Abstract
The preovulatory human follicular fluid contains only HDLs as a lipoprotein class with a typically high proportion of preβ HDL. We first examined the role of follicular fluid and HDL subfractions on human spermatozoa capacitation, a process characterized by a hyperactivation of the flagellar movement and a depletion of plasma membrane cholesterol. Whole follicular fluid and isolated HDL, used at constant free cholesterol concentration, were both able to promote an early flagellar hyperactivation. Moreover, incubation of [3H]cholesterol-labeled spermatozoa with follicular fluid induced a rapid cholesterol efflux from spermatozoa that was confirmed by mass measurements of cholesterol transfer. Using isolated HDL, the cholesterol efflux had a similar time course and represented 70% of that mediated by whole follicular fluid. We then analyzed the time course of radioactive labeling of HDL subfractions. In the first minute of incubation, we found that the preβ HDL fraction incorporated the main part of the radioactivity (60%), with the rest being found in α-HDL, but strikingly, the labeling of α-HDL increased with time at the expense of preβ HDL.Thus, our results indicate that HDLs are involved in both spermatozoa hyperactivation and cholesterol effl ux and suggest the role of preβ-HDL particles as fi rst cellular cholesterol acceptors.
Keywords: oocyte, capacitation, cholesterol depletion, cholesterol acceptor, preβ-HDL, α-HDL
Sperm capacitation plays a key role in fertilization and is associated with dramatic changes in membrane lipid, especially with a loss of membrane cholesterol (1–4). These lipid modifications increase membrane fluidity and induce the externalization of binding sites for zona pellucida (5, 6), allowing acrosome reaction and fertilization (for a review see Ref. 7). Human follicular fluid (FF) is an efficient capacitating agent (8, 9). It acts, at least in part, by inducing an efflux of cholesterol from spermatozoa to different acceptors like HDL, albumin (10), or lipid transfer protein-I (11, 12). In the human mature follicle, the FF surrounds the oocyte and is in direct contact with granulosa cells, which are separated from the thecal blood capillaries by a basal membrane. FF is currently considered an extravascular compartment, which originates from blood plasma transport through the follicle basal membrane, since the major FF proteins are those of plasma. However, its lipoprotein profile seems to be specific since HDLs are the only class of lipoproteins present in the FF obtained from human preovulatory follicles (13, 14).
HDLs are involved in the reverse cholesterol transport from peripheral cells to the liver (15), and this process may involve several HDL subfractions (16). Particularly, HDL particles play a key role in the first step of the reverse cholesterol transport also called cellular cholesterol efflux. Human plasma HDLs are heterogeneous in terms of particle size, density, lipid content, and apolipoprotein (apo) composition (17). On the basis of their electrophoretic mobility through agarose, HDLs have been divided into two main populations, a major subfraction with α mobility, and a minor one with preβ mobility (preβ HDL) (16, 18). Most of HDL in plasma are α-HDLs, whereas preβ HDLs represent only 2–10% of total apoA-I, the main apo HDL (19–21). These preβ HDLs are further resolved by two-dimensional electrophoresis into preβ1, preβ2, and preβ3 HDL particles (22). Preβ1 HDLs are considered to be the first acceptors of cellular cholesterol (16, 18) and of interest, we previously isolated and characterized the preβ1 HDL from the FF where they are relatively abundant (14, 23). However, whether the HDL particles of the FF can take part in spermatozoa hyperactivation as well as in the cholesterol efflux has not been yet investigated. Hence, in this study, we confirmed that HDL particles of the FF are involved in both processes and deciphered the contribution of each HDL subfraction in the cholesterol efflux. Finally, we revealed the distinctive role of preβ HDL particles.
MATERIALS AND METHODS
Reagents
FFs and plasma were obtained from consent and informed patients undergoing an in vitro fertilization program as previously described by Parinaud et al. (24). Human serum albumin, aprotinin, and leupeptin were obtained from Sigma (St. Louis, MO). The 125I- labeled goat anti-mouse F(ab’)2 and [3H] cholesterol were purchased from Amersham (Les Ulis, France). Monoclonal antibodies against human apoA-I were kindly provided by Dr. Ross Milne and Dr. Yves Marcel (Ottawa, Canada). Commercial kits of cholesterol oxydase and esterase were obtained from Boehringer (Mannheim, Germany). Calibration standards for PAGE electrophoresis were obtained from Pharmacia (St. Quentin-en-Yvelines, France).
Isolation of FF HDL
HDL were obtained from FF, after the addition of an antiprotease cocktail containing EDTA (0.2 mM), sodium azide (0.01%, w/v), PMSF (0.1 mM), iodoacetamide (1 mM), 1,10-phenanthroline (1 mM), leupeptin (0.1 mM), and pepstatin A (1 µM). HDLs were isolated by a single step of FF ultracentrifugation at a density of 1.21 g/ml, at 120,000 g, for 40 h at 7°C.
Human spermatozoa collection
Human sperm ejaculates were obtained by masturbation after 2 to 4 days of sexual abstinence, from consent and informed normozoospermic men, and were allowed to liquefy for about 20 min at 37°C. Briefly, sperm was applied onto a three-layer Percoll gradient (90, 80, and 60%, v/v) and centrifuged at 300 × g during 20 min. The 90% Percoll fraction was kept and washed with Tyrode's buffer prior to centrifugation at 400 × g during 5 min. Then, the pellet was resuspended in modified Menezo B2 medium without albumin, cholesterol, and phenol red (25) and maintained at 37°C under 5% CO2, at a final concentration of 1.5 × 107 spermatozoa/ml for motility studies and 5 × 107 spermatozoa/ml for the [3H]cholesterol labeling procedure.
