Abstract
Several psychiatric disorders increase the risk of cardiovascular disease, including posttraumatic stress disorder and major depression. While the precise mechanism for this association has not yet been established, it has been shown that certain disorders promote an unfavorable lipid profile. To study the interaction of stress and lipid dysregulation, we utilized chronic social defeat stress (CSDS), a mouse model of chronic stress with features of posttraumatic stress disorder and major depression. Following exposure to CSDS, mice were given access to either regular chow or a Western-style diet high in fat and cholesterol (HFD). The combination of social stress and HFD resulted in significant perturbations in lipid regulation, including two key features of the metabolic syndrome: increased plasma levels of non–HDL cholesterol and intrahepatic accumulation of triglycerides. These effects were accompanied by a number of changes in the expression of hepatic genes involved in lipid regulation. Transcriptional activity of LXR, SREBP1c, and ChREBP were significantly affected by exposure to HFD and CSDS. We present CSDS as a model of social stress induced lipid dysregulation and propose that social stress alters lipid metabolism by increasing transcriptional activity of genes involved in lipid synthesis.
Keywords: anxiety, cholesterol, depression feeding, liver
Patients with psychiatric disorders have a significantly elevated risk of death from cardiovascular disease (1). A Swedish study of 9,911 patients reported an increased standardized mortality ratio of 120% from cardiovascular disease in patients with anxiety neurosis (2). A review of 4,328 male military veterans in the United States reported that a diagnosis of posttraumatic stress disorder (PTSD) was associated with a significant increase in the risk of death from early-age heart disease (3). Coryell and colleagues reported that patients with panic disorder were more than twice as likely to die from cardiovascular disease (4).
One potential mechanism linking anxiety and cardiovascular mortality is the development of metabolic syndrome. The metabolic syndrome consists of several risk factors that predispose an individual to cardiovascular disease and diabetes, including abdominal obesity, atherogenic dyslipidemia, hypertension, and insulin resistance (5). Studies have concluded that patients with psychiatric disorders, such as PTSD (6) and major depression, (7, 8) have an increased risk of developing the metabolic syndrome. Despite the clinical evidence supporting a link between mental illness and metabolic syndrome, the underlying mechanism has not been identified. Recently we have validated a chronic social defeat stress (CSDS) protocol for use in mice (9). CSDS shares features with many psychiatric disorders, including posttraumatic stress disorder, social anxiety, and major depression with comorbid anxiety (10, 11). In this model, mice subjected to repeated social aggression develop behavioral deficits similar to symptoms observed in human mental illness, including social avoidance and decreased preference for natural rewards such as sucrose.
Utilizing this model we now report the effect of social stress on plasma lipid profiles and lipid regulation. We have selected the C57BL/6 mouse line for these studies because of its vulnerability to the effects chronic social stress. Additionally, we have analyzed the effect of a Western-style diet high in cholesterol and triglyceride (high-fat diet, HFD) in both groups. CSDS by itself induces insulin resistance, as demonstrated by elevated fasting glucose levels in mice exposed to CSDS. The combination of CSDS with HFD induces a synergistic elevation in the plasma level of non–HDL cholesterol and intrahepatic accumulation of triglycerides—two key features of the metabolic syndrome. We present CSDS as a model of social stress–induced metabolic dysregulation.
MATERIALS AND METHODS
Animals and housing
Male 8–10-week-old C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) and CD1 retired breeder mice (Charles River, Wilmington, MA) were housed at the University of Texas Southwestern (UTSW) vivarium in a temperature-controlled environment (lights on: 0400–1600) with ad libitum access to water and standard chow (4% fat diet #7001, Harlan-Teklad, Madison, WI) or high-fat diet (protein/carbohydrate/fat: 15.2/42.7/42.0%kcal with 0.2% (w/w) cholesterol, TD.88137, Harlan-Teklad). At the completion of the 40-day study, mice were fasted overnight, and early the following morning, euthanized to acquire of blood and tissues. All animal procedures were carried out in accordance with the UT Southwestern Institutional Animal Care and Use Committee (IACUC) guidelines.
Hormone levels
Serum was collected from trunk blood after a 3-h fast. 4-(hydroxymercuri)benzoic acid Sodium Salt (#55540, Sigma Aldrich, St. Louis, MO) was added to preserve neuropeptides hormones. Insulin (#90060 Crystal Chem, Downers Grove, IL) and corticosterone (AC-15F1, Immunodiagnostic Systems, Fountain Hills, AZ) concentrations were determined from serum using ELISA kits as per the manufacturer's instructions. RIA kit (Cat. #GL-32K, Millipore, Billerica, MA) was used to measure glucagon levels.
Body composition and serum levels
Data reported was acquired by the UTSW Mouse Metabolic Phenotyping Core (http://www.utsouthwestern.edu/utsw/home/research/metstudcore/index.html). Body composition was determined by an mq10 series Bruker Minispec (Bruker Optics, The Woodlands, TX) 28 days after defeat.
Chronic social defeat stress
CSDS was carried out using a method reported recently (12). Test mice were exposed to a different CD1 strain aggressor mouse each day prior to lights out (1600) for 5 min over a total of 10 days. After the 5 min of physical contact, test mice were separated from the aggressor and placed across a plastic separator with holes, where they remained in sensory contact with the CD1 aggressor for the remainder of the 24 h. Controls were handled daily in the palm of the hand for 30 s and housed in equivalent cages with members of the same strain. After the last defeat, all mice were housed individually, and a social interaction test was performed to measure the behavioral consequences of the chronic defeat stress. Briefly, mice were placed in a new arena with a small animal cage at one end, and their movements tracked for 2.5 min in the absence of another mouse, followed by 2.5 min in the presence of a caged, unfamiliar target CD1 mouse. Social interaction was quantified by comparing the amount of time the test mouse spent in the interaction zone near the small-animal cage in the presence versus the absence of the target CD1 mouse using Ethovision 3.0 software (Noldus, Leesburg, VA).
