Abstract
The peroxisome proliferator-activated receptor δ (PPARδ) is implicated in regulation of mitochondrial processes in a number of tissues, and PPARδ activation is associated with decreased susceptibility to ectopic lipid deposition and metabolic disease. Here, we show that PPARδ is the PPAR subtype expressed at the highest level in insulinoma cells and rat pancreatic islets. Furthermore, PPARδ displays high transcriptional activity and acts in pronounced synergy with retinoid-X-receptor (RXR). Interestingly, unsaturated fatty acids mimic the effects of synthetic PPARδ agonists. Using short hairpin RNA-mediated knockdown, we demonstrate that the ability of unsaturated fatty acids to stimulate fatty acid metabolism is dependent on PPARδ. Activation of PPARδ increases the fatty acid oxidation capacity in INS-1E β-cells, enhances glucose-stimulated insulin secretion (GSIS) from islets, and protects GSIS against adverse effects of prolonged fatty acid exposure. The presented results indicate that the nuclear receptor PPARδ is a fatty acid sensor that adapts β-cell mitochondrial function to long-term changes in unsaturated fatty acid levels. As maintenance of mitochondrial metabolism is essential to preserve β-cell function, these data indicate that dietary or pharmacological activation of PPARδ and RXR may be beneficial in the prevention of β-cell dysfunction.
Keywords: peroxisome proliferator-activated receptor δ, β-cells, β-oxidation, fatty acids, glucose-stimulated insulin secretion
The peroxisome proliferator-activated receptors (PPARs) are a family of transcription factors belonging to the nuclear receptor superfamily. The PPAR family consists of three subtypes, PPARα, PPARγ, and PPARβ/δ, all of which are regulated by fatty acids and their derivatives. The PPARs bind to peroxisome proliferator response elements (PPREs) as heterodimers with the retinoid-X-receptor (RXR). Upon ligand binding, the PPAR-RXR heterodimers stimulate expression of each their subset of genes involved in glucose and fatty acid metabolism, insulin signaling, cell differentiation, or cell proliferation (reviewed in Ref. 1). The PPAR subtypes have distinct transactivation characteristics and display preference for distinct subsets of target genes (2–4).
All three PPAR subtypes are expressed in pancreatic β-cells (5, 6). We previously reported that PPARα governs β-cell fatty acid catabolism, while activation of PPARγ primarily directs fatty acids toward esterification and accumulation as triglycerides (7). These specific roles are reflected in the metabolic control of PPARα and PPARγ expression. Under hypoglycemic fasting conditions, where there is increased demand for energy production from nonglucose substrates, PPARα is most abundant, whereas PPARγ is upregulated in β-cells under hyperglycemic and hyperlipidemic conditions (6, 8, 9).
PPARδ is widely expressed in mammalian tissues and cell types; however, until recently, the function of PPARδ in metabolic regulation has been elusive. Evidence now accumulates in favor of a pivotal role for PPARδ in basal lipid oxidation. The importance of PPARδ in lipid catabolism has been described for metabolically important tissues, such as skeletal muscle (10), adipocytes (11), as well as cardiomyocytes (12), pointing at PPARδ as a potential drug target in treatment of metabolic disease. Interestingly, hypolipidemic drugs, unsaturated fatty acids, and derivatives hereof directly activate PPARδ as ligands (13, 14).
In the endocrine pancreas, a key regulator of whole-body metabolism, the role of PPARδ remains unexplored. It is recognized that the PPARδ gene is expressed in pancreatic β-cells (5, 9, 15), but the function of PPARδ in β-cell physiology has not been studied. PPARs, in particular PPARα and PPARδ, have been shown to mediate effects of unsaturated fatty acids on gene expression in some cell types (13, 14), but it is unknown whether this is the case in pancreatic β-cells.
Fatty acids have profound effects on β-cell function and viability. While they acutely stimulate insulin release (16, 17), long-term exposure of β-cells to elevated levels of fatty acids leads to lipid accumulation, β-cell dysfunction, and eventually apoptosis (18–20). These long-term effects are associated with changes in β-cell gene expression. Moreover, the effects of fatty acids on β-cell function depend on their chain length and degree of saturation (17, 21, 22). Saturated fatty acids, such as palmitic acid, negatively affect insulin production and β-cell viability (23, 24), whereas unsaturated fatty acids raise the basal insulin secretion, thereby desensitizing the β-cell to glucose without causing apoptosis (20, 25). Notably, the adverse effects of fatty acids on β-cell function are generally inversely related to the rate of fatty acid oxidation (19, 22).
In this study, we show that PPARδ is abundant in pancreatic β-cells. We show that PPARδ activity is highly inducible and that PPARδ displays a pronounced synergy with agonists of RXRα in the activation of endogenous target genes in isolated rat islets and clonal β-cells. Interestingly, using short hairpin RNA (shRNA)-mediated knockdown, we find that the ability of unsaturated fatty acids to stimulate expression of genes involved in fatty acid metabolism is dependent on PPARδ and is potentiated by RXR agonists. The transcriptional regulation by PPARδ increases β-cell fatty acid oxidation capacity, potentiates glucose-stimulated insulin secretion (GSIS), and counteracts fatty acid-induced alterations in insulin secretion. Our results show that the nuclear receptor PPARδ is both a main transcriptional effector of unsaturated fatty acids and a central regulator of fatty acid metabolism in pancreatic β-cells. The modulation of PPARδ activity using selective agonists hence provides new perspectives for protection of β-cell function and prevention of diabetes.
