ABCA1 (Mm00442646), ABCG1 (Mm00437390), CYP27A1 (Mm00470430), and APOE (Mm00437573) (Applied Biosystems). Relative gene expression was quantified as described (26). Briefly, the control group (C57Bl/6) values were calculated by subtracting the raw cycle (CT) data for the housekeeping gene (GAPDH, 4352339E) from the cycle data for the gene of interest. The ensuing values (ΔCT) were averaged and normalized to 1.0. Data from MacPPARγ KO were quantified in a similar fashion and expressed as relative mRNA expression compared with wild-type. For these experiments, BAL cells were isolated from individual MacPPARγ KO mice and compared with pooled samples of C57Bl/6 BAL cells.
Cholesterol efflux assay
Pooled BAL cells (3.5 × 105/well) were plated in 48-well cell culture plates in complete DMEM media (Invitrogen, Carlsbad, CA) and maintained at 37°C and 5% CO2. Nonadherent cells were removed after 1 h. Cells were incubated for 24 h in 2 μCi/ml of [1,2-3H(N)]cholesterol (NEN, Perkin Elmer, Waltham, MA), equilibrated in serum-free media for 24 h, and incubated in the presence of 10% fetal bovine serum (FBS), apolipoprotein A-I (ApoA-I) (25 μg/ml) (Sigma) or HDL (25 μg/ml) (Intracel, Frederick, MD) for 24 h. Supernatant fluids were harvested and centrifuged at 1800 rpm for 5 min to remove cellular debris. Cells were washed with PBS and lysed in a 0.2 M sodium hydroxide (NaOH) and 0.1% SDS solution for 1 h at room temperature. Supernatant and cell-associated radioactivity was measured by liquid scintillation. Cholesterol efflux was expressed as the percentage of radioactivity in the supernatant divided by the total radioactivity of the cells and supernatant. Each assay was performed in duplicate, and results from three independent assays were used to calculate percentage efflux.
Immunoblotting
For analysis of BAL cell protein, samples were loaded based on equal total protein determined using a modified Lowry assay (Dc Protein Assay, Bio-Rad Laboratories, Hercules, CA). Gels were eletrophoresed under reducing conditions using a 10% Bis-Tris gel (Bio-Rad) with MOPS buffer (Invitrogen). The following primary antibodies and dilutions were used: 1:500 ABCG1 (sc-11150) and 1:500 ABCA1 (sc-5491, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Bands corresponding to ABCG1 were normalized to β-actin as the loading control, and intensity of the protein bands were quantified using ImageQuant TL (GE Healthcare, Little Chalfont, UK). Bands corresponding to ABCA1 were analyzed in the same manner using ImageJ.
Lipid extraction
Total lipids were extracted from BAL cells and fluid using a modified method of Bligh and Dyer in HPLC-grade chloroform/methanol/1M sodium chloride (NaCl) (2/1/1.25, v/v/v) (Sigma) (27). The organic phase was obtained by centrifugation at 1500 rpm. Lipids were dried under a gentle stream of nitrogen gas. The Phospholipids C kit (Wako Pure Chemicals, Osaka, Japan) was used according to manufacturer's instructions. Phospholipid content was expressed as mg phospholipid per mg protein.
Cholesterol content analysis
The total cholesterol content from BAL cells and fluid was analyzed using the Amplex Red Cholesterol Assay (Invitrogen) according to the manufacturer's protocol. Samples were assayed in serial dilution in 96-well plates. Cholesterol content was expressed as μg cholesterol per mg protein.
Statistical analysis
Data were analyzed by Student's t-test using Prism software (GraphPad, Inc., San Diego, CA). Significance was defined as P ≤ 0.05.