Sperm kinematics
Spermatozoa were adjusted to 1.5 × 107 spermatozoa/ml, and 20 µL of each sample was put into a in 2-well microcell of 20 µm maintained at 37°C (BICEF, L'Aigle, France). Motility parameters were assessed at 37°C using a Hamilton Thorn Motility Analyzer, version 10.8 (Hamilton Thorn Research, Beverly, MA). Machine parameters were a frame rate of 25/s, minimum contrast 8, minimum size 6, low/high size gates 0.5/1.7, low/high intensity gates 0.4/1.7, nonmotile head size 9, nonmotile intensity 200, and a path velocity >5 µm/s to be counted as motile. The variables measured included the percent motile, percent progressive motility, straight line velocity (VSL), mean path velocity (VAP), curvilinear velocity (VCL), mean linearity, mean amplitude of lateral head displacement (ALH), and percent of motile sperm with a path velocity >25µm/s defined as the rapid motility. Hyperactivated motility was defined as follows: curvilinear velocity > 100 µ/s, linearity <65%, and amplitude of lateral head displacement >7.5 µm (26). Spermatozoa hyperactivation assays were performed at 37°C, using whole FF and isolated HDL at different concentrations varying from 5–70% (v/v), during incubation times from 1–300 min, depending on experiments. Finally, all the given rates of hyperactivation take into account those measured with B2 medium, free of FF or of isolated HDL.
Spermatozoa cholesterol efflux assays
Spermatozoa concentration was adjusted to 5 × 107 cells/ml by dilution with modified B2 medium and labeled during 1 h incubation at 37°C with [3H] free cholesterol in ethanol (10 µCi/ml). At the end of the labeling period, sperm cells were centrifuged during 5 min at 400 × g. Spermatozoa were resuspended in modified B2 medium and recentrifuged to eliminate unbound labeled cholesterol. After three washes with albumin-free B2 medium and centrifugation, spermatozoa bearing [3H]cholesterol were resuspended in medium at 5 × 107 cells/ml and were used for the cholesterol efflux studies. The sperm cells were able to incorporate an average of 37% of the [3H]cholesterol during the labeling procedure. The specific radioactivity range was about 2–3 × 105 dpm per nmol of cholesterol. Each efflux assay was performed on the basis of 1.2 × 107 cells/ml incubated with isolated FF HDL or whole human FFs used at the following concentrations: 1, 2.5, 5, 10, 15, 20, 25, and 50% (v/v). For each concentration and sample, we have measured the fractional cholesterol efflux (FE) at different incubation times, 1, 5, 15, 30, and 60 min. At the end of the efflux experiment, microcentrifuge tubes from Millipore (Ultrafree-MC centrifugal filter units, Durapore membrane, 0.45 µm) were used for filtrate collection of the efflux medium to ensure the absence of contamination by labeled spermatozoa. After washing sperm cells with Tyrode's buffer, the radioactivity associated with cells was counted. Fractional cholesterol efflux (FE) was calculated as the ratio between the label released to the medium and the total radioactivity recovered in media plus cells. Finally, all the given FEs take into account those measured with medium free of FF or isolated HDL at all the incubation time with cells.
Agarose electrophoresis and passive transfer
Electrophoresis was carried out on 0.75% (w/v) low-medium resolution agarose (1.5 mm thickness) at a constant voltage of 150 V in Barbital buffer 50 mM (pH 8.6) and at 4°C, using GelBond as a support. After efflux experiments, the samples (12 µl) were added to a 1 cm sample channel and run at 150 V for 1 h 30 min in a S60 electrophoresis tank (Sebia, Issy-les-Moulineaux, France). After electrophoresis, the gel was divided in two parts. Part of the gel was transferred by pressure onto nitrocellulose membrane and was immunoblotted against apoA-I according to Barrans et al. (27), using as first antibody a mixture of monoclonal antibodies raised against human apoA-I and then 125I-labeled goat anti-mouse F(ab’)2 as second antibody. Membranes were exposed overnight to a PhosphorImager screen (Molecular Dynamics, France), and the cassettes were imaged by PhosphorImager. Quantification of apoA-I was performed using ImageQuant 1.0, and data were expressed as pixel points determined by the computer and were linearly correlated with the dpm of the 125I cpm associated with the antigen-antibody complex. From the other part of the gel, about 12 strips of agarose by lane (0.5 × 1 cm) were cut out and the [3H] radioactivity was counted.
Extraction and analysis of neutral lipids by gas-liquid chromatography
After cholesterol efflux assays, lipids of each sample of FF and spermatozoa were extracted according to Bligh and Dyer protocol after an acidification of the aqueous phase by formic acid. Neutral lipids were then quantified by a gas-liquid chromatographic method that we previously reported (28).
Analytical methods
Free and total cholesterol were also measured by cholesterol esterase/cholesterol oxydase techniques. Measurement of proteins was carried out according to Lowry procedure using BSA as a standard. Lipid extraction was performed as described above. Phospholipids were measured according to their phosphorus content (29) following lipid extraction. ApoA-I was measured by a rate nephelometry method using the IMMAGE® immunochemistry system (Beckman Coulter, Villepinte, France), which is widely spread (30).