MATERIALS AND METHODS
Cell culture, islet isolation, and agonist stimulation
The cell line INS-1E was cultured as previously described (26). The cells were at passage numbers between 50 and 78 and were used for experiments at 70–80% confluence. Rat islets were isolated from adult male Wistar rats by collagenase perfusion and cultured for 24 h in RPMI with 11 mM glucose (27). Medium and supplements were from Invitrogen-GIBCO and serum from HyClone. L165041, a selective PPARδ agonist, was kindly provided by Merck Research Laboratories, PPARγ and RXRα agonists BRL49653 and LG100268 were kindly provided by Novo Nordisk, and WY14.643, a PPARα-specific agonist, was purchased from Calbiochem. All fatty acids were purchased from Sigma. Agonists were used in the following concentrations [EC50 values for the relevant PPAR subtype in parentheses (28)]; L165041, 1 μM (3.8 μM); WY14.643, 30 μM (0.63 μM); BRL49653, 1 μM (0.076 μM); LG100268, 0.2 μM; and oleic acid, 100 μM. A table of EC50 values can be found in supplementary Table I. Fatty acids were added directly to the medium containing 10% FBS. Unless otherwise indicated, cells were stimulated with agonists for 24 h before harvest or further experiments. Rat handling was carried out in a certified animal facility according to procedures that were approved by the animal care and experimentation authorities of the Canton of Geneva.
Quantitative PCR
RNA isolation and cDNA synthesis were performed at described previously (7). Quantitative three-step PCR was performed on the ABI-7700 Prism quantitative PCR instrument using 2xSyBRgreen master mix (Sigma) and Sigma passive reference according to instructions from the manufacturer. PCR reactions were made in duplicates. Primers for quantitative PCR were designed using Primer Express 2.0 (Applied Biosystems) and specificity and efficacy validated before use. For each primer set, a standard curve was generated from cDNA stepwise diluted 10-fold over a 10,000-fold range. The slope of the standard curve for each primer set was used to determine the efficacy, which in turn was used to calculate expression levels from Ct values. Only primer sets with efficacies 0.95 < E < 1.05 were used for analysis. All quantifications were performed with TFIIB as internal standard and presented as fold over control. TFIIB expression remained unaffected under the described experimental conditions. Primer sequences and gene information are also found in the supplementary data.
Transient transfection and luciferase reporter assay
Cells were seeded in 24-well plates and grown until 70% confluence. Medium was changed the day before transfection. Transfection with the 3xACO-PPRE luciferase reporter construct (described in Ref. 29) was performed in triplicate using Lipofectamine2000 from Invitrogen for 4 h, and cells were then incubated for 24 h in new media containing DMSO or indicated agonists. Transfected cells were harvested in lysis buffer from Tropix, and cell lysates were assayed for luciferase activity. All measurements were performed as duplicates in 96-well microtiter plates in a LUMIstar luminometer from BMG-Labtech. It was verified that the observed reporter stimulation by the agonists was mediated through the PPRE elements with no stimulation of the parent vector activity (thymidine kinase promoter-driven luciferase reporter) or plasmid uptake (data not shown). All transfections were repeated a minimum of three times.
Adenovirus generation and transduction
pSuper-shPPARα and pSuper-shPPARδ were constructed with specific oligos directed against sequences 5′-GAGGCAGAGGTCCGATTCT-3′ and 5′-GAGGAGAAAGAGGAAGTGG-3′, respectively, as described (30). The H1 promoter cassette was then excised using SmaI and HindIII and ligated into pShuttle (EcoRV and HindIII). Recombinant adenoviruses containing shRNA against PPARα or -δ were generated using the AdEasy cloning system from Stratagene. The linarized plasmids were transfected into 293-HEK cells, and the viruses were amplified and purified. The viruses were initially titrated by a plaque assay-based approach. Subsequently, relative titers of functional viruses were equalized based on quantification of the adenoviral transcript AdE4 by quantitative PCR. Twenty-four hours after transduction with AdshEmpty, AdshPPARα, or AdshPPARδ, agonists (L165041, LG100268, or oleic acid) were added to the medium, and cells were incubated further 24 h before harvest for RNA and protein extraction.
Protein analysis by Western blotting and ECL detection
For total protein extraction, INS-1E cells were harvested in hypotonic lysis buffer containing 2.5% SDS. Nuclear extracts were prepared essentially as described in Ref. 31. Protein extracts were separated by SDS-PAGE and proteins blotted onto polyvinylidene difluoride membranes (Millipore) and probed with the indicated antibodies. Primary antibodies anti-PPARγ (SC-7273) and anti-TFIIB (SC-225) were obtained from Santa Cruz and secondary horseradish peroxidase-coupled anti-Fcγ antibodies, anti-mouse (P0447) and anti-rabbit (P0399), were obtained from DAKO Cytomation. SC-7273 (E-8) is raised against the PPARγ C terminus, which is highly conserved between the PPAR subtypes. Therefore, SC-7273 detects all three PPAR subtypes with a slight preference for PPARα over PPARδ and PPARγ, as determined by comparing ECL signals of HA-tagged versions of the three subtypes (data not shown).
[1-14C]Oleic acid oxidation
INS-1E cells were treated with agonists or vehicle for 24 h. The fatty acid oxidation capacity was subsequently determined by incubating the cells with [1-14C]oleic acid complexed to BSA in serum free medium containing 5 mM glucose for 4 h and collecting 14CO2 essentially as described by Berge et al. (32). Data were normalized to total cellular protein. Dishes with medium but without cells were treated in parallel and used for background subtraction. Experiments were performed in triplicate.
GSIS
INS-1E cells and primary rat islets were treated with fatty acids or ligands as indicated for 24 h before insulin secretion. After an incubation for 30 min in KRBS with basal glucose (2.5 mM for INS-1E cells and 2.8 mM for rat islets) or stimulatory glucose (15 mM for INS-1E cells and 11.2 mM for rat islets), secreted insulin was measured by radioimmunoassay (Linco Research) using rat insulin as standard as described (26) and normalized to total insulin content or to the number of islets. Experiments were performed in triplicate.