RESULTS
PPARγ deficiency results in lipid accumulation and dysregulation of lipid transporters in alveolar macrophages
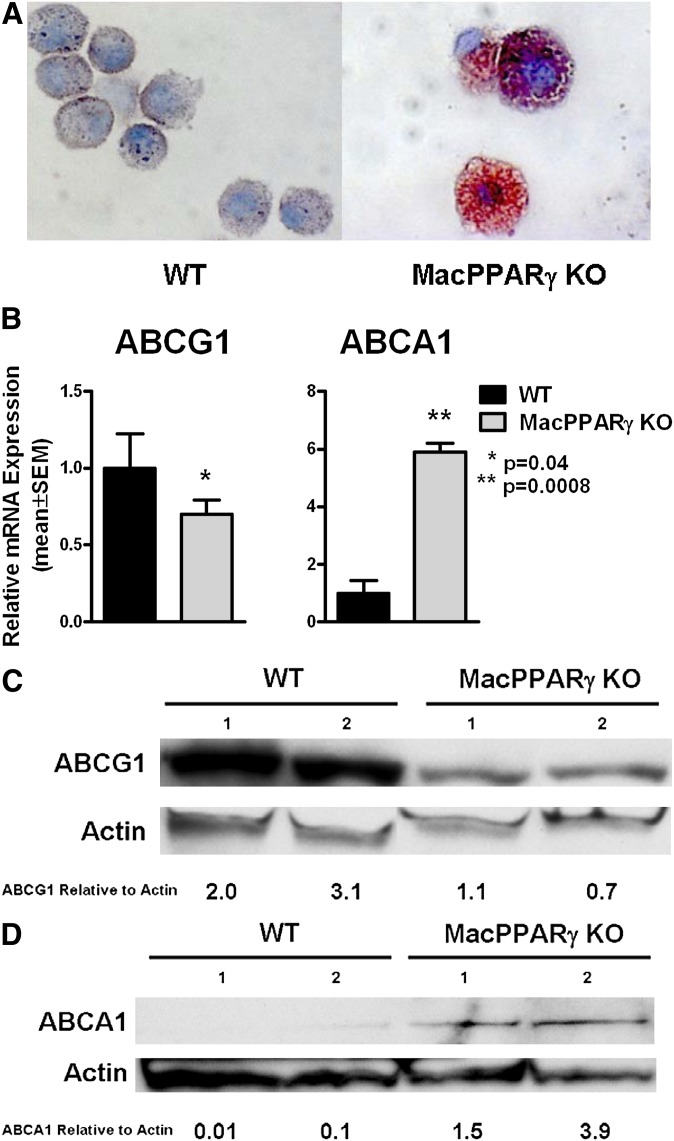
Wright-Giemsa staining revealed large foamy alveolar macrophages and Oil Red O staining showed that 88.8 ± 1.7% of MacPPARγ KO alveolar macrophages stained positive, compared with 2.4 ± 1.0% of wild type, indicating neutral lipid accumulation in the MacPPARγ KO (P < 0.0001) (Fig. 1A). Because of the lipid accumulation, we evaluated mRNA expression of the lipid transporters ABCG1 and ABCA1, which are known to be involved in lipid metabolism in macrophages and are downstream targets of PPARγ (28). ABCG1 mRNA was decreased by 30%; in contrast, ABCA1 was increased 5.9-fold (Fig. 1B). Decreased ABCG1 and increased ABCA1 protein expression were confirmed by immunoblotting (Fig. 1C–D).
Fig. 1.
PPARγ deficiency results in dysregulation of lipid metabolism in alveolar macrophages. (A) Marked Oil Red O staining of alveolar macrophages from MacPPARγ KO indicates neutral lipid accumulation compared with wild-type (n = 3). (B) ABCG1 is decreased whereas ABCA1 expression is enhanced in MacPPARγ KO compared with wild-type as measured by RT-PCR (n = 6). Data represent mean ± SEM. (C) ABCG1 protein is decreased and (D) ABCA1 protein is increased in MacPPARγ KO alveolar macrophages as shown in a representative immunoblot from one of two experiments. The numbers above the bands refer to sets of pooled BAL cells from each genotype. The relative ratios of the ABCG1 and ABCA1 to actin are indicated. ABC, ATP binding cassette; KO, knockout; MacPPARγ, macrophage-specific PPARγ; WT, wild-type.