Cholesterol and triglyceride levels
Lipids were extracted from 100–200 mg of frozen tissue using the Folch method. Cholesterol and triglyceride concentrations were obtained using the Triglyceride and Cholesterol Reagent (Fischer Scientific, Pittsburgh, PA) with readings at 515 nm (triglyceride) and 500 nm (cholesterol).
Quantitative PCR analysis
The epididymal white adipose, brown adipose, gastrocnemius muscle and liver of each mouse were collected, snap-frozen in liquid nitrogen, and stored at −85°C. Total RNA was isolated from these tissues using RNA STAT-60 (Tel-Test Inc., Friendswood, TX). RNA concentrations were determined by absorbance at 260 nm with a Thermo Scientific Nanodrop 100 Spectrophotometer. The total RNA was treated with RNase-free DNase (Roche, Palo Alto, CA) and reverse-transcribed into cDNA with SuperScript II reagents (Invitrogen, Carlsbad, CA) as previously described (13). Quantitative real-time PCR was performed using an Applied Biosystems 7900HT sequence detection system and SYBR-green chemistry (Applied Biosystems, Foster City, CA). The nucleotide sequences of the various primers used are presented in supplementary Table I. The mRNA levels are expressed relative to the housekeeping gene 36B4, calculated by the comparative threshold cycle (Ct) method (14).
Western blot
Snap-frozen mouse liver samples were lysed in cold lysis buffer, and membrane fractions were prepared as previously described (15). Protein concentrations were measured using the BCA protein assay kit (Pierce, Rockford, IL). For immunoblot analysis, 20 µg of liver membrane proteins were separated on an 8% denaturing SDS-polyacrylamide gel and were transferred to a polyvinylidene difluoride (#RPN2020F, GE Healthcare Life Sciences, Pittsburgh, PA) membrane. Rabbit polyclonal antiserum for LDL-R (16) was used with horseradish peroxidase-conjugated donkey anti-rabbit IgG as the secondary antibody. Antiserum against the C-terminal of human LDL receptor-related protein (LRP) was used for the detection of the invariant control protein (17). ECL western blot substrate (Pierce) was used to generate a signal by autoradiograph, and signal densities of bands were determined by analyzing scanned images with ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis of data
Data are reported as the mean ± SEM for the specified number of animals. GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA) was used to perform all statistical analyses. A two-way (15)(14) analysis of variance (ANOVA) was performed using CSDS and diet as factors. If a statistical interaction was observed between factors, comparison of all four groups was performed by Tukey posthoc comparison, and statistically different groups are indicated by different letters (P < 0.05). If no significant interaction between factors (CSDS and diet) was observed, statistical effects of one or both factors are denoted as an inset for each graph. Not significant (n.s.) was defined as P > 0.05; significant was defined as P < 0.05.
RESULTS
Metabolic consequences of CSDS
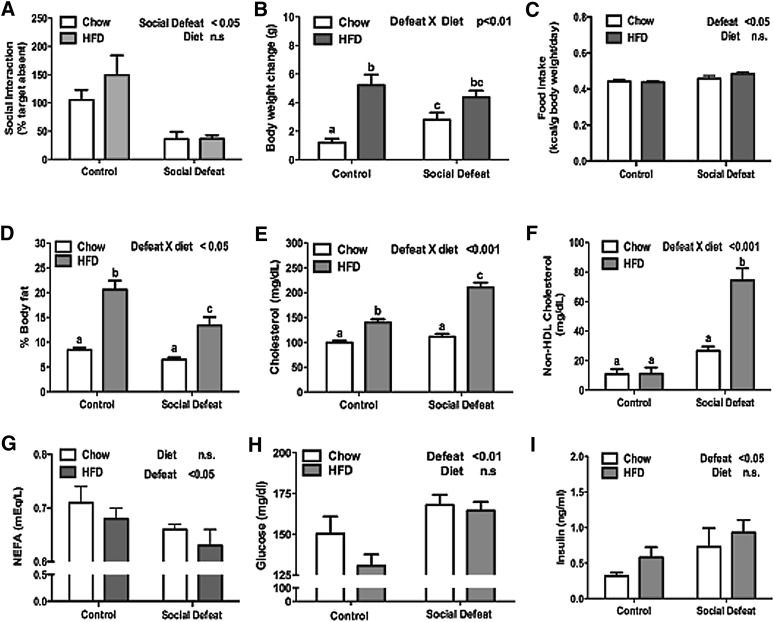
Following a 10-day exposure to CSDS, mice were tested for social interaction. Mice subjected to CSDS spent more time interacting with an inanimate object than with an unfamiliar mouse (Fig. 1A). This social avoidance is considered a measure of depression-like behavior because it can be reversed by chronic, but not acute, treatment with antidepressants (18). After testing for social avoidance, the mice were randomized into two equal groups based on interaction ratios. They received either low-fat, standard rodent chow or a diet high in fat and cholesterol (HFD). As expected, control mice given access to HFD gained more weight and body fat than chow-fed mice (Fig. 1B, D). Defeated mice given either diet showed an increase in calories consumed per gram body weight; both groups of defeated mice ate a similar number of calories (Fig. 1C). While the overall number of calories was similar between the CSDS groups, there was a significant difference in body composition. As noted, control mice fed HFD significantly increased body fat. In contrast, defeated mice maintained on HFD demonstrated a repartitioning of energy stores with significantly less body fat compared with control mice maintained on HFD (Fig. 1D).
Fig. 1.
Effect of social stress on metabolic parameters. Eight-week-old C57BL/6 male mice were subjected to CSDS (n = 14) or nonaggressive encounters (control, n = 14) and then randomized into groups receiving either chow or high-fat diet for 30 days. CSDS alters (A) social avoidance at day 11 and (B) body weight change at day 40. A subgroup of animals (n = 6/group) were further analyzed for (C) food intake, (D) body composition, (E) total cholesterol, (F) non–HDL cholesterol, (G) nonesterified fatty acids, (H) glucose, and (I) insulin. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups first tested by two-way ANOVA. If a statistical interaction was observed between factors, comparison of all four groups was performed by one-way ANOVA with Tukey posthoc comparison, and statistically different groups are indicated by different letters. CSDS, chronic social defeat stress; HFD, high-fat diet.