RESULTS
Effects of FF or HDL on kinetics of spermatozoa hyperactivation
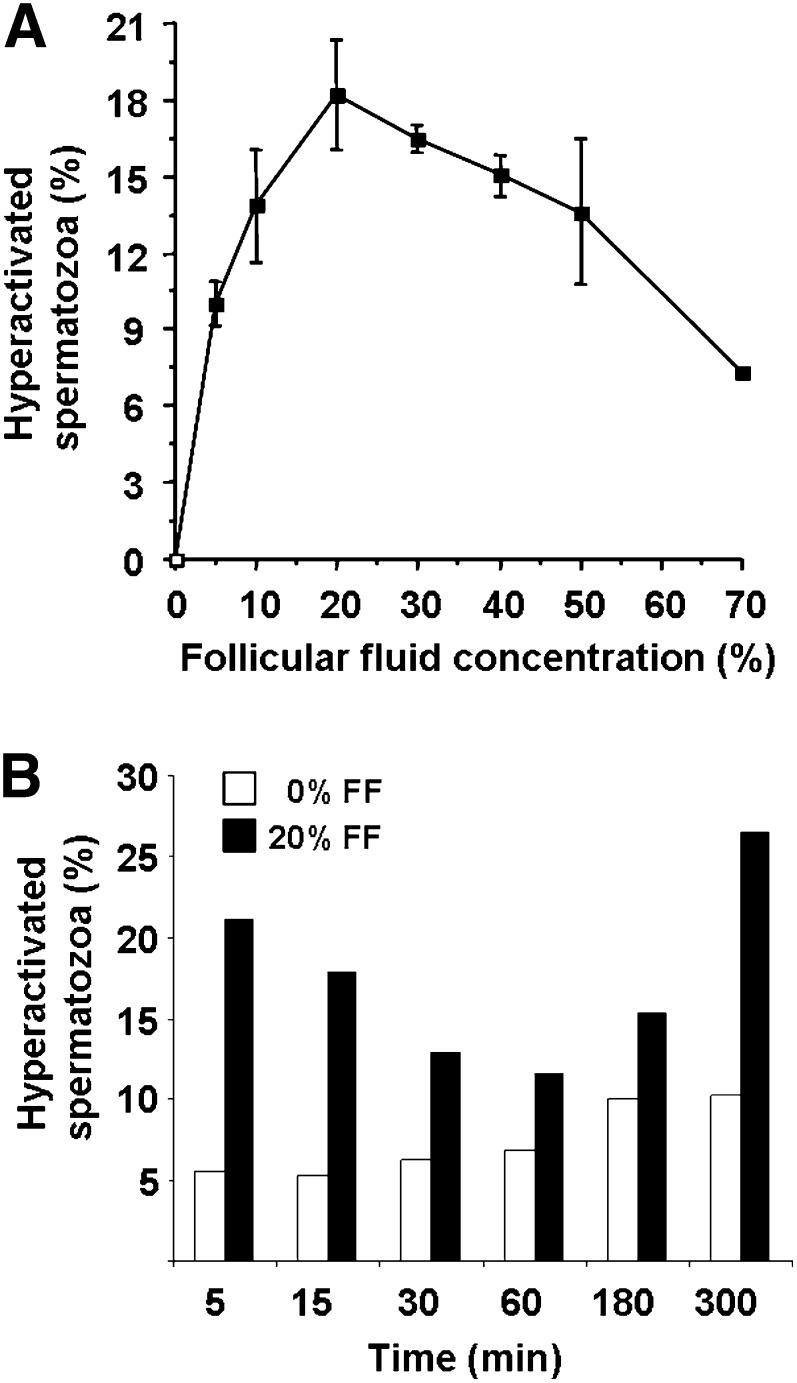
We first evaluated the effects of varying FF concentrations (5, 10, 20, 30, 40, and 50%, v/v in modified B2 medium) on spermatozoa hyperactivation process after 5 min of incubation at 37°C (Fig. 1A). The maximal rate of hyperactivation was obtained with 20% of FF. Then, spermatozoa (1.5 × 107 cells/ml) were incubated at 37°C with this FF dilution, and the hyperactivation process was analyzed as a function of the incubation time. This experiment revealed a biphasic kinetics (Fig. 1B) with a rapid increase of the hyperactivation in the first 5 min (21% versus 6% for control) followed by a second peak that appeared later, after 300 min of incubation.
Fig. 1.
A: Effect of follicular fluid concentration on spermatozoa hyperactivation. Human spermatozoa (1.5 × 107cells/ml) were incubated for 5 min with the indicated dilution (v/v) of FF in B2 medium. Results are expressed as mean ± SE of three experiments. B: Kinetics of spermatozoa hyperactivation. Spermatozoa (1.5 × 107cells/ml) were incubated with or without a 20% dilution of FF from 0–300 min at 37°C. Results represent a typical experiment of two.
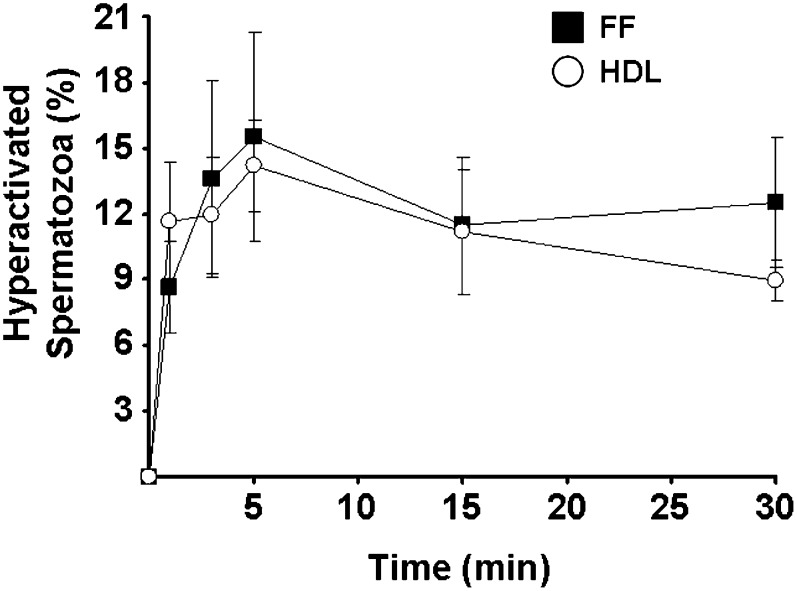
We then compared FF and HDL effects on the first wave of spermatozoa hyperactivation (Fig. 2). FF induced a rapid process that reached a peak at 5 min (16 ± 5%) followed by a slight decrease and a plateau up to 30 min (13 ± 3%). Of interest, when HDL isolated from FF were used at the same free cholesterol concentration than that of the 20%-FF dilution (i.e., 13.6 µmol/l of HDL-free cholesterol), level and kinetics of spermatozoa hyperactivation were in the same range (14 ± 2% at 5 min). We noticed that FF with HDL free cholesterol concentration lower than 3.4 μmol/l was unable to promote the spermatozoa hyperactivation (data not shown).