Statistical analysis
Statistical evaluation of the data was performed using two-way ANOVA with Bonferroni corrections where applicable. Data relative to control samples are presented as means ± SD.
RESULTS
PPARδ is expressed as the most abundant PPAR subtype in pancreatic β-cells
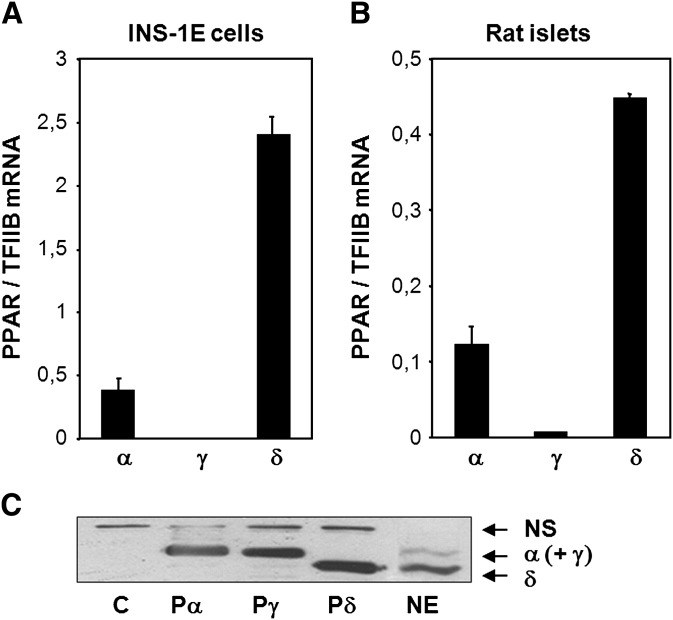
PPARδ has recently been implicated in the regulation of fatty acid metabolism in metabolically important tissues, such as skeletal muscle, white adipose tissue, and heart. In pancreatic β-cells, PPARδ is reported to be highly expressed at the mRNA level (5). Figure 1 shows that PPARδ is abundantly expressed at the RNA level in INS-1E insulinoma cells and in isolated rat islets. PPARα is also expressed at significant levels, whereas PPARγ is expressed at very low levels (Fig. 1A, B). The primers used for quantitative PCR were subtype specific and tested for equal efficacy of amplification. Previous reports have indicated that PPARδ may be subject to translational control (33). Thus, to investigate if the PPAR protein levels reflected the PPAR mRNA abundances, nuclear protein extracts were prepared from INS-1E cells, and proteins were analyzed by Western blotting. As control for protein migration, protein extracts from NIH-3T3 cells ectopically expressing mPPARα, mPPARγ, or mPPARδ were run in parallel. An antibody recognizing all three PPAR subtypes with a slight preference for PPARα over PPARγ and PPARδ (data not shown) was used for ECL detection. In keeping with the relative mRNA abundances, the lower band corresponding to PPARδ had the highest intensity (Fig. 1C). The less intense upper band corresponds to nonseparated PPARα and PPARγ proteins. Hence, PPARδ is the predominant PPAR subtype in INS-1E insulin-secreting cells under normal culture conditions.
Fig. 1.
PPARδ is the most abundant PPAR subtype in pancreatic β-cells. Total RNA was extracted from INS-1E cells (A) and Wistar rat islets (B) cultured in RPMI with 11 mM glucose and supplemented as described. Expression of the PPAR subtypes α, γ, and δ was quantified by quantitative PCR and normalized to TFIIB expression. RNA was harvested in duplicates and range is indicated. The presented results are representative of at least three independent experiments. C: Nuclear proteins were extracted from INS-1E cells (NE) and separated by SDS-PAGE, and PPAR levels were visualized by immunoblotting. Protein extracts from NIH-3T3 cells ectopically expressing murine PPARα (Pα), PPARγ (Pγ), PPARδ (Pδ), or control adenoviral vector (E) were run in parallel as control for protein migration. Arrows indicate the PPAR subtypes (NS, nonspecific band).