Next, we analyzed the lipid profiles of mice subjected to CSDS. The combination of CSDS and HFD produced significant increases in both total cholesterol and non–HDL cholesterol levels (Fig. 1E, F). Serum trigylcerides were not significantly different in any group (data not shown), which suggests that the majority of the increase in non–HDL cholesterol was composed of LDL cholesterol. While there was no difference in trigylceride levels, mice exposed to CSDS had significantly lower levels of nonesterified fatty acids (NEFA) than control animals (Fig. 1G). This finding is consistent with a previous report that demonstrated restraint stress in male rats resulted in an acute decrease in NEFA (19).
Finally, CSDS was associated with increased fasting glucose levels in both diets, accompanied by elevated insulin levels, which suggests a degree of insulin resistance (Fig. 1H, I). We attempted to confirm this finding by performing insulin and glucose tolerance tests. Unfortunately, mice subjected to CSDS are hypersensitive to the effects of handling, which resulted in large increases in glucose levels even after injection of saline (data not shown) that confounded efforts to determine insulin and glucose tolerance.
Regulation of non–HDL cholesterol
These findings demonstrate that the combination of CSDS and access to a HFD induce two key components of the metabolic syndrome: insulin resistance and elevated non–HDL cholesterol. To better understand the signaling pathways underlying these metabolic disturbances, we collected tissue samples of liver, white adipose tissue, brown adipose tissue, and muscle from mice at the conclusion of the experiment. As shown in Fig. 1F, the combination of social defeat stress and HFD induced a significant increase in the serum level of non–HDL cholesterol.
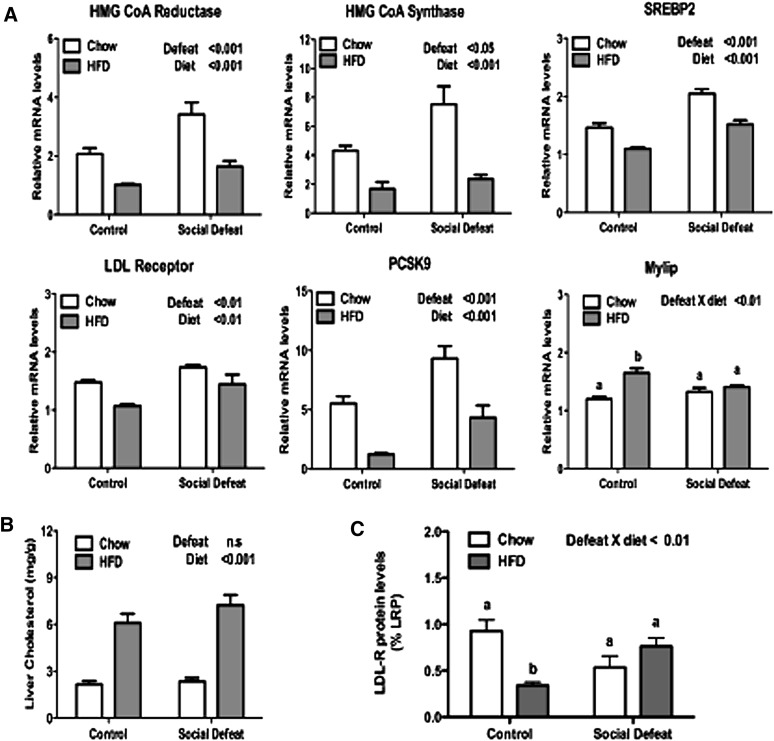
Using quantitative real-time PCR, we first measured the expression levels of genes involved in cholesterol synthesis. Cholesterol synthesis in the liver is controlled by a series of genes regulated by the transcription factor sterol regulatory element binding protein 2 (SREBP2) (20). The expression level of both of the cholesterol synthesis genes we measured, HMG-CoA reductase and HMG-CoA synthase, showed a similar pattern of regulation (Fig. 2A). Consistent with known results, a Western-style HFD rich in cholesterol suppressed mRNA levels of both genes. Social defeat, however, increased their expression, especially when fed regular chow.
Fig. 2.
Effects of CSDS on cholesterol homeostasis. After 30 days on either chow or HFD, mice were fasted 3 h and liver was collected. (A) Significant differences were noted by qPCR for genes involved in cholesterol homeostasis, including HMG-CoA reductase, HMG-CoA synthase, SREBP2, LDL receptor, PCSK9, and MYLIP. (B) Intrahepatic levels of cholesterol. (C) Relative levels of LDL-R protein by western blot with human LDL receptor-related protein (LRP) used as control. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups first tested by two-way ANOVA. If a statistical interaction was observed between factors, comparison of all four groups was performed by one-way ANOVA with Tukey posthoc comparison, and statistically different groups are indicated by different letters. CSDS, chronic social defeat stress; HFD, high-fat diet; MYLIP, myosin regulatory light chain interacting protein; PCSK9, proprotein convertase subtilisin/kexin type 9; SREBP2, sterol-regulatory element binding protein 2.
Next we analyzed expression levels of the LDL receptor, which is responsible for clearing cholesterol containing LDL particles from the blood stream. Similar to cholesterol synthesis genes, LDL receptor expression is reduced in mice maintained on HFD, but it is elevated by CSDS. LDL receptor, as well as HMG-CoA reductase and HMG-CoA synthase, are targets of the transcription factor SREBP2. Transcriptional control of the SREBP2 gene is autoregulated, and accordingly, SREBP2 levels displayed the same pattern of expression with HFD decreasing and social defeat increasing mRNA levels (Fig. 2A).