Fig. 2.
Comparison of FF and isolated HDL effects on spermatozoa hyperactivation. Hyperactivation measurements were performed after incubation, for 0 to 30 min, of 1.5 × 107 sperm cells with diluted FF and isolated HDL, both used at a constant free cholesterol concentration of 13.6 µM. Results are expressed as mean ± SE of four independent experiments, each performed in duplicate.
Fractional cholesterol efflux from spermatozoa to isolated HDL compared with whole FF
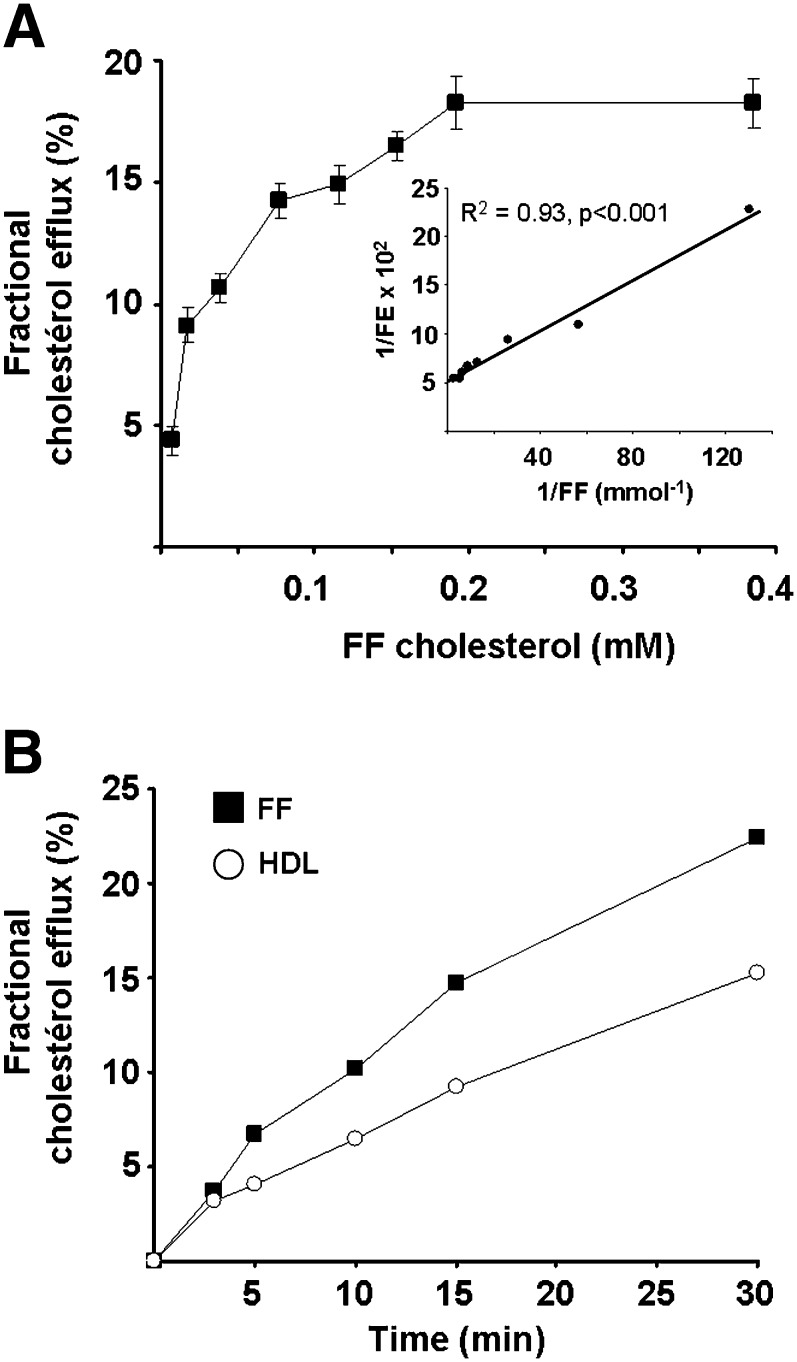
We tested the effects of FF, used at total cholesterol concentrations ranging from 0.0076–0.38 mmol/l (i.e., 1–50% v/v of FF in B2 medium), on the spermatozoa cholesterol efflux during 30 min of incubation at 37°C. As shown in Fig. 3A, the fractional cholesterol efflux increased along with FF cholesterol concentrations up to 0.2 mmol/l and tended to saturate above this concentration. These data were linearized according to the Lineweaver-Burk plot (Fig. 3A, inset), and a mean value of 19% of cell 3H cholesterol was estimated for the maximal efflux, while 0.023 mmol/l of FF cholesterol was required to promote a 50% cholesterol efflux. Of interest, the free cholesterol/phospholipid molar ratio of FF increased from 0.124 to 0.138 after 30 min incubation with spermatozoa, whereas the spermatozoa free cholesterol/phospholipid molar ratio decreased from 0.53–0.43 in 30 min, representing a cholesterol depletion of 19% (data not shown), which is in good agreement with the radioactive assay.
Fig. 3.
A: Dose-response curve for the release of labeled cholesterol from sperm in function of FF cholesterol content. Sperm cells (1.2 × 107 cells/ml) were incubated 30 min with the indicated FF concentrations. Experimental values (mean of three experiments ± SE) are expressed as the fractional efflux of [3H]cholesterol from cells versus total cholesterol concentration of FF pool (n = 6). Inset, Lineweaver-Burk plot of dose-response data. Labeling procedure of spermatozoa is detailed in Methods. B: Fractional cholesterol efflux from spermatozoa to isolated HDL compared with whole FF. Experimental values are expressed as the fractional efflux of [3H]cholesterol from spermatozoa (1.2 × 107 cells/ml) versus incubation time with isolated HDL and whole FF, both used at constant free cholesterol concentration (13.6 µM). Results are the mean of two independent experiments, each performed in duplicate. The variation between the two experiments is lower than 10%.