Surfactant lipids accumulate in the lungs of MacPPARγ KO mice
The composition of the lipid accumulating in the lungs of the MacPPARγ KO was determined by measuring both extracellular and intracellular cholesterol and phospholipid levels in BAL fluids and alveolar macrophages. Compared with wild-type mice, cellular content of free cholesterol was significantly increased in MacPPARγ KO mice (0.39 ± 0.07 versus 5.80 ± 1.69 μg/mg protein) while the cholesteryl ester content was not significantly different (0.12 ± 0.01 versus 0.58 ± 0.29 μg/mg protein) (Fig. 2A). Free cholesterol was also elevated in the BAL fluid of MacPPARγ KO mice (59.6 ± 5.7 μg/mg protein) compared with the wild-type mice (17.8 ± 1.3 μg/mg protein) (Fig. 2B). Cholesteryl esters were not detected in the BAL fluid of wild-type or MacPPARγ KO mice. The cellular phospholipid content in MacPPARγ KO alveolar macrophages was significantly increased over wild-type (0.03 ± 0.01 versus 0.26 ± 0.07 mg/mg protein) (Fig. 2C). Extracellular phospholipids were elevated in the BAL fluid of MacPPARγ KO mice (257.5 ± 28.9 mg/mg protein) compared with wild-type (174.2 ± 16.0 mg/mg protein) (Fig. 2D).
Fig. 2.
Surfactant lipids accumulate in the lungs of MacPPARγ KO mice. (A–B) The free cholesterol content of MacPPARγ KO alveolar macrophages (n = 3 sets) and BAL fluid (n = 5) is increased. Total and free cholesterol were measured and cholesteryl ester was determined by subtraction (51). (C–D) The phospholipid content is also increased in the alveolar macrophages (n = 4 sets) and BAL fluid (n = 11) of MacPPARγ KO mice. Data represent mean ± SEM. BAL, bronchoalveolar lavage; KO, knockout; MacPPARγ, macrophage-specific PPARγ; WT, wild-type.
PPARγ deficiency results in decreased cholesterol efflux to HDL from alveolar macrophages
The accumulation of cholesterol in the lungs and alveolar macrophages of the MacPPARγ KO and decreased expression of key cholesterol efflux mediators led us to evaluate the cholesterol efflux system. Baseline cholesterol efflux (no acceptor) was increased in the MacPPARγ KO alveolar macrophages (8.3 ± 0.8%) compared with wild-type (4.5 ± 0.3%), and the overall cholesterol efflux to media supplemented with FBS was decreased in the MacPPARγ KO (59.5 ± 1.7%) relative to wild-type (70.5 ± 3.5%) (Fig. 3). We next measured the efflux of cholesterol to acceptor molecules HDL and ApoA-I. Cholesterol efflux to ApoA-I in MacPPARγ KO (25.7 ± 1.7%) was significantly increased over wild-type (17.3 ± 1.5%), and efflux to HDL was significantly decreased in MacPPARγ KO (46.2 ± 1.5%) compared with wild-type (56.7 ± 3.6%). These results suggest impairment of ABCG1-mediated cholesterol efflux.
Fig. 3.
PPARγ deficiency results in decreased cholesterol efflux to HDL from alveolar macrophages. The efflux of 3H labeled cholesterol was measured in MacPPARγ KO alveolar macrophages and compared with wild type (n = 3). Apo, apolipoprotein; KO, knockout; MacPPARγ, macrophage-specific PPARγ; WT, wild-type.
PPARγ deficiency results in dysregulated LXRalpha and LXRbeta expression
Given the increased expression of ABCA1 in MacPPARγ KO alveolar macrophages, we next investigated the expression of the LXR transcription factors, which may regulate cholesterol metabolism in macrophages in part by mediating transcription of the ABC transporters (29). RT-PCR analysis revealed LXRα mRNA was downregulated by 40% and LXRβ mRNA was upregulated 2.1-fold in MacPPARγ KO alveolar macrophages (Fig. 4A). RT-PCR analysis also revealed increased expression in sterol 27-hydroxylase (CYP27A1) (2.3-fold) and apolipoprotein E (ApoE) mRNA (34-fold) in MacPPARγ KO alveolar macrophages, indicating that the LXR pathway is enhanced (Fig. 4B).
Fig. 4.