PPARδ is transcriptionally highly active and displays a high degree of synergy with RXR in insulin-secreting cells
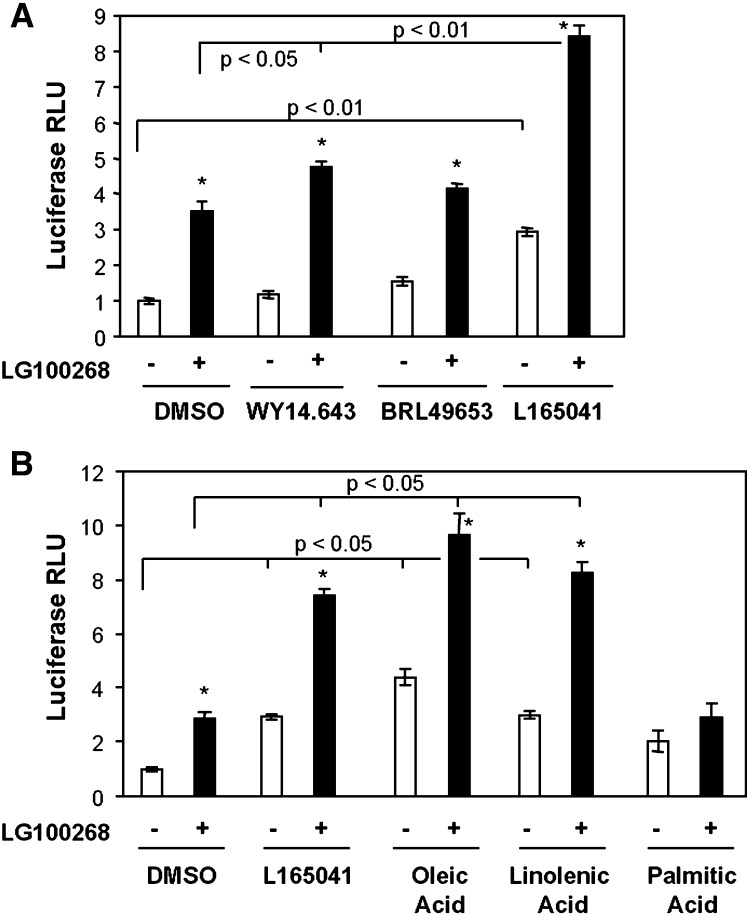
The finding that PPARδ is the most abundant PPAR subtype in β-cells prompted us to investigate if this is reflected at the functional level, i.e., whether endogenous PPARδ is also transcriptionally highly active in insulin secreting cells. We therefore transiently transfected INS-1E cells with a luciferase reporter construct containing a multimerized PPRE and incubated 24 h with DMSO or specific agonists for PPARα (WY14.643), PPARγ (BRL49653), or PPARδ (L165041) in the presence or absence of the RXRα agonist LG100268. PPARα and PPARγ agonists were administered at concentrations that give maximal but specific activation of the respective receptors, whereas the PPARδ agonist was kept at a suboptimal concentration to ensure specific activation of PPARδ (28). Whereas neither the PPARα nor the PPARγ agonist had any significant effect alone, activation of either RXRα or PPARδ increased the activity of the reporter construct (Fig. 2A). Simultaneous activation of both PPARδ and RXRα resulted in a pronounced (8.5-fold) synergistic activation. The combined activation of PPARα and RXRα resulted in a much weaker but still significant activation of the reporter, while activation of PPARγ and RXRα was without effect compared to RXRα activation alone.
Fig. 2.
A PPARδ-specific agonist and unsaturated fatty acids activate a PPAR-responsive reporter in synergy with RXRα in INS-1E cells. INS-1E cells were transfected with the 3xACO-PPRE luciferase reporter construct and incubated in RPMI (11 mM glucose) for 24 h with DMSO, PPAR subtype-specific agonists, or fatty acids in the presence or absence of the RXRα agonist LG100268 (0.2 μM). A: The specific PPAR agonists used were for PPARα, WY14.643 (30 μM); for PPARγ, BRL49653 (1 μM); and for PPARδ, L165041 (1 μM). B: The fatty acids used were oleic acid (100 μM), γ-linolenic acid (100 μM), or palmitic acid (100 μM). Luciferase activities were measured and presented relative to DMSO control. Transfections were performed in triplicate, and standard deviations are indicated. Results are representative of three independent experiments, and P values were calculated using two-way ANOVA. Addition of LG100268 differs from corresponding DMSO control (*P < 0.05).
Unsaturated fatty acids have previously been described as potential endogenous ligands of the PPARs (34). Interestingly, oleic acid (C18:1), γ-linolenic acid (C18:3), and arachidonic acid (C20:4; data not shown) all activated the PPRE reporter construct alone and synergistically with LG100268 to a similar degree as the specific PPARδ agonist L165041 (Fig. 2B). By contrast, the saturated fatty acid palmitic acid (C16:0) did not have any significant effect on reporter activity. In keeping with unsaturated fatty acids acting through PPARδ, there was no additive effect of combining L165041 and fatty acids neither in the absence nor in the presence of LG100268 (data not shown). These results indicate that PPARδ is a transcriptionally highly responsive PPAR subtype in INS-1E cells and that the effect of unsaturated fatty acids on the expression of PPAR target genes in β-cells may be mediated by PPARδ in synergy with RXRα.
The expression of endogenous PPAR target genes is synergistically activated by selective agonists of PPARδ and RXRα as well as by unsaturated fatty acids
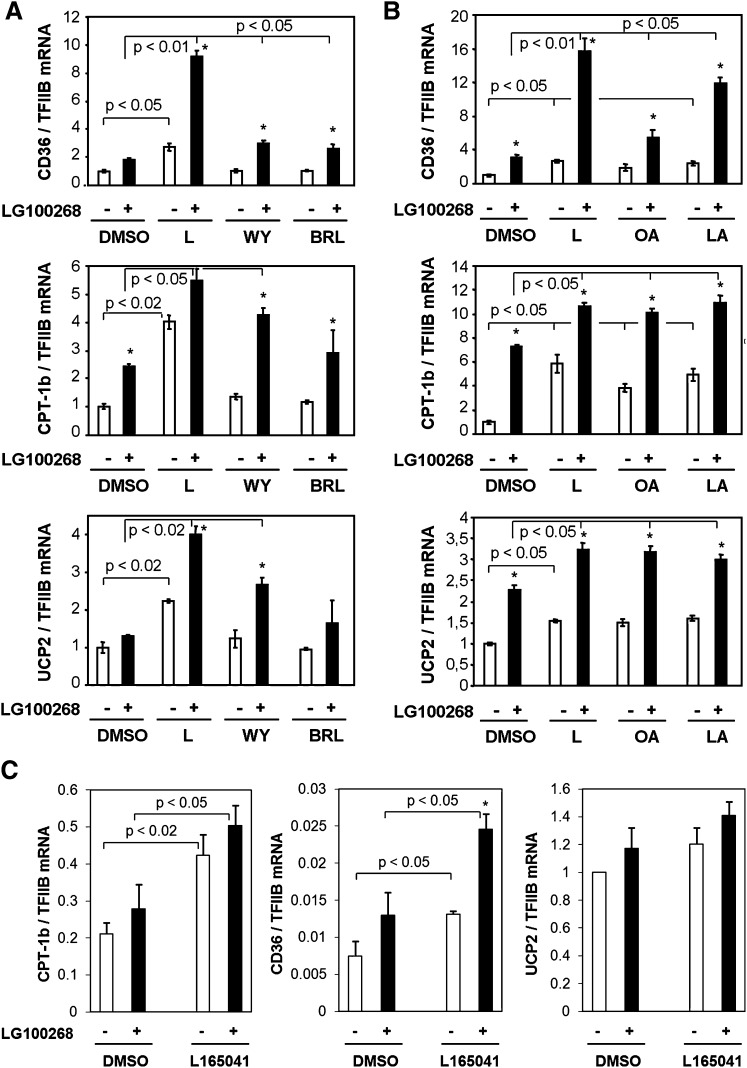