We showed above that FF-derived HDLs were able to promote sperm cell early hyperactivation at the same extent as whole FF (Fig. 2). Therefore, we compared the capacity of these HDLs to promote cellular cholesterol efflux, up to 30 min, to that of whole FF (both used at a similar free cholesterol HDL concentration of 13.6 µmol/l). As shown in Fig. 3B, the fractional cholesterol efflux to HDL increased from 3–15%, between 3 and 30 min, whereas it reached 22% with whole FF. Thus, one can assume that HDLs contribute to about 70% of the fractional cholesterol efflux mediated by whole FF.
Distribution of the radiolabeled cholesterol from spermatozoa to the FF HDL subfractions
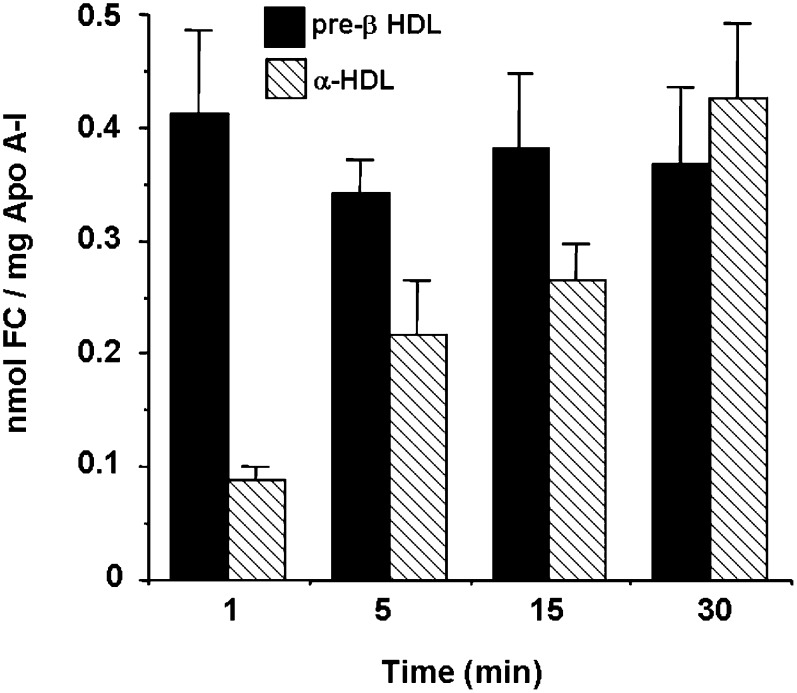
Spermatozoa were radiolabeled with [3H]cholesterol and incubated with whole FFs during 1, 5, 15, and 30 min at 37°C. At the end of each efflux period, an aliquot of the efflux medium was submitted to agarose electrophoresis to assess the [3H]cholesterol distribution in FF HDL subfractions. Following migration on a nondenaturating agarose gel electrophoresis to separate the different HDL subfractions, apoA-I was immunoblotted. We evidenced the presence of preβ HDL and α-HDL on the apoA-I immunoblots. After quantitation by PhosphorImager, the proportion of apoA-I in preβ1 HDL after 1 and 30 min of incubation of FF with spermatozoa was not significantly different (27 ± 2% and 30 ± 1% of total HDL apoA-I, n = 4), respectively. When FF was incubated for 1 min with spermatozoa labeled with [3H]cholesterol, there was a rapid appearance of radioactivity in the medium, and about 79 ± 2% was recovered in both α and preβ electrophoretic zones. The remaining radioactivity was found at the gel origin and did not contain apoA-I. After 1 min of incubation, labeled cholesterol incorporated in the preβ HDL represented 60% of the radioactivity associated with whole HDL compared with 40% in the α-HDL (Table 1).With time, the rate of [3H]cholesterol increased in the α-HDL at the expense of preβ HDL until 30 min where α-HDL retained 72 ± 3% of the radiolabeling versus 28 ± 3% for preβ HDL (P < 0.001). To get a more precise insight of the contribution of each HDL subfraction in cholesterol efflux, we analyzed the free cholesterol/apoA-I ratio during the incubation of labeled spermatozoa with FF. As shown in Fig. 4, preβ HDLs were early saturated with [3H]cholesterol and kept this high level of labeling during the whole incubation, whereas α-HDLs were gradually labeled. To slow down the kinetics of cholesterol exchange, we decreased the number of radiolabeled spermatozoa from 1.5 × 107 to 0.5 × 107. After 5 min of incubation with FF (as described in Table 1), the radioactivity associated with the preβ HDL fraction was significantly higher and reached 56 ± 6% (data not shown). Thus, a slower kinetics leads to an accumulation of labeled cholesterol in preβ HDL. These results strongly suggest that FF preβ HDLs behave as the first acceptors of spermatozoa cholesterol with a further transfer to α-HDL. However, we cannot completely exclude that the latter is able to directly remove a minor fraction of cholesterol from spermatozoa.
TABLE 1.
[3H]Cholesterol incorporation into FF-derived HDL subfractions after incubation with labeled spermatozoa
| Pre-β HDL (%) | α-HDL (%) | |
|---|---|---|
| 1 min | 59.83 ± 5.62 | 40.18 ± 5.62 |
| 5 min | 41.91 ± 4.71 | 58.09 ± 4.71 |
| 15 min | 36.47 ± 4.67 | 63.53 ± 4.67 |
| 30 min | 28.19 ± 2.60 | 71.81 ± 2.60 |
Spermatozoa cholesterol efflux experiments were carried out with whole FF (n = 4) used at 10% (v/v) during incubation time varying from 1–30 min. An aliquot of each efflux medium was submitted to agarose gel electrophoresis. Strips of agarose were cut out, and associated radioactivity was counted. Results were expressed as the mean ± SE of the ratio between the labeled cholesterol incorporated in preβ or α-HDL and total HDL associated radioactivity.