PPARγ deficiency results in dysregulated LXRα and LXRβ expression. (A) Gene expression of LXRα and LXRβ in BAL cells from wild-type (n = 4) and MacPPARγ KO mice (n = 6) were analyzed by RT-PCR. LXRα mRNA expression is decreased in MacPPARγ KO alveolar macrophages while LXRβ is increased. (B) CYP27A1 and ApoE mRNA expression are increased in MacPPARγ KO alveolar macrophages (n = 6) compared with wild type (n = 5). Data represent mean ± SEM. Apo, apolipoprotein; KO, knockout; LXR, liver X receptor; MacPPARγ, macrophage-specific PPARγ; WT, wild-type.
DISCUSSION
In the present study we show that the targeted knockout of PPARγ in macrophages results in the accumulation of surfactant-like material in the alveolar spaces of the lung and within the alveolar macrophages. This is the first report directly linking the deficiency of PPARγ to lipid accumulation in the lung. MacPPARγ KO alveolar macrophages phenotypically resemble those of PAP patients in that they are foamy and Oil Red O–positive for neutral lipid accumulation (11). Additionally, surfactant lipid components (phospholipid and cholesterol) are increased within extracellular BAL fluids. Finally, the alveolar macrophages of MacPPARγ KO mice are ABCG1-deficient and exhibit reduced ABCG1-mediated cholesterol efflux to HDL. Our results support the hypothesis that PPARγ-mediated regulation of ABCG1 expression is critical for surfactant catabolism in alveolar macrophages.
Previous studies have suggested that PPARγ is a key mediator of surfactant clearance and catabolism by alveolar macrophages (12, 14). Surfactant accumulates in alveolar macrophages of PAP patients. PPARγ is deficient in the alveolar macrophages of these patients and is associated with the presence of neutralizing auto-antibodies against the hematopoietic growth factor GM-CSF (12). PPARγ deficiencies were also demonstrated in the GM-CSF KO mouse model of the disease (14).
GM-CSF also promotes cell survival, proliferation, and differentiation of alveolar macrophages and promotes the transcription of PPARγ in macrophages (13, 30, 31). The biological loss of GM-CSF has been reported to impair the differentiation of alveolar macrophages through dysregulation of the transcription factor PU.1 (4). It was further demonstrated that PU.1 is deficient in the alveolar macrophages of PAP patients and GM-CSF KO mice (32, 33); however, no deficiency in GM-CSF or PU.1 mRNA expression was observed in the alveolar macrophages of the MacPPARγ KO mice. GM-CSF was upregulated 2.7 ± 0.25-fold (n = 5, P = 0.02) while PU.1 expression was not different from wild-type mice (n = 3). These data suggest that maturation of the MacPPARγ KO alveolar macrophages is not disrupted as it is in PAP patients and GM-CSF KO mice (4). This is consistent with current literature, which suggests that although PPARγ is not necessary for the differentiation of monocytes, a variation in the expression levels of PPARγ may modulate differentiation (34–36).
Consistent with the findings in PAP patients and GM-CSF KO mice (37–41), the MacPPARγ KO mice exhibit elevated levels of the major lipid components of surfactant, including cholesterol and phospholipid, in the BAL fluid and alveolar macrophages. The alveolar macrophages from the MacPPARγ KO mice had significant accumulation of free (unesterified) cholesterol. Cellular deposition of cholesterol is considered to be an initial step in foam-cell formation (42) and is consistent with the foamy phenotype of the MacPPARγ KO alveolar macrophages. The impact that free cholesterol accumulation has on cell signaling, plasma membrane rigidity, and induction of pro-apoptotic cascades warrants further study (43). The pattern of lipid accumulation both in the alveolar space and alveolar macrophages of the lungs of MacPPARγ KO mice suggests deficient or incomplete surfactant catabolism by the alveolar macrophages.
RT-PCR analysis of the MacPPARγ KO alveolar macrophages revealed similar gene expression patterns of downstream PPARγ direct and indirect targets to those previously reported from PAP patients and GM-CSF KO mice with decreased expression of ABCG1 mRNA and increased expression in ABCA1 mRNA (12, 14). These results, which are consistent with several studies, indicate that deficiency of one ABC transporter is compensated by the other transporter and is mediated by the sterol-sensing nuclear transcription factor LXR in response to the buildup of oxysterol ligands (44) or the oxidation of cholesterol metabolites by CYP27A1 in foamy macrophages (45). The two isoforms of LXR—LXRα and LXRβ—have overlapping roles in promoting cellular cholesterol export through regulation of the ABC transporters and ApoE (29).