PPARδ potently activated transcription from an artificial PPRE in synergy with RXRα. To investigate the impact of PPARδ activation on the expression of endogenous genes, we treated INS-1E cells with DMSO or the indicated agonists for 24 h. Based on previous results (7), expression of PPAR-regulated genes involved in β-cell fatty acid metabolism was investigated using quantitative PCR and normalized to TFIIB expression. CD36, muscle carnitine palmitoyltransferase 1b (CPT-1b), and uncoupling protein 2 (UCP2) were all significantly induced by the specific PPARδ agonist L165041, but only minimally, or not at all, by specific activators of PPARα and PPARγ (Fig. 3A). The RXR-specific agonist LG100268 alone resulted in a minor activation of all three genes; however, the combination of PPARδ and RXR specific agonists resulted in a significant and synergistic activation of target genes. Simultaneous activation of PPARα and RXRα also resulted in significant, although less pronounced, synergistic activation of the genes, as we have shown previously (7). In contrast, no synergy was observed between the specific PPARγ and RXR agonists.
Fig. 3.
PPARδ activation and fatty acids stimulate expression of fatty acid handling genes in synergy with RXRα in INS-1E cells and rat islets. INS-1E cells were incubated for 24 h in RPMI (11 mM glucose) with DMSO, PPAR subtype-specific agonists, or fatty acids in the presence or absence of the RXRα agonist LG100268 (0.2 μM). A: The specific PPAR agonists used were for PPARα, WY14.643 (WY, 30 μM); for PPARγ, BRL49653 (BRL, 1 μM); and for PPARδ, L165041 (L, 1 μM). B: The fatty acids used were oleic acid (OA, 100 μM) or γ-linolenic acid (LA, 100 μM). Total RNA was extracted, and expression levels of CD36, muscle CPT-1, and UCP2 were quantified using quantitative PCR and normalized to TFIIB expression. Expression levels are presented relative to DMSO control and are representative of three independent experiments. For each experiment, RNA was harvested in triplicates. C: Rat islets were isolated and incubated for 24 h in RPMI (11 mM glucose) with the indicated ligands. Results are means of three independent experiments, and P values are calculated using two-way ANOVA. Addition of LG100268 differs from corresponding DMSO control (*P < 0.05).
Notably, as observed in the transient transfections, unsaturated fatty acids, such as oleic acid and γ-linolenic acid, mimicked selective activation of PPARδ and activated all the PPAR target genes investigated (Fig. 3B).
In keeping with high expression of PPARδ in primary cells (see Fig. 1B), PPARδ and RXRα agonists significantly activated CPT-1b and CD36 expression in isolated rat islets (Fig. 3C). These results indicate that the response to PPARδ activation is comparable between primary β-cells and INS-1E cells.
Taken together, these results show that PPARδ regulates genes involved in fatty acid metabolism in both rat islets and INS-1E β-cells and is a probable mediator of transcriptional effects of unsaturated fatty acids.
Oleic acid induces fatty acid handling genes through activation of PPARδ
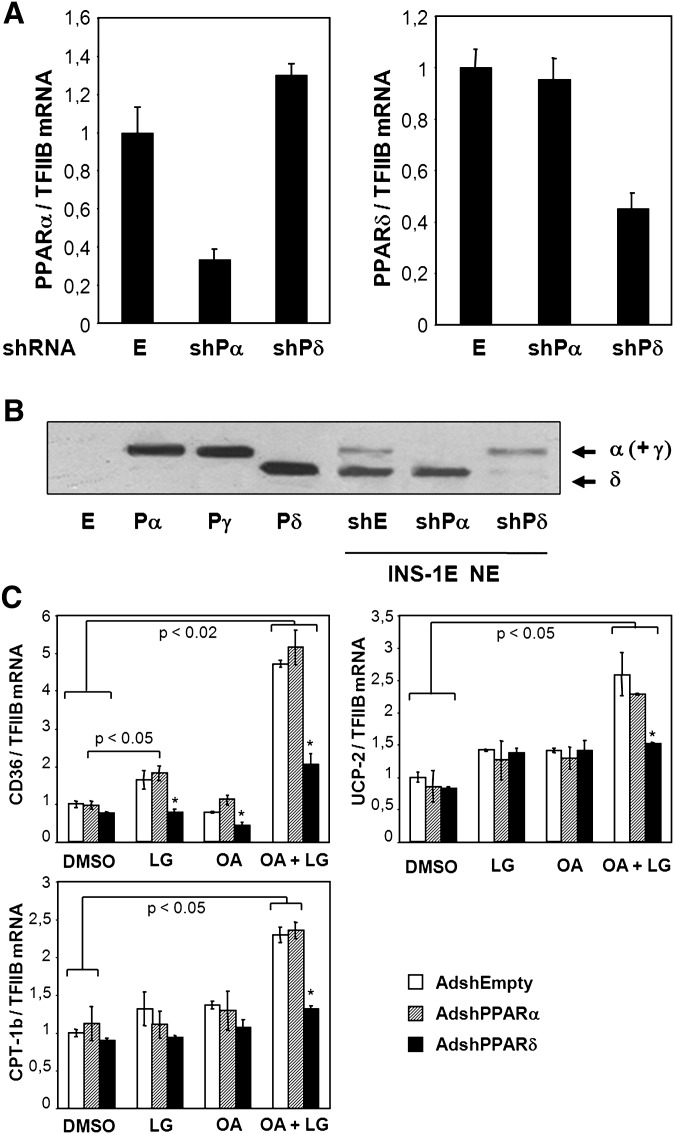
The above results suggest that PPARδ, and possibly also PPARα, are mediators of the transcriptional effects of unsaturated fatty acids. To determine the relative importance of PPARα and PPARδ in mediating these effects, we constructed adenoviral vectors for acute knockdown of each of these receptors in INS-1E cells. Adenoviral transfer of shRNA targeting specifically PPARα or PPARδ resulted in knockdown of mRNA expression levels by 70% and 60%, respectively, compared with control-infected cells (Fig. 4A). A nearly complete depletion of either of the two PPAR subtypes was confirmed at the protein level (Fig. 4B). Notably, the slower migrating protein band corresponding to PPARα and PPARγ was totally absent in the PPARα knockdown cells, confirming that PPARγ is virtually absent in INS-1E cells under the described culture conditions.