Fig. 4.
Evolution of free cholesterol/apoA-I ratio of HDL subfractions during the incubation of labeled spermatozoa with FF (n = 4). The ratio was calculated using the data from Table 1 and according to FF apoA-I content, apoA-I repartition among HDL subfractions, and specific radioactivity of [3H]cholesterol.
DISCUSSION
Free cholesterol depletion from plasma membrane appears to be one of the essential mechanisms for sperm cell capacitation (31, 32). The fact that FF is in vitro the best capaciting agent (8) can be explained, at least in part, by the presence of cholesterol acceptors, such as HDL and albumin, that we and others found out (10, 13, 33). Moreover, we evidenced that HDL is the single class of lipoprotein during the preovulatory period (13) and that preβ HDLs represent about 18% of total HDL of the FF (14). This subfraction plays a key role in the cellular cholesterol efflux since it promotes the early phases of this process (16, 18).
In this study, we have shown the early effects of whole FF and FF-derived HDL on the spermatozoa cholesterol efflux as well as on their hyperactivation. Indeed, FF (used at 10%) was able to promote cholesterol efflux from sperm cells, increasing with time up to 14% after 1 h. Using 20% FF, a 22% cholesterol efflux was reached after 30 min of incubation. The Km value for cholesterol efflux was estimated at 3.2% FF, which corresponds to an average value of 24 µM total cholesterol concentration, which is 3-fold less than the value estimated with hepatoma cells Fu5AH (64 µM) (34). Thus, spermatozoa represent one of the best models of cellular cholesterol efflux. Of interest, this efflux could be already measured after the first minute of incubation with cells, which represented about 4% of cell-labeled cholesterol. With respect to this early event, and taking into account the role of plasma preβ HDL as initial cellular cholesterol acceptors, we tested the early incorporation of labeled cholesterol into the FF HDL subfractions, following efflux experiments with whole FF. When FF (10%, v/v) was incubated for 1 min at 37°C with [3H]cholesterol labeled spermatozoa, a major fraction of the radioactivity (60%) was recovered in the preβ HDL fraction, compared with 40% in the α-HDL (Table 1). Preβ HDLs thus appeared as first acceptors for spermatozoa cholesterol. These preβ HDLs were always highly enriched in label (relative to their apoA-I content) compared with the major part of α-HDL (Fig. 3). These results are in good agreement with those obtained by Castro and Fielding (16) using the fibroblast efflux model. Since the rate of labeled cholesterol incorporated in the α-HDL increased with time at the expense of that in preβ HDL and since the proportion of apoA-I in preβ HDL remains constant during incubation time, we can thus hypothesize that preβ HDLs act as a shuttle of cholesterol molecules between cells and α-HDL as previously reported in plasma. However, we cannot exclude that α-HDLs are able to directly capture a minor part of cholesterol from spermatozoa. The efflux capacity of isolated HDLs compared with that of whole FF, used at constant free cholesterol-HDL concentration, revealed that HDL contributed to 70% of the whole process. Part of these differences might arise from preβ HDL loss during ultracentrifugal isolation of HDL. Moreover, as we recovered upon electrophoresis only 80% of radiolabeled cholesterol in α-HDL and preβ HDL, we cannot exclude other efflux mediators. Interestingly, we have previously observed apoA-IV in FF in significantly higher amounts (corrected per mg of apoA-I) than in serum (34). Considering the role of apoA-IV or apoA-IV-and apoAI-containing lipoprotein particles in promoting cholesterol efflux from various cell lines (35–37), apoA-IV may also act in modulating spermatozoa cholesterol efflux to FF. Moreover, expression of apoJ has been recently reported in the rat reproductive tract (38). Since apoJ is able to promote cholesterol efflux from mouse macrophage foam cells (39), it may be involved in spermatozoa capacitation, but this hypothesis has not been yet explored in humans. Finally, a part of the non-apoA-I radioactivity may correspond to prostasomes that remained bound to spermatozoa during the preparation and were released in the presence of FF (S. M. Hamdi and X. Collet, unpublished observations). Indeed, prostasomes are small vesicles of prostate origin that exhibit an unusual membrane composition with a very high cholesterol-to-phospholipid ratio.
Whole FF and isolated HDLs were able to promote spermatozoa hyperactivation with comparable kinetics and extent, which allowed us to conclude that this process was mediated by HDL. As cholesterol efflux and hyperactivation were both early events stimulated by HDL, this may reflect their close dependence. Moreover, it has been shown that the action of HDLs, as cholesterol acceptors, is coupled to an adenylyl cyclase/cAMP/protein kinase A signaling that supports spermatozoa capacitation (32). In this setting, an interesting hypothesis to explore is that the FF preβ HDL subfraction is an early activator of this signaling pathway. Another issue remains to be solved is the identity of transporters that mediate cholesterol efflux from spermatozoa membrane to FF preβ HDL. A recent report showed that three members of the ABC transporter superfamily, ABCA1, ABCA7, and ABCG1, are involved in cholesterol transport from mouse spermatozoa to apoA-I (40). Further studies are needed to confirm that these transporters are also implicated in the cholesterol efflux from human spermatozoa. Finally, our study raises the question of the fertility of female patients with HDL deficiency or hypoalphalipoproteinemia. Unfortunately, this question has not been systematically addressed. In the three familial disorders in which patients exhibit very low plasmatic HDL levels (apoA-I deficiency, LCAT deficiency, and Tangier disease), the most often reported clinical features are premature and severe atherosclerosis and neuropathy but no fertility abnormalities (41). Since the reproductive function is very robust, one can hypothesize that a compensatory mechanism substitutes for HDL in cholesterol efflux from spermatozoa. However, owing to the very low incidence of these disorders, as well as ethical issues, it will be difficult to decipher the interaction with spermatozoa of FFs from HDL-deficient women.