In contrast to the increased LXRα expression reported in PAP and GM-CSF KO (14), LXRα was downregulated in the MacPPARγ KO mice. The differential expression of LXRα, which is regulated in part by PPARγ (16), may be explained by the varying levels of PPARγ in these systems: PPARγ is deficient in PAP and GM-CSF KO while it is absent in MacPPARγ KO. This is supported by the finding that LXRβ expression, which is regulated independently of PPARγ (16), is increased 2-fold in the MacPPARγ KO alveolar macrophages. The expression of LXRβ has not been reported in PAP or GM-CSF KO alveolar macrophages.
While more study is needed to elucidate the possible mechanisms and differential regulation of the LXRs, it has been shown that the function and expression of the LXR isoforms are tissue-dependent (29, 46, 47). LXRβ is expressed at higher levels than LXRα in macrophages and is more effective than LXRα at upregulating ABCA1 in response to sterol ligands (48). While the contributions of the individual LXR isoforms are unknown, as specific gene targets have yet to be identified, the LXR pathway overall is enhanced in the MacPPARγ KO, as evidenced by increased expression of downstream targets ABCA1 and ApoE. We show that increased expression of the LXR pathway is not sufficient to maintain surfactant catabolism in the absence of PPARγ.
The accumulation of cholesterol in the lungs and alveolar macrophages of MacPPARγ KO mice and the dysregulation of several cholesterol transport genes led us to investigate the efflux of cholesterol in MacPPARγ KO alveolar macrophages in vitro. PPARγ promotes lipid influx and efflux in macrophages through transcriptional regulation of ABC transporters and LXRs. ABCG1 mediates transport of cholesterol to extracellular acceptor HDL, and ABCA1 transports cholesterol to lipid-free ApoA-I (19, 22, 23, 49, 50). In the present study, overall cholesterol efflux to FBS (10% serum) was decreased compared with wild-type, consistent with the high levels of cholesterol in the macrophages in vivo. ABCA1-mediated efflux to ApoA-I was increased, while ABCG1-mediated cholesterol efflux to HDL was reduced. These findings indicate that the reduction in cholesterol efflux in the MacPPARγ KO alveolar macrophages may be because of deficient transporter-mediated cholesterol efflux pathways, specifically transport mediated by ABCG1.
Interestingly, similar patterns of cholesterol and phospholipid accumulation and altered lipid efflux have been reported in ABCG1 KO models (18, 20). A reduction in total cholesterol efflux and a specific reduction in efflux to HDL were noted in ABCG1 KO peritoneal macrophages (20). Importantly, the authors also noted a significantly increased efflux to ApoA-I in ABCG1 KO, which indicated compensation by ABCA1. Taken together, deficiencies in ABCG1 may result in dysregulated or insufficient cholesterol efflux and, therefore, cholesterol accumulation in the lung.
A summary of the differential gene expression of various lipid regulators and transporters in the alveolar macrophages from MacPPARγ KO mice, GM-CSF KO mice, and PAP patients is presented in Table 1. Comparison of the data supports the hypothesis that PPARγ-mediated regulation of ABCG1 is necessary to prevent the accumulation of surfactant. Additionally, the LXR pathway is enhanced in all of the groups, as evidenced by increased expression in ABCA1. An interesting difference, however, is the expression of LXRα, which is increased in PAP and GM-CSF KO (PPARγ-deficient) alveolar macrophages and decreased in the MacPPARγ KO model. We speculate that lipid accumulation activates the LXR-ABCA1 pathway as a compensation mechanism and that, in the absence of PPARγ, LXRβ is the predominant isoform driving the upregulation of ABCA1 and ApoE.
TABLE 1.