Elevated cellular levels of cholesterol inhibit SREBP2 maturation through a posttranslational process and, therefore, negatively regulate intracellular cholesterol levels by inhibiting both cholesterol synthesis and cholesterol uptake by LDL receptor. The failure to fully suppress SREBP2 activity after CSDS could be explained by a decrease in intrahepatic cholesterol in mice exposed to CSDS. We tested this possibility by measuring intrahepatic levels of cholesterol. Access to HFD elevated intrahepatic cholesterol levels in both control and CSDS mice (Fig. 2B). This finding suggests that the negative regulation of SREBP2 activity by intracellular cholesterol is impaired after CSDS.
In addition to transcriptional regulation, levels of LDL receptor protein are controlled by two additional pathways. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an SREBP2 target gene that increases serum levels of LDL by reducing LDL receptor protein levels (21–23). Interestingly, CSDS significantly elevates levels of PCSK9 (Fig. 2A) consistent with other SREBP2 target genes, which suggests that CSDS increases transcriptional activity of the SREBP2 transcription factor. LDL receptor protein levels are also regulated by the liver X receptor (LXR) target gene myosin regulatory light chain interacting protein (MYLIP, also known as IDOL). MYLIP is an E3 ubiquitin ligase that reduces LDL receptor levels by targeting it for degradation by the proteosome (24). The combination of HFD and CSDS caused an interactive effect with HFD elevating MYLIP in the control, but not CSDS, mice (Fig. 2A). Because PCSK9 and MYLIP demonstrated differential regulation by diet and stress, it is difficult to predict the overall effect on LDL protein levels. Therefore, we measured protein levels of LDL receptor by Western blot (Fig. 2C). HFD and CSDS caused a significant interaction, with HFD reducing LDL receptor levels only in the control mice. This pattern is most consistent with an effect of MYLIP reducing LDL receptor protein levels. Interestingly, elevated expression of PCSK9 mRNA after CSDS seemed to have no effect on LDL receptor levels.
Fatty acid regulation
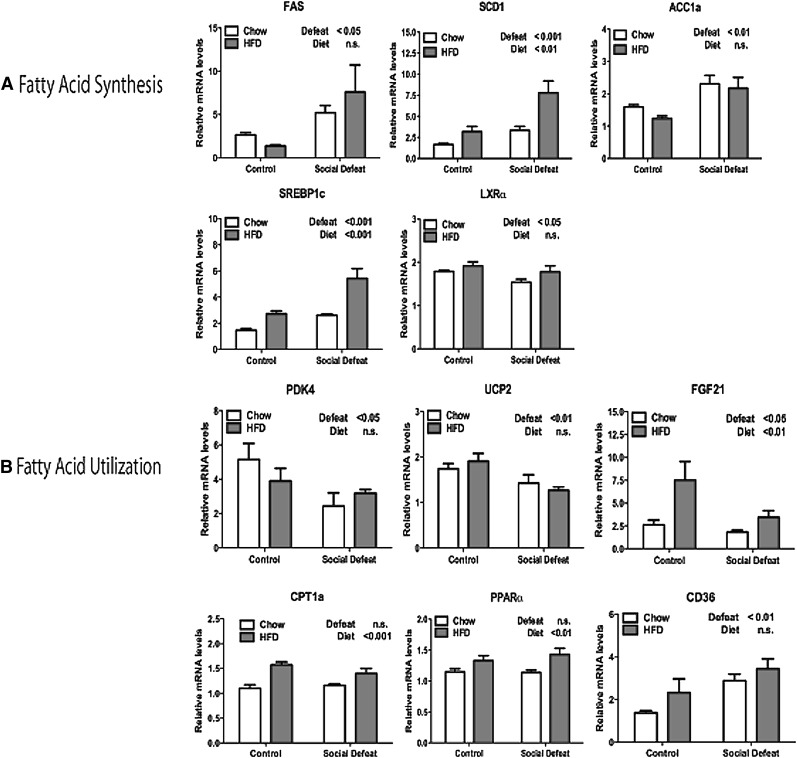
Next we examined insulin signaling and fatty acid regulation. Insulin signaling is a key regulator of fatty acid synthesis in the liver. Exposure to CSDS resulted in significantly elevated expression of several insulin-regulated genes involved in fatty acid synthesis including the transcription factor SREBP1c and its downstream targets fatty acid synthase, stearoyl CoA desaturase 1 (SCD1), and acetyl CoA carboxylase 1a (ACC1a) (Fig. 3A). Interestingly, LXRα was slightly decreased by CSDS despite the fact that many genes controlled by LXRα were actually upregulated after stress, including SREBP1c. This observation is likely due to the fact that LXRα activity is regulated mainly by the binding of its endogenous ligand oxysterols (25).
Fig. 3.
Expression of genes that control fatty acid levels in liver after CSDS. (A) Significant differences in expression of genes involved in fatty acid synthesis: FAS, SCD1, ACC1a, SREBP1c, and LXRα. (B) Expression of genes that regulate fatty acid utilization: PDK4, UCP2, FGF21, CPT1a, PPARα, and CD36. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups tested by two-way ANOVA. ACC1a, acetyl CoA carboxylase 1a; CPT1a, carnitine palmitoyltransferase 1a; CSDS, chronic social defeat stress; FGF21, fibroblast growth factor 21; LXRα, liver X receptor α; PDK4, pyruvate dehydrogenase kinase 4; PPARα, peroxisome proliferator-activated receptor α; SCD1, stearoyl CoA desaturase 1; SREBP1c, sterol-regulatory element binding protein 1c; UCP2, uncoupling protein 2.
To complement the data on the expression of lipogenic genes, we examined the expression of genes involved in fatty acid oxidation. Many components of the fatty acid oxidation pathway were decreased by CSDS, including pyruvate dehydrogenase kinase 4 (PDK4), uncoupling protein 2 (UCP2), and fibroblast growth factor 21 (FGF21) (Fig. 3B). These genes are targets of the transcription factor peroxisome proliferator-activated receptor α (PPARα). Interestingly, the level of PPARα was actually increased slightly by HFD (Fig. 3B). The discrepancy between mRNA levels and apparent activity suggests the possibility that CSDS decreases the transcriptional activity of PPARα, which thereby decreases fatty acid oxidation.