Whatever the mechanism is by which HDLs promote spermatozoa hyperactivation, we have shown that they stimulate this process from the first minute of incubation with sperm as well as an early cholesterol efflux, mainly through the preβ HDL subfraction. Of great interest, a recent report raised the possibility that HDLs are involved in human oocyte health (42). Thus, by ascribing a new role of HDL in the fertilization process, our study confirms their significance in human reproduction and opens new avenues for infertility as well as preventing conception research.
Acknowledgments
X.C. was a recipient of a “Contrat d'Interface” from CHU of Toulouse. The authors thank Dr. Pascale Benlian (Paris, France) for helpful discussions and Guy Cholez for HDL studies.
REFERENCES
- 1.Davis B. K. 1981. Timing of fertilization in mammals: sperm cholesterol/phospholipid ratio as a determinant of the capacitation interval. Proc. Natl. Acad. Sci. USA. 78: 7560–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoshi K., Aita T., Yanagida K., Yoshimatsu N., Sato A. 1990. Variation in the cholesterol/phospholipid ratio in human spermatozoa and its relationship with capacitation. Hum. Reprod. 5: 71–74. [DOI] [PubMed] [Google Scholar]
- 3.Benoff S. 1993. Preliminaries to fertilization. The role of cholesterol during capacitation of human spermatozoa. Hum. Reprod. 8: 2001–2006. [DOI] [PubMed] [Google Scholar]
- 4.Cross N. L. 1998. Role of cholesterol in sperm capacitation. Biol. Reprod. 59: 7–11. [DOI] [PubMed] [Google Scholar]
- 5.Gamzu R., Yogev L., Paz G., Yavetz H., Lichtenberg D. 1997. Reduction of sperm cholesterol:phospholipid ratio is a possible mechanism for enhancement of human sperm binding to the zona pellucida following incubation with phosphatidylcholine liposomes. Biol. Reprod. 57: 539–546. [DOI] [PubMed] [Google Scholar]
- 6.Benoff S., Hurley I., Cooper G. W., Mandel F. S., Rosenfeld D. L., Hershlag A. 1993. Head-specific mannose-ligand receptor expression in human spermatozoa is dependent on capacitation-associated membrane cholesterol loss. Hum. Reprod. 8: 2141–2154. [DOI] [PubMed] [Google Scholar]
- 7.Primakoff P., Myles D. G. 2002. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296: 2183–2185. [DOI] [PubMed] [Google Scholar]
- 8.Ghetler Y., Ben-Nun I., Kaneti H., Jaffe R., Gruber A., Fedjin M. 1990. Effect of sperm preincubation with follicular fluid on the fertilization rate in human in vitro fertilization. Fertil. Steril. 54: 944–946. [DOI] [PubMed] [Google Scholar]
- 9.Kulin S., Bastiaans B. A., Hollanders H. M., Janssen H. J., Goverde H. J. 1994. Human serum and follicular fluid stimulate hyperactivation of human spermatozoa after preincubation. Fertil. Steril. 62: 1234–1237. [DOI] [PubMed] [Google Scholar]
- 10.Langlais J., Kan F. W., Granger L., Raymond L., Bleau G., Roberts K. D. 1988. Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation. Gamete Res. 20: 185–201. [DOI] [PubMed] [Google Scholar]
- 11.Hamamah S., Lanson M., Barthelemy C., Garrigue M. A., Muh J. P., Royere D., Lansac J. 1995. Analysis of the lipid content and the motility of human sperm after follicular fluid treatment. Andrologia. 27: 91–97. [DOI] [PubMed] [Google Scholar]
- 12.Ravnik S. E., Albers J. J., Muller C. H. 1993. A novel view of albumin-supported sperm capacitation: role of lipid transfer protein-I. Fertil. Steril. 59: 629–638. [DOI] [PubMed] [Google Scholar]
- 13.Perret B. P., Parinaud J., Ribbes H., Moatti J. P., Pontonnier G., Chap H., Douste-Blazy L. 1985. Lipoprotein and phospholipid distribution in human follicular fluids. Fertil. Steril. 43: 405–409. [DOI] [PubMed] [Google Scholar]
- 14.Jaspard B., Collet X., Barbaras R., Manent J., Vieu C., Parinaud J., Chap H., Perret B. 1996. Biochemical characterization of pre-beta 1 high-density lipoprotein from human ovarian follicular fluid: evidence for the presence of a lipid core. Biochemistry. 35: 1352–1357. [DOI] [PubMed] [Google Scholar]
- 15.Miller N. E., La Ville A., Crook D. 1985. Direct evidence that reverse cholesterol transport is mediated by high density lipoprotein in the rabbit. Nature. 314: 109–111. [DOI] [PubMed] [Google Scholar]
- 16.Castro G. R., Fielding C. J. 1988. Early incorporation of cell-derived cholesterol into pre-beta-migrating high- density Lipoprotein. Biochemistry. 27: 25–29. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg S. 1984. High density lipoprotein metabolism. J. Lipid Res. 25: 1017–1058. [PubMed] [Google Scholar]
- 18.Huang Y., von Eckardstein A., Assmann G. 1993. Cell-derived unesterified cholesterol cycles between different HDLs and LDL for its effective esterification in plasma. Arterioscler. Thromb. 13: 445–458. [DOI] [PubMed] [Google Scholar]
- 19.Kunitake S. T., La Sala K. J., Kane P. J. 1985. Apolipoprotein A-I containing Lipoproteins with pre-beta electrophoretic mobility. J. Lipid Res. 26: 549–555. [PubMed] [Google Scholar]
- 20.Ishida B. Y., Frolich J., Fielding C. J. 1987. Prebeta-migrating high density lipoprotein: quantitation in normal and hyperlipidemic plasma by solid phase radioimmunoassay following electrophoretic transfer. J. Lipid Res. 28: 778–785. [PubMed] [Google Scholar]