Summary of expression levels of key lipid regulator and transporter genes in the alveolar macrophages from MacPPARγ KO mice, GM-CSF KO mice, and PAP patients
| Lipid Regulator | MacPPARγ KO Mice | GM-CSF KO Mice | PAP Patients |
|---|---|---|---|
| GM-CSF | Increased | Not expressed (30) | Decreaseda (52) |
| PPARγ | Decreased | Decreased (14) | Decreased (12) |
| ABCG1 | Decreased | Decreased (14) | Decreased (14) |
| ABCA1 | Increased | Increased (14) | Increased (14) |
| LXRα | Decreased | Increased (14) | Increased (14) |
| LXRβ | Increased | Not reported | Not reported |
Abbreviations: ABC, ATP binding cassette; GM-CSF, granulocyte-macrophage colony-stimulating factor; KO, knockout; LXR, liver X receptor; MacPPARγ, macrophage-specific PPARγ; PAP, pulmonary alveolar proteinosis. Numbers in parentheses are reference citations.
In the MacPPARγ KO mouse model, the absence of PPARγ results in reduced expression levels of ABCG1 and LXRα. Despite increased expression of LXRβ and ABCA1 and increased ABCA1-mediated cholesterol efflux, surfactant components accumulate in the alveolar macrophages and BAL fluid of MacPPARγ KO mice. Our results indicate that as part of surfactant catabolism, ABCG1-mediated cholesterol efflux to HDL may be a pathway for cholesterol efflux in alveolar macrophages. Thus PPARγ-mediated regulation of ABCG1 expression may be critical to the maintenance of surfactant homeostasis. This is the first report directly linking PPARγ deficiency in alveolar macrophages to lipid accumulation in the lungs. Understanding the role of PPARγ in normal surfactant homeostasis contributes to our knowledge of the pathophysiology of PAP and identifies a potential target for therapy.
Footnotes
Abbreviations:
- ABC
- ATP binding cassette
- apo
- apolipoprotein
- BAL
- bronchoalveolar lavage
- CE
- cholesteryl ester
- Cyp27A1
- cytochrome P450 sterol 27-hydroxylase
- FC
- free cholesterol
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- LXR
- liver X receptor
- PAP
- pulmonary alveolar proteinosis
- PPARγ
- peroxisome proliferator-activated receptor-gamma
- SP
- surfactant-associated protein
This work was supported by Faculty Recruitment Grant 2005-FRG-1013 (M.J.T.) from the North Carolina Biotechnology Center.
REFERENCES
- 1.Kitamura T., Tanaka N., Watanabe J., Uchida K., Kanegasaki S., Yamada Y., Nakata K. 1999. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 190: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomassen M. J., Yi T., Raychaudhuri B., Malur A., Kavuru M. S. 2000. Pulmonary alveolar proteinosis is a disease of decreased availability of GM-CSF rather than an intrinsic cellular defect. Clin. Immunol. 95: 85–92. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen G. L., Husby S., Holmskov U. 2007. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology. 212: 381–416. [DOI] [PubMed] [Google Scholar]
- 4.Trapnell B. C., Whitsett J. A. 2002. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 64: 775–802. [DOI] [PubMed] [Google Scholar]
- 5.Veldhuizen R., Nag K., Orgeig S., Possmayer F. 1998. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta. 1408: 90–108. [DOI] [PubMed] [Google Scholar]
- 6.Hawgood S., Poulain F. R. 2001. The pulmonary collectins and surfactant metabolism. Annu. Rev. Physiol. 63: 495–519. [DOI] [PubMed] [Google Scholar]
- 7.Trapnell B. C., Whitsett J. A., Nakata K. 2003. Pulmonary alveolar proteinosis. N. Engl. J. Med. 349: 2527–2539. [DOI] [PubMed] [Google Scholar]
- 8.Alberti A., Luisetti M., Braschi A., Rodi G., Iotti G., Sella D., Poletti V., Benori V., Baritussio A. 1996. Bronchoalveolar lavage fluid composition in alveolar proteinosis. Early changes after therapeutic lavage. Am. J. Respir. Crit. Care Med. 154: 817–820. [DOI] [PubMed] [Google Scholar]
- 9.Huffman J. A., Hull W. M., Dranoff G., Mulligan R. C., Whitsett J. A. 1996. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J. Clin. Invest. 97: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida M., Ikegami M., Reed J. A., Chroneos Z. C., Whitsett J. A. 2001. GM-CSF regulates protein and lipid catabolism by alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 280: L379–L386. [DOI] [PubMed] [Google Scholar]