Fig. 4.
Oleic acid activation of fatty acid handling genes is dependent on PPARδ in insulin secreting cells. INS-1E cells were transduced with adenoviral vectors (∼20 pfu/cell) expressing shRNA targeting PPARα [AdshPPARα (shPα)] or PPARδ [AdshPPARδ (shPδ)] or with empty control virus [AdshEmpty (E)] and cultured for 48 h in RPMI (11 mM glucose) before harvest of total RNA and nuclear protein extracts. A: Expression levels of PPARα and PPARδ were quantified using quantitative PCR, normalized to TFIIB expression, and presented relative to AdshEmpty control transduced cells. B: Nuclear proteins (NE) were separated by SDS-PAGE, and PPAR levels were visualized by immunoblotting. Protein extracts from NIH-3T3 cells ectopically expressing murine PPARα (Pα), PPARγ2 (Pγ), PPARδ (Pδ), or control adenoviral vector (E) were run in parallel as control for protein migration. Arrows indicate migration of the subtypes. C: INS-1E cells were transduced with the indicated adenoviral vectors and cultured for 24 h in RPMI (11 mM glucose) before incubation with DMSO or the fatty acid oleic acid (OA, 100 μM) in the presence or absence of the RXRα agonist LG100268 (0.2 μM) for additional 24 h. Total RNA was extracted, and expression levels of CD36, muscle CPT-1, and UCP2 were quantified using quantitative PCR and normalized to TFIIB expression. Expression levels are presented relative to DMSO control. Results are means of three independent experiments, and P values were calculated by two-way ANOVA. Addition of LG100268 differs from corresponding DMSO control (*P < 0.05).
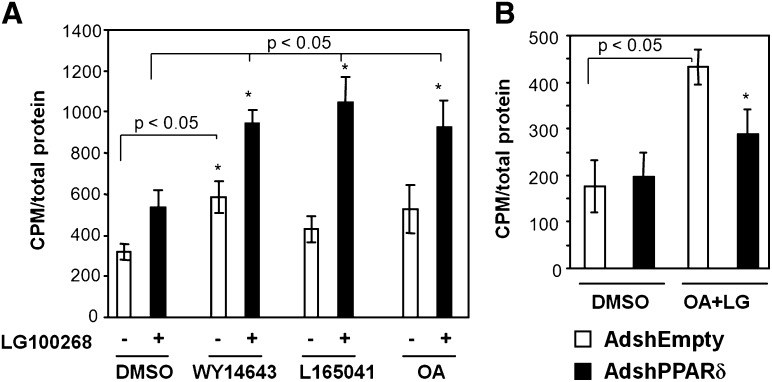
The basal expression levels of the investigated PPAR target genes were unaffected by PPARδ knockdown as well as by PPARα knockdown. Importantly, however, PPARδ knockdown abolished induction of these genes in response to oleic acid and the RXRα agonist LG100268 (Fig. 4C) and in response to γ-linolenic acid and LG100268 (data not shown). By contrast, PPARα knockdown had no effect on the induction of these PPAR target genes by unsaturated fatty acids or the RXR agonist under the experimental conditions described. These results show that in insulin-secreting cells; PPARδ is an endogenous mediator of the induction of genes involved in fatty acid oxidation by unsaturated fatty acids.
Activation of PPARδ increases fatty acid oxidation capacity in insulin secreting cells
Others and we have previously shown that activation of PPARα in pancreatic β-cells is associated with an increased potential to oxidize fatty acids (7). Activation of the endogenous PPARδ in INS-1E cells by the specific agonist L165041 or unsaturated fatty acids induced genes involved in fatty acid uptake and oxidation. To investigate whether the PPARδ-induced changes in gene expression affected INS-1E cell fatty acid oxidation capacity, we incubated cells for 24 h with DMSO, the PPARα agonist WY14.643, the PPARδ agonist L165041, or oleic acid in the presence or absence of the RXRα agonist LG100268, respectively, and subsequently determined [1-14C]oleic acid oxidation. In keeping with previous observations (7), activation of PPARα with or without concomitant RXRα activation increased oleic acid oxidation (Fig. 5A), whereas agonists for either PPARδ or RXRα alone only modestly affected fatty acid oxidation. However, simultaneous activation of RXRα and PPARδ by L165041 or oleic acid synergistically increased fatty acid oxidation to the same extent as PPARα/RXRα activation. The effect of incubation with oleic acid and LG100268 was blunted when the PPARδ level was reduced by shRNA-mediated knockdown (Fig. 5B). These results establish PPARδ as a lipostat required for oleic acid-induced fatty acid oxidation in INS-1E cells, and the pronounced synergy between PPARδ and RXRα reflects our observations at the transcriptional level.