Insulin signaling
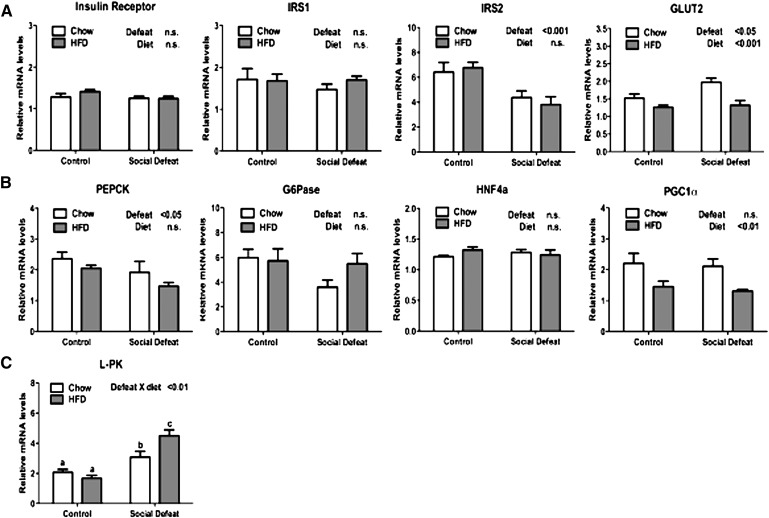
The combination of fasting hyperglycemia and hyperinsulinemia (Fig. 1H, I) observed after CSDS suggested insulin resistance in these mice. Insulin signaling in the liver, however, seemed to be intact based upon increased expression of SREBP1c, a sensitive target of insulin action. Therefore, we next analyzed components of the insulin signaling pathway. Genes involved in insulin signaling were largely unaffected by CSDS (Fig. 4A), including insulin receptor and insulin receptor substrate 1 (IRS1). Insulin receptor substrate 2 (IRS2) expression was reduced after CSDS; however, the significance of this result is unclear given the redundancy of IRS1 and 2. The glucose transporter 2 (GLUT2) was elevated after exposure to social defeat, but it also decreased by access to HFD.
Fig. 4.
Expression of genes that regulate carbohydrate metabolism in liver after CSDS. (A) Relative mRNA levels of genes involved in insulin signaling, including insulin receptor, IRS1, IRS2, and GLUT2. (B) Expression of genes involved in gluconeogenesis: PEPCK, G6Pase, HNF4a, and PGC1a. (C) Expression of the carbohydrate response element binding protein target L-PK. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups first tested by two-way ANOVA. If a statistical interaction was observed between factors, comparison of all four groups was performed by one-way ANOVA with Tukey posthoc comparison, and statistically different groups are indicated by different letters. CSDS, chronic social defeat stress; G6Pase, glucose-6-phophatase; GLUT2, glucose transporter 2; HNF4a, hepatocyte nuclear factor 4a; IRS, insulin receptor substrate; L-PK, liver pyruvate kinase; PGC1a, PPARγ coactivator 1a; PEPCK, phosphoenolpyruvate carboxykinase; PPAR, peroxisome proliferator-activated receptor.
Genes involved in gluconeogenesis showed a mixed pattern of regulation (Fig. 4B). While phosphoenolpyruvate carboxykinase (PEPCK) was significantly downregulated after CSDS, no effect was observed for glucose-6-phophatase (G6Pase). Additionally, CSDS had no effect on the expression of transcription factors [hepatocyte nuclear factor 4a (HNF4α) and PPARγ coactivator 1a (PGC1α)] that regulate gluconeogenesis. Finally, we measured the levels of liver pyruvate kinase (L-PK), a target of the carbohydrate-response element binding protein (ChREBP) (26). L-PK was significantly elevated in chow fed animals after social defeat and further increased in defeated mice maintained on HFD (Fig. 4C). Because L-PK enhances the conversion of glucose into pyruvate (the precursor of de novo fatty acid synthesis), this finding is consistent with a role for social stress in promoting fatty acid accumulation.
Peripheral regulation of NEFA
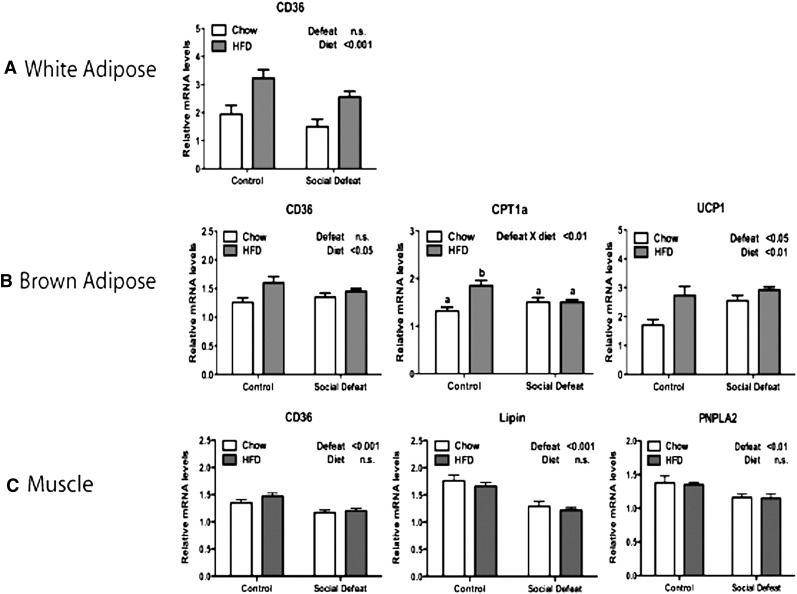
We noted that in mice receiving HFD, CSDS altered body composition with significantly decreased levels of body fat (Fig. 1D). However, the levels of NEFA, a product of lipolysis, were also lower in defeated animals. The combination of low body fat and low NEFA indicated the possibility that peripheral tissues were utilizing NEFA as an energy source. We evaluated this possibility by examining the expression of CD36, a gene involved in NEFA uptake. While HFD upregulated CD36 in white adipose tissue and brown adipose tissue, there was no effect of CSDS (Fig. 5A, B). CD36 levels in muscle tissue of mice exposed to CSDS were actually significantly lower than control mice (Fig. 5C). Additionally, genes involved in NEFA utilization, including carnitine palmitoyltransferase 1a (CPT1a) (brown adipose), lipin, and patatin-like phospholipase domain containing 2 (PNPLA2) (muscle), were decreased by CSDS. In contrast, CD36 expression is significantly elevated in liver of mice exposed to CSDS (Fig. 3B).