- 11.Iyonaga K., Suga M., Yamamoto T., Ichiyasu H., Miyakawa M., Ando M. 1999. Elevated bronchoalveolar concentrations of MCP-1 in patients with pulmonary alveolar proteinosis. Eur. Respir. J. 14: 383–389. [DOI] [PubMed] [Google Scholar]
- 12.Bonfield T. L., Farver C. F., Barna B. P., Malur A., Abraham S., Raychaudhuri B., Kavuru M. S., Thomassen M. J. 2003. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 29: 677–682. [DOI] [PubMed] [Google Scholar]
- 13.Ricote M., Huang J., Fajas L., Li A., Welch J., Najib J., Witztum J. L., Auwerx J., Palinski W., Glass C. K. 1998. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidixed low density lipoprotein. Proc. Natl. Acad. Sci. USA. 95: 7614–7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomassen M. J., Barna B. P., Malur A., Bonfield T. L., Farver C. F., Malur A., Dalrymple H., Kavuru M. S., Febbraio M. 2007. ABCG1 is deficient in alveolar macrophages of GM-CSF knock-out mice and patients with pulmonary alveolar proteinsosis. J. Lipid Res. 48: 2762–2768. [DOI] [PubMed] [Google Scholar]
- 15.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla A., Boisvert W. A., Laffitte B. A., Barak Y., Liao D., Nagy L., Edwards P. A. 2001. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 7: 161–171. [DOI] [PubMed] [Google Scholar]
- 17.Chinetti G., Lestavel S., Bocher V., Remaley A. T., Neve B., Torra I. P., Teissier E., Minnich A., Jaye M., Duverger N., et al. 2001. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 7: 53–58. [DOI] [PubMed] [Google Scholar]
- 18.Baldan A., Tarr P., Vales C. S., Frank J., Shimotake T. K., Hawgood S., Edwards P. A. 2006. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J. Biol. Chem. 281: 29401–29410. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy M. A., Barrera G. C., Nakamura K., Baldan A., Tarr P., Fishbein M. C., Frank J., Francone O. L., Edwards P. A. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131. [DOI] [PubMed] [Google Scholar]
- 20.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., Tall A. R. 2007. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117: 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klucken J., Buchler C., Orso E., Kaminski W. E., Porsch-Ozcurumez M., Liebisch G., Kapinsky M., Diederich W., Drobnik W., Dean M., et al. 2000. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA. 97: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malur A., Mccoy A. J., Arce S., Barna B. P., Kavuru M. S., Malur A. G., Thomassen M. J. 2009. Deletion of PPARγ in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J. Immunol. 182: 5816–5822. [DOI] [PubMed] [Google Scholar]
- 25.Haslam P. L., Baughman R. P. 1999. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur. Respir. J. 14: 245–248. [DOI] [PubMed] [Google Scholar]
- 26.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 27.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama T. E., Sakai S., Lambert G., Nicol C. J., Matsusue K., Pimprale S., Lee Y. H., Ricote M., Glass C. K., Brewer H. B., et al. 2002. Conditional disruption of the peroxime proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22: 2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castrillo A., Tontonoz P. 2004. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu. Rev. Cell Dev. Biol. 20: 455–480. [DOI] [PubMed] [Google Scholar]
- 30.Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A., et al. 1994. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 264: 713–716. [DOI] [PubMed] [Google Scholar]
- 31.Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. 1985. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 230: 1171–1173. [DOI] [PubMed] [Google Scholar]
- 32.Bonfield T. L., Raychaudhuri B., Malur A., Abraham S., Trapnell B. C., Kavuru M. S., Thomassen M. J. 2003. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am. J. Physiol. Lung Cell. Mol. Physiol. 285: L1132–L1136. [DOI] [PubMed] [Google Scholar]