Fig. 5.
Oleic acid activation of PPARδ increases fatty acid oxidation capacity in synergy with RXRα in insulin-secreting cells. A: INS-1E cells were incubated for 24 h in RPMI (11 mM glucose) with DMSO, the specific PPARα agonist WY14.643 (30 μM), or the specific PPARδ agonist L165041 (1 μM) in the presence or absence of the RXRα agonist LG100268 (0.2 μM). Cells were incubated with [1-14C]oleic acid for 4 h in serum-free RPMI1640 containing 5 mM glucose, and oxidation was measured in triplicate, background was subtracted, and values were normalized to total cellular protein. B: INS-1E cells were transduced with adenoviral vectors (∼20 pfu/cell) expressing shRNA against PPARδ (AdshPPARδ) or a control shRNA vector (AdshEmpty). After 24 h in RPMI (11 mM glucose), DMSO or oleic acid (OA; 100 μM) together with LG100268 (LG; 0.2 μM) was added to the media. Cells were incubated for another 24 h, and fatty acid oxidation was measured as described above. The experiments were performed in triplicate, and standard deviations are indicated. Results are representative of three independent experiments, and P values were calculated using two-way ANOVA. Addition of LG100268 differs from corresponding DMSO control (*P < 0.05).
PPARδ protects β-cells against fatty acid-induced changes in insulin secretion
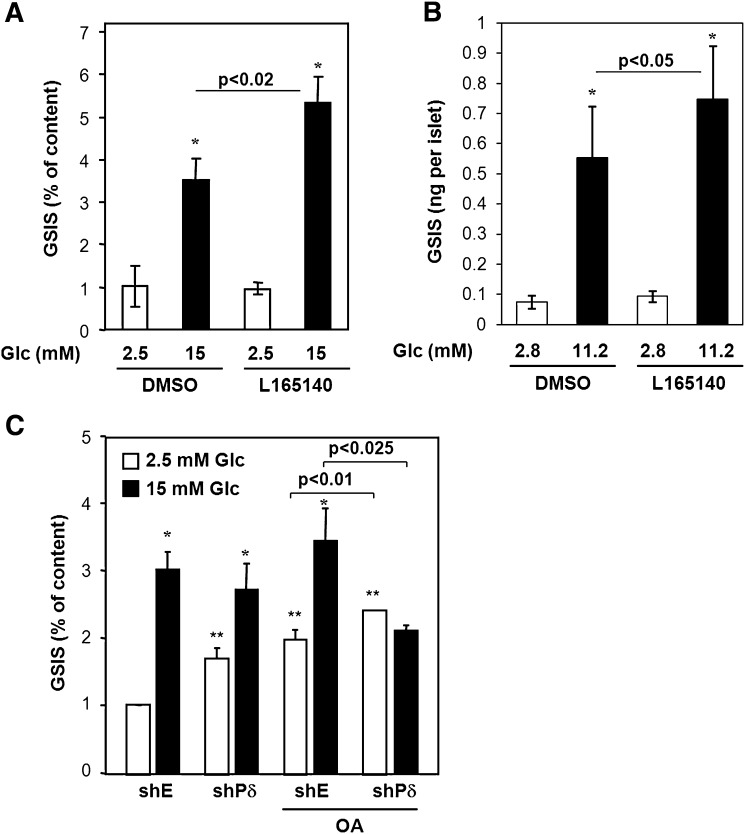
Prolonged exposure of β-cells to fatty acids is known to have profound effect on both basal insulin secretion and GSIS (35). The notion that excess fatty acids ablate mitochondrial function and that these effects are inversely related to fatty acid oxidation prompted us to investigate the effects of PPARδ activity on insulin secretion. INS-1E cells were preincubated for 24 h with the PPARδ agonist L165041 or DMSO. Cells were then exposed to basal 2.5 mM or stimulatory 15 mM glucose for 30 min and supernatant was collected. The secreted insulin was quantified and calculated as percentage of total cellular insulin content (Fig. 6A). The pretreatment with the PPARδ agonist increased GSIS 1.5-fold (P < 0.02) without affecting basal insulin secretion. To extend this finding to primary β-cells, we isolated rat pancreatic islets and pretreated these for 24 h with DMSO or the PPARδ agonist L165041. The rat islets were then subjected to basal 2.8 mM or stimulatory 11.2 mM glucose for 1 h and supernatant was collected. Similar to INS-1E cells, the PPARδ agonist had no effect on basal insulin secretion but increased GSIS by 35% (P < 0.05) (Fig. 6B).
Fig. 6.