Fig. 5.
Gene expression profile in white adipose, brown adipose, and muscle after CSDS. After 30 days on either chow or HFD, mice were fasted 3 h and tissue was collected as noted. (A) Changes in gene expression in white adipose tissue: CD36. (B) Changes in gene expression in brown adipose tissue: CD36, CPT1a, and UCP1. (C) Changes in gene expression in muscle: CD36, Lipin, and PNPLA2. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups first tested by two-way ANOVA. If a statistical interaction was observed between factors, comparison of all four groups was performed by one-way ANOVA with Tukey posthoc comparison, and statistically different groups are indicated by different letters. CPT1a, carnitine palmitoyltransferase 1a; CSDS, chronic social defeat stress; HFD, high-fat diet; PNPLA2, patatin-like phospholipase domain containing 2; UCP1, uncoupling protein 1.
Intrahepatic triglyceride levels
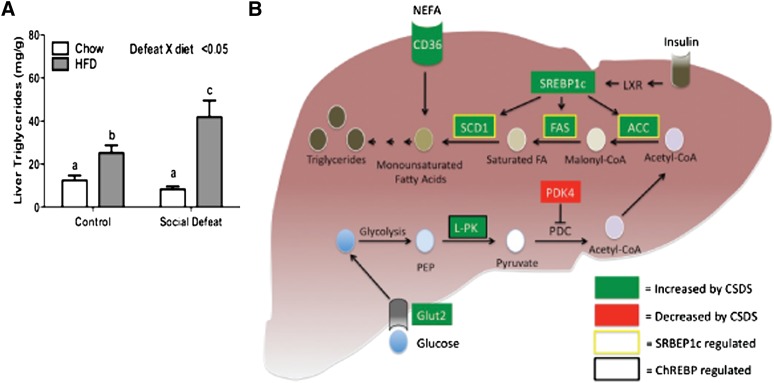
While mRNA levels do not necessarily correlate with protein changes, taken together, the gene expression profiles are consistent and indicate that CSDS decreases the capacity of several tissues, including white adipose, brown adipose, and muscle, to take up and utilize plasma NEFA. NEFA may be redistributed to the liver where elevated expression of CD36 promotes accumulation of intrahepatic levels of fatty acids. Increased transcriptional activity of LXRα, SREBP1c, and ChREBP further increases fatty acid synthesis. The combination of increased NEFA uptake and fatty acid synthesis would be predicted to lead to accumulation of intrahepatic lipids. To test this possibility, we measured triglyceride levels in the liver. The interaction of CSDS and HFD produced a significant increase in triglyceride levels in the liver (Fig. 6A), which is consistent with the increased uptake and production and decreased utilization predicted by the observed patterns of gene expression (Fig. 6B).
Fig. 6.
Intrahepatic triglyceride accumulation after CSDS. (A) Concentration of triglycerides in the liver. (B) Summary of changes in liver after CSDS. Data are presented as mean ± SEM, with significant differences as an insert on the chart (P < 0.05, P < 0.01, P < 0.001). Groups first tested by two-way ANOVA. If a statistical interaction was observed between factors, comparison of all four groups was performed by one-way ANOVA with Tukey posthoc comparison, and statistically different groups are indicated by different letters. ACC, acetyl CoA carboxylase; ChREBP, carbohydrate-response element binding protein; CSDS, chronic social defeat stress; HFD, high-fat diet; PDC, pyruvate dehydrogenase complex; PDK4, pyruvate dehydrogenase kinase 4; PEP, phosphoenolpyruvate; SCD1, stearoyl CoA desaturase 1; SREBP1c, sterol-regulatory element binding protein 1c.
DISCUSSION
Historically, most psychiatric research has focused on the affective and cognitive consequences of mental illness. However, mental illness also dramatically increases the risk of mortality from medical illnesses such as cardiovascular disease (1). We now present a model for better understanding at least one component of this risk: the relationship between mental illness and lipid dysregulation, a major component of the metabolic syndrome. Fig. 6B highlights major aspects of this model. Chronic stress reduces levels of body fat and increases utilization of NEFA. This extra energy may be redistributed to the liver through increased expression of CD36. Additionally, increased levels of GLUT2 promote the uptake of glucose and conversion to phosphoenolpyruvate by glycolysis. Increased activity of ChREBP induces the conversion of phosphoenolpyruvate to pyruvate by upregulating liver-pyruvate kinase. Pyruvate is converted to acetyl-CoA and used for synthesis of fatty acids as a result of increased activity of LXRα and SREBP1c. Elevated intracellular fatty acids are then converted to triglycerides and accumulate in the liver.
The effect of CSDS in chow-fed animals
While the combination of stress and HFD produces significant metabolic perturbations, exposure to social stress by itself caused several interesting findings. In animals maintained on standard chow, CSDS produced a significant increase in body weight (Fig. 1B) with a slight decrease in body fat (8.47% ± 0.41 versus 6.51% ± 0.44) (Fig. 1D). This decrease in body fat was accompanied by a decrease in plasma NEFA as well (Fig. 1G), suggesting the possibility of increased utilization of NEFA by peripheral tissues. However, expression profiling of liver (Fig. 3B) and white adipose, brown adipose, and muscle (Fig. 5) was most consistent with CSDS either reducing or having no effect on fatty acid utilization. Increased CD36 expression in liver (Fig. 3B) suggested the possibility that hepatic uptake may reduce NEFA levels. However, there was not a significant increase in intrahepatic triglyceride levels after CSDS (Fig. 6A). Additional work will need to be done to expand beyond analyses of metabolite and mRNA levels to better understand the processes by which CSDS alters utilization of NEFA.