- 33.Shibata Y., Berclaz Y. P., Chroneos Z. C., Yoshida M., Whitsett J. A., Trapnell B. C. 2001. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 15: 557–567. [DOI] [PubMed] [Google Scholar]
- 34.Chawla A., Barak Y., Nagy L., Liao D., Tontonoz P., Evans R. M. 2001. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat. Med. 7: 48–52. [DOI] [PubMed] [Google Scholar]
- 35.Moore K. J., Rosen E. D., Fitzgerald M. L., Randow F., Andersson L. P., Altshuler D., Milstone D. S., Mortensen R. M., Spiegelman B. M., Freeman M. W. 2001. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat. Med. 7: 41–47. [DOI] [PubMed] [Google Scholar]
- 36.Szanto A., Nagy L. 2005. Retinoids potentiate peroxisome proliferator-activated receptor gamma action in differentiation, gene expression, and lipid metabolic processes in developing myeloid cells. Mol. Pharmacol. 67: 1935–1943. [DOI] [PubMed] [Google Scholar]
- 37.Crouch E., Persson A., Chang D. 1993. Accumulation of surfactant protein D in human pulmonary alveolar proteinosis. Am. J. Pathol. 142: 241–248. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B. M., Stern E. J., Schmidt R. A., Pierson D. J. 1997. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest. 111: 460–466. [DOI] [PubMed] [Google Scholar]
- 39.Doyle I. R., Davidson K. G., Barr H. A., Nicholas T. E., Payne K., Pfitzner J. 1998. Quantity and structure of surfactant proteins vary among patients with alveolar proteinosis. Am. J. Respir. Crit. Care Med. 157: 658–664. [DOI] [PubMed] [Google Scholar]
- 40.Meaney S., Bonfield T. L., Hansson M., Babiker A., Kavuru M. S., Thomassen M. J. 2004. Serum cholestenoic acid as a potential marker of pulmonary cholesterol homeostasis: increased levels in patients with pulmonary alveolar proteinosis. J. Lipid Res. 45: 2354–2360. [DOI] [PubMed] [Google Scholar]
- 41.Abe A., Hiraoka M., Wild S., Wilcoxen S. E., Paine R., III, Shayman J. A. 2004. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J. Biol. Chem. 279: 42605–42611. [DOI] [PubMed] [Google Scholar]
- 42.Ross R. 1995. Cell biology of atherosclerosis. Annu. Rev. Physiol. 57: 791–804. [DOI] [PubMed] [Google Scholar]
- 43.Tabas I. 2002. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J. Clin. Invest. 110: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranalletta M., Wang N., Han S., Yvan-Charvet L., Welch C., Tall A. R. 2006. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler. Thromb. Vasc. Biol. 26: 2308–2315. [DOI] [PubMed] [Google Scholar]
- 45.Fu X., Menke J. G., Chen Y., Zhou G., MacNaul K. L., Wright S. D., Sparrow C. P., Lund E. G. 2001. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 276: 38378–38387. [DOI] [PubMed] [Google Scholar]
- 46.Mak P. A., Laffitte B. A., Desrumaux C., Joseph S. B., Curtiss L. K., Mangelsdorf D. J., Tontonoz P., Edwards P. A. 2002. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J. Biol. Chem. 277: 31900–31908. [DOI] [PubMed] [Google Scholar]
- 47.Laffitte B. A., Repa J. J., Joseph S. B., Wilpitz D. C., Kast H. R., Mangelsdorf D. J., Tontonoz P. 2001. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA. 98: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costet P., Luo Y., Wang N., Tall A. R. 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275: 28240–28245. [DOI] [PubMed] [Google Scholar]
- 49.Wang N., Silver D. L., Costet P., Tall A. R. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275: 33053–33058. [DOI] [PubMed] [Google Scholar]
- 50.Oram J. F., Lawn R. M., Garvin M. R., Wade D. P. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275: 34508–34511. [DOI] [PubMed] [Google Scholar]
- 51.Bates S. R., Tao J. Q., Collins H. L., Francone O. L., Rothblat G. H. 2005. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289: L980–L989. [DOI] [PubMed] [Google Scholar]
- 52.Thomassen M. J., Raychaudhuri B., Bonfield T. L., Malur A., Abraham S., Barna B. P., Kavuru M. S. 2003. Elevated IL-10 inhibits GM-CSF synthesis in pulmonary alveolar proteinosis. Autoimmunity. 36: 285–290. [DOI] [PubMed] [Google Scholar]