- 21.Neary R., Bhatnagar D., Durrington P., Ishola M., Arrol S., Mackness M. 1991. An investigation of the role of lecithine: cholesterol acyl transferase and triglyceride-rich lipoproteins in the metabolism of pre-beta high density lipoproteins. Atherosclerosis. 89: 35–48. [DOI] [PubMed] [Google Scholar]
- 22.Francone O. L., Gurakar A., Fielding C. J. 1989. Distribution and functions of lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in plasma lipoproteins. Evidence for a functional unit containing these activities together with apolipoproteins A-I and D that catalyzes the esterification and transfer of cell-derived cholesterol. J. Biol. Chem. 264: 7066–7072. [PubMed] [Google Scholar]
- 23.Barrans A., Jaspard B., Barbaras R., Chap H., Perret B., Collet X., Pre-beta H. D. L. 1996. Structure and metabolism. Biochim. Biophys. Acta. 1300: 73–85. [DOI] [PubMed] [Google Scholar]
- 24.Parinaud J., Perret B. P., Ribbes H., Chap H., Pontonnier G., Douste-Blazy L. 1987. High density lipoprotein and low density lipoprotein utilization by human granulosa cells for progesterone synthesis in serum-free culture: respective contributions of free and esterified cholesterol. J. Clin. Endocrinol. Metab. 64: 409–417. [DOI] [PubMed] [Google Scholar]
- 25.Bussenot I., Azoulay-Barjonet C., Parinaud J. 1993. Modulation of the steroidogenesis of cultured human granulosa-lutein cells by gonadotropin-releasing hormone analogs. J. Clin. Endocrinol. Metab. 76: 1376–1379. [DOI] [PubMed] [Google Scholar]
- 26.Burkman L. J. 1991. Discrimination between nonhyperactivated and classical hyperactivated motility patterns in human spermatozoa using computerized analysis. Fertil. Steril. 55: 363–371. [PubMed] [Google Scholar]
- 27.Barrans A., Collet X., Barbaras R., Jaspard B., Manent J., Vieu C., Chap H., Perret B. 1994. Hepatic lipase induces the formation of pre-beta 1 high density lipoprotein (HDL) from triacylglycerol-rich HDL2. A study comparing liver perfusion to in vitro incubation with lipases. J. Biol. Chem. 269: 11572–11577. [PubMed] [Google Scholar]
- 28.Vieu C., Jaspard B., Barbaras R., Manent J., Chap H., Perret B., Collet X. 1996. Identification and quantification of diacylglycerols in HDL and accessibility to lipase. J. Lipid Res. 37: 1153–1161. [PubMed] [Google Scholar]
- 29.Böttcher C. J. F., Van Gent C. M., Pries C. 1961. A rapid sensitive submicrophosphorus determination. Anal. Chim. Acta. 24: 203–204. [Google Scholar]
- 30.Kim B. J., Hwang S. T., Sung K. C., Kim B. S., Kang J. H., Lee M. H., Park J. R. 2005. Comparison of the relationships between serum apolipoprotein B and serum lipid distributions. Clin. Chem. 51: 2257–2263. [DOI] [PubMed] [Google Scholar]
- 31.Benoff S. 1993. Preliminaries to fertilization. The role of cholesterol during capacitation of human spermatozoa. Hum. Reprod. 8: 2001–2006. [DOI] [PubMed] [Google Scholar]
- 32.Travis A. J., Kopf G. S. 2002. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 110: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrenwald E., Foote R. H., Parks J. E. 1990. Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol. Reprod. Dev. 25: 195–204. [DOI] [PubMed] [Google Scholar]
- 34.Jaspard B., Fournier N., Vieitez G., Atger V., Barbaras R., Vieu C., Manent J., Chap H., Perret B., Collet X. 1997. Structural and functional comparison of HDL from homologous human plasma and follicular fluid. A model for extravascular fluid. Arterioscler. Thromb. Vasc. Biol. 17: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz A., Barbaras R., Ghalim N., Clavey V., Fruchard J. C., Ailhaud G. 1990. Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J. Biol. Chem. 265: 7859–7863. [PubMed] [Google Scholar]
- 36.Duverger N., Ghalim N., Ailhaud G., Steinmetz A., Fruchart J-C., Castro G. 1993. Characterization of apo A-IV-containing lipoprotein particles isolated from human plasma and interstitial fluid. Arterioscler. Thromb. 13: 126–132. [DOI] [PubMed] [Google Scholar]
- 37.von Eckardstein A., Huang Y., Wu S., Sarmadi A. S., Schwarz S., Steinmetz A., Assman G. 1995. Lipoproteins containing apolipoprotein A-IV but not apolipoprotein A-I take up and esterify cell-derived cholesterol in plasma. Arterioscler. Thromb. Vasc. Biol. 15: 1755–1763. [DOI] [PubMed] [Google Scholar]
- 38.Argraves W. S., Morales C. R. 2004. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol. Reprod. Dev. 69: 419–427. [DOI] [PubMed] [Google Scholar]
- 39.Gelissen I. C., Hochgrebe T., Wilson M. R., Easterbrook-Smith S. B., Jessup W., Dean R. T., Brown A. J., Apolipoprotein J. 1998. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: a potential anti-atherogenic function? Biochem. J. 331: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales C. R., Marat A. L., Ni X., Yu Y., Oko R., Smith B. T., Argraves W. S. 2008. ATP-binding cassette transporters ABCA1, ABCA7, and ABCG1 in mouse spermatozoa. Biochem. Biophys. Res. Commun. 376: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frohlich J., Westerlund J., Sparks D., Pritchard P. H. 1990. Familial hypoalphalipoproteinemias. Clin. Invest. Med. 13: 202–210. [PubMed] [Google Scholar]
- 42.Browne R. W., Shelly W. B., Bloom M. S., Ocque A. J., Sandler J. R., Huddleston H. G., Fujimoto Y. 2008. Distributions of high-density lipoprotein particle components in human follicular fluid and sera and their associations with embryo morphology parameters during IVF. Hum. Reprod. 23: 1884–1894. [DOI] [PubMed] [Google Scholar]