Role of β-adrenergic signaling
Several lines of evidence suggest that increased activity of the autonomic nervous system mediates the reduction in adipose tissue we observed: (1) activity of the sympathetic nervous is increased in both social defeat stress and in human patients with anxiety disorders (27–29); (2) the β adrenergic receptor agonist isoproterenol decreases activity of lipoprotein lipase in white adipose (30); and (3) we recently demonstrated that the β3-adrenergic receptor antagonist SR59230A blocks CSDS induced reductions in adipose tissue mass (31). If the autonomic arousal often associated with psychiatric illnesses, such as PTSD or panic disorder, inhibits the ability of white adipose tissue to appropriately store excess calorie intake as lipid, then excess energy stores may be redistributed into alternate organs such as the liver. Consistent with this hypothesis, we found that the expression of genes in the liver associated with lipogenesis is significantly elevated after CSDS, leading to increased uptake and synthesis of fatty acids in the liver. Accumulation of triglycerides in nonadipose tissues is of great interest to the field of diabetes mellitus type II as it relates to insulin resistance and β-cell failure by lipotoxicity (32, 33).
Mounting evidence now indicates that a brain-liver axis regulates hepatic glucose production through the vagal nerve (34, 35). Parasympathetic activity from the vagal nerve suppresses glucose production, countering the gluconeogenic effect of autonomic signaling through α-adrenergic signaling (36, 37). Recently, Ghia et al. demonstrated reduction of parasympathetic vagal tone in two mouse models of depression that could be reversed by antidepressant treatment (38). This finding suggests certain mental illnesses, such as depression and anxiety, could cause an imbalance of sympathetic and parasympathetic tone that directly alters metabolic regulation of peripheral tissues.
Role of glucocortioids and glucagon
Two additional signaling pathways affected by stress are known to alter metabolism and may therefore contribute to the observed changes. Corticosterone levels were slightly decreased (see supplementary Fig. I) in both CSDS groups, which is consistent with findings in human patients with PTSD who show diminished cortisol levels (39). Glucocorticoids play a key role in preserving levels of pyruvate for gluconeogenesis by inhibiting the pyruvate dehydrogenase complex via pyruvate dehydrogenase kinase 4 (40). The combination of elevated insulin and lower corticosterone levels suppresses expression of PDK4 and results in disinhibition of the pyruvate dehydrogenase complex and conversion of pyruvate to acetyl-CoA for use in synthesis of fatty acids. Finally, glucagon is released in response to stimulation of the α-2 adrenergic receptor (41). Chronic elevations in sympathetic nervous activity could increase glucagon levels and alter glucose and fatty acid metabolism. However, on day 40 no significant difference was noted in glucagon levels in any group (see supplementary Fig. II).
CONCLUSION
These findings provide a framework to better understand the interaction of stress and diet in the development of metabolic illness. Chronic stress, in the presence of a HFD, alters energy portioning with decreased levels of body fat, accumulation of intrahepatic triglyceride, and reduced suppression of SREBP2-signaling by intracellular cholesterol. Characterization of this process may eventually lead to improved treatment of medical illness in patients with mental illness.
Supplementary Material
Acknowledgments
The authors thank Jay Horton for helpful discussion of the manuscript.
Footnotes
Abbreviations:
- ACC1a
- acetyl CoA carboxylase 1a
- ChREBP
- carbohydrate-response element binding protein
- CPT1a
- carnitine palmitoyltransferase 1a
- CSDS
- chronic social defeat stress
- FGF21
- fibroblast growth factor 21
- G6Pase
- glucose-6-phophatase
- GLUT2
- glucose transporter 2
- HFD
- high-fat diet
- HNF4a
- hepatocyte nuclear factor 4a
- IRS
- insulin receptor substrate
- L-PK
- liver pyruvate kinase
- LXR
- liver X receptor
- MYLIP
- myosin regulatory light chain interacting protein
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- PDK4
- pyruvate dehydrogenase kinase 4
- PEPCK
- phosphoenolpyruvate carboxykinase
- PGC1a
- PPARγ coactivator 1a
- PNPLA2
- patatin-like phospholipase domain containing 2
- PPARα
- peroxisome proliferator-activated receptor α
- PTSD
- posttraumatic stress disorder
- SCD1
- stearoyl CoA desaturase 1
- SREBP
- sterol-regulatory element binding protein
- UCP2
- uncoupling protein 2
This work was supported by the following grants: K08 MH084058-1A1, 1RL1DK081185-01, 1PL1DK081182-01, 8–UL1-DE019584-02, R37 DK53301; R01DK071320, RL1 DK081182, UL1 RR024923, RL1 DK081185, K08 DK068069-01A2; the NARSAD Young Investigator Award; the Physician Scientist Training Program; the Disease Oriented Clinical Scholars Program; and the Klarman Family Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
REFERENCES
- 1.Harris E. C., Barraclough B. 1998. Excess mortality of mental disorder. Br. J. Psychiatry. 173: 11–53. [DOI] [PubMed] [Google Scholar]
- 2.Allgulander C. 1994. Suicide and mortality patterns in anxiety neurosis and depressive neurosis. Arch. Gen. Psychiatry. 51: 708–712. [DOI] [PubMed] [Google Scholar]
- 3.Boscarino J. A. 2008. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 70: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coryell W., Noyes R., Clancy J. 1982. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch. Gen. Psychiatry. 39: 701–703. [DOI] [PubMed] [Google Scholar]
- 5.Grundy S. M. 2008. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 28: 629–636. [DOI] [PubMed] [Google Scholar]
- 6.Heppner P. S., Crawford E. F., Haji U. A., Afari N., Hauger R. L., Dashevsky B. A., Horn P. S., Nunnink S. E., Baker D. G. 2009. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med. 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldbacher E. M., Bromberger J., Matthews K. A. 2009. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom. Med. 71: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiskanen T. H., Niskanen L. K., Hintikka J. J., Koivumaa-Honkanen H. T., Honkalampi K. M., Haatainen K. M., Viinamaki H. T. 2006. Metabolic syndrome and depression: a cross-sectional analysis. J. Clin. Psychiatry. 67: 1422–1427. [DOI] [PubMed] [Google Scholar]
- 9.Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S. A., Anderson J. G., Jung S., Birnbaum S., Yanagisawa M., Elmquist J. K., Nestler E. J., et al. 2008. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 11: 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avgustinovich D. F., Gorbach O. V., Kudryavtseva N. N. 1997. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol. Behav. 61: 37–43. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan V., Berton O., Nestler E. 2008. The use of animal models in psychiatric research and treatment. Am. J. Psychiatry. 165: 1109. [DOI] [PubMed] [Google Scholar]
- 12.Lutter M., Krishnan V., Russo S. J., Jung S., McClung C. A., Nestler E. J. 2008. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 28: 3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurrasch D. M., Huang J., Wilkie T. M., Repa J. J. 2004. Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol. 389: 3–15. [DOI] [PubMed] [Google Scholar]
- 14.Schmittgen T. D., Livak K. J. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 15.Engelking L. J., Kuriyama H., Hammer R. E., Horton J. D., Brown M. S., Goldstein J. L., Liang G. 2004. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Invest. 113: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. 1984. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 37: 577–585. [DOI] [PubMed] [Google Scholar]
- 17.Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. 1988. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7: 4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berton O., McClung C. A., Dileone R. J., Krishnan V., Renthal W., Russo S. J., Graham D., Tsankova N. M., Bolanos C. A., Rios M., et al. 2006. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 311: 864–868. [DOI] [PubMed] [Google Scholar]
- 19.Hershock D., Vogel W. H. 1989. The effects of immobilization stress on serum triglycerides, nonesterified fatty acids, and total cholesterol in male rats after dietary modifications. Life Sci. 45: 157–165. [DOI] [PubMed] [Google Scholar]
- 20.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank-Kamenetsky M., Grefhorst A., Anderson N. N., Racie T. S., Bramlage B., Akinc A., Butler D., Charisse K., Dorkin R., Fan Y., et al. 2008. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA. 105: 11915–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNutt M. C., Kwon H. J., Chen C., Chen J. R., Horton J. D., Lagace T. A. 2009. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 284: 10561–10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S. W., Moon Y. A., Horton J. D. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279: 50630–50638. [DOI] [PubMed] [Google Scholar]
- 24.Zelcer N., Hong C., Boyadjian R., Tontonoz P. 2009. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 325: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tontonoz P., Mangelsdorf D. J. 2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17: 985–993. [DOI] [PubMed] [Google Scholar]
- 26.Cha J. Y., Repa J. J. 2007. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 282: 743–751. [DOI] [PubMed] [Google Scholar]
- 27.Keeney A. J., Hogg S., Marsden C. A. 2001. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol. Behav. 74: 177–184. [DOI] [PubMed] [Google Scholar]
- 28.Sgoifo A., Koolhaas J., De Boer S., Musso E., Stilli D., Buwalda B., Meerlo P. 1999. Social stress, autonomic neural activation, and cardiac activity in rats. Neurosci. Biobehav. Rev. 23: 915–923. [DOI] [PubMed] [Google Scholar]
- 29.Davies S., Esler M., Nutt D. 2009. Anxiety – bridging the heart/mind divide. J. Psychopharmacol. In press. [DOI] [PubMed] [Google Scholar]
- 30.Deshaies Y., Geloen A., Paulin A., Marette A., Bukowiecki L. J. 1993. Tissue-specific alterations in lipoprotein lipase activity in the rat after chronic infusion of isoproterenol. Horm. Metab. Res. 25: 13–16. [DOI] [PubMed] [Google Scholar]
- 31.Chuang J. C., Krishnan V., Yu H. G., Mason B., Cui H., Wilkinson M. B., Zigman J. M., Elmquist J. K., Nestler E. J., Lutter M. 2010. A beta(3)-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol. Psychiatry. Epub ahead of print. doi:10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger R. H. 2002. Lipotoxic diseases. Annu. Rev. Med. 53: 319–336. [DOI] [PubMed] [Google Scholar]
- 33.Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. 2009. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA. 106: 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caspi L., Wang P. Y., Lam T. K. 2007. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 35.Wang P. Y., Caspi L., Lam C. K., Chari M., Li X., Light P. E., Gutierrez-Juarez R., Ang M., Schwartz G. J., Lam T. K. 2008. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 452: 1012–1016. [DOI] [PubMed] [Google Scholar]
- 36.Erraji-Benchekroun L., Couton D., Postic C., Borde I., Gaston J., Guillet J. G., Andre C. 2005. Overexpression of beta2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am. J. Physiol. Endocrinol. Metab. 288: E715–E722. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi A., Ishimaru H., Ikarashi Y., Kishi E., Maruyama Y. 1996. Effects of hepatic nerve stimulation on blood glucose and glycogenolysis in rat liver: studies with in vivo microdialysis. J. Auton. Nerv. Syst. 61: 181–185. [DOI] [PubMed] [Google Scholar]
- 38.Ghia J. E., Blennerhassett P., Collins S. M. 2008. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Invest. 118: 2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda R., Teicher M. H., Trestman R. L., Levengood R. A., Siever L. J. 1996. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry. 40: 79–88. [DOI] [PubMed] [Google Scholar]
- 40.Huang B., Wu P., Bowker-Kinley M. M., Harris R. A. 2002. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 51: 276–283. [DOI] [PubMed] [Google Scholar]
- 41.Hirose H., Maruyama H., Itoh K., Koyama K., Kido K., Saruta T. 1992. Alpha-2 adrenergic agonism stimulates islet glucagon release from perfused rat pancreas: possible involvement of alpha-2A adrenergic receptor subtype. Acta Endocrinol. (Copenh.). 127: 279–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.