PPARδ activity potentiates GSIS and protects against fatty acid-induced insulin secretion defects. A: INS-1E cells were incubated for 24 h in RPMI (11 mM glucose) with DMSO or the specific PPARδ agonist L165041 (1 μM). Insulin secretion over 30 min at 2.5 and 15 mM glucose was determined and normalized to total insulin content. GSIS is significantly different from basal insulin secretion (*P < 0.01). GSIS in cells preincubated with L165041 is elevated compared with DMSO-treated cells (P < 0.02). B: Rat islets were incubated for 24 h in RPMI (11 mM glucose) with DMSO or the specific PPARδ agonist L165041 (1 μM). Insulin secretion over 30 min at 2.8 and 11.2 mM glucose was determined and normalized to islet number. GSIS is significantly different from basal insulin secretion (*P < 0.01). GSIS in cells preincubated with L165041 is elevated compared with DMSO-treated cells (P < 0.05). C: INS-1E cells were transduced with adenoviral vectors (∼20 pfu/cell) expressing shRNA against PPARδ (shPδ) or control shRNA (shE). After 24 h of incubation in RPMI (11 mM glucose) with DMSO or oleic acid (OA; 400 μM), insulin secretion over 30 min at 2.5 and 15 mM glucose was determined and normalized to total insulin content. Where indicated, GSIS is significantly different from basal insulin secretion (*P < 0.05) as assessed by two-way ANOVA. ** Significantly different from control transduced, DMSO-treated cells at 2.5 mM glucose (P < 0.001). Insulin secretion was significantly reduced at 15 mM glucose (P < 0.025) and increased at 2.5 mM glucose (P < 0.01) for shPδ cells compared with wild-type cells treated with oleic acid. The results are means of three independent experiments.
Knowing that a PPARδ agonist enhances GSIS, we wanted to see if PPARδ activity was important for preservation of insulin secretion when β-cells were exposed to fatty acids. INS-1E cells were transduced with adenovirus expressing shPPARδ or control virus. Cells were then cultured in medium with or without 0.4 mM oleic acid for 24 h, and insulin secretion at 2.5 or 15 mM glucose was determined. Treatment of control cells for 24 h with oleic acid increased basal insulin secretion (P < 0.001) without affecting insulin secretion stimulated by 15 mM glucose, thereby lowering the fold increase normally induced by glucose (Fig. 6C). This phenotype was resembled by knockdown of PPARδ in the absence of oleic acid, as these cells still responded to 15 mM glucose but the overall response was blunted. Importantly, oleic acid completely abolished the insulin response to glucose from cells lacking PPARδ. The basal insulin secretion was even further elevated compared with oleic acid-treated control cells (P < 0.01), and the glucose stimulation was completely ablated. Insulin content was unaffected under all of the described experimental conditions. These data show that PPARδ expression is important for the β-cells to cope with the increased fatty acid load possibly by adjusting the fatty acid turnover.
DISCUSSION
The presence of abundant levels of PPARδ, mRNA in pancreatic islet is well established (9, 15), but until now, no function has been ascribed to this nuclear receptor in pancreatic β-cells. In this work, we show that PPARδ is the most abundant PPAR subtype in pancreatic β-cells at both the mRNA and protein level. Consistently, selective activation of PPARδ by the use of a specific agonist leads to efficient induction of genes involved in lipid metabolism. By contrast, little activation of target genes was observed by activation of PPARα and PPARγ. Gene activation by PPARδ is synergistically potentiated by co-stimulation of RXRα. Also, this synergism is more pronounced than the synergy observed by agonist activation of the other PPAR subtypes and RXR but is similar to the synergy between ectopically expressed PPARα and RXRα in INS-1E cells (7). Notably, mono- and polyunsaturated fatty acids behave highly similar to the specific PPARδ agonist in both luciferase reporter assays and in the induction of endogenous genes in INS-1E cells. The sensing of unsaturated fatty acids at physiological levels and their influence on gene expression is synergistically potentiated by co-stimulation of RXRα and abolished by specific knockdown of PPARδ. These data show that PPARδ is an important mediator of the transcriptional effects by unsaturated fatty acids in INS-1E insulinoma cells. In accordance with these findings in insulin secreting cells, PPARδ was previously described as a fatty acid sensor also in macrophages (36). In contrast, the saturated fatty acid palmitic acid had minimal effect on PPARδ activity, although saturated and unsaturated fatty acids reportedly are bound by the PPARδ ligand-binding domain with comparable affinities (37, 38). Possibly, PPARδ binding of different fatty acids could give rise to different structural conformations and recruitment of different cofactor complexes as it has been described for PPARγ (39). Moreover, the fatty acids used in our study could be derivatized prior to PPARδ ligand-binding domain binding, and little can be concluded about the bona fide PPARδ agonist.
In some tissues, PPARδ has been implicated in regulation of growth and differentiation (40, 41). Recently, however, evidence has accumulated that PPARδ is also involved in the maintenance of mitochondrial oxidative capacity, in particular fatty acid oxidation. This has been described in various tissues, including white adipose tissue (11), skeletal muscle (10), and cardiomyocytes (12). Importantly, stimulation of PPARδ activity protects against diet-induced obesity and insulin resistance, whereas loss of PPARδ causes ectopic lipid accumulation, obesity, and cardiac lipotoxicity. These findings are in agreement with our observations suggesting a similar role for PPARδ in pancreatic β-cells. PPARδ is a central regulator of genes involved in mitochondrial handling of fatty acids and, hence, important for lipid partitioning in pancreatic β-cells. Indeed, activation of PPARδ with a specific agonist or the monounsaturated oleic acid increases the potential for fatty acid oxidation in INS-1E cells. The induction of fatty acid oxidation by PPARδ and PPARα is of similar magnitude. As PPARδ agonists induced PPAR target genes involved in fatty acid metabolism more potently than the PPARα agonist but produced a comparable increase in fatty acid oxidation capacity, other parameters must be limiting for PPARδ-induced fatty acid oxidation under the experimental conditions described. Notably, the effects of PPARδ/RXR activation appear to be confined to enhancement of mitochondrial substrate utilization with no effect on mitochondrial density (data not shown).
As PPARδ activation by unsaturated fatty acids or synthetic ligands in INS-1E cells increased mitochondrial capacity to oxidize fatty acids, we wanted to investigate if PPARδ activation would also affect GSIS, which is tightly coupled to mitochondrial metabolism. Indeed, activation of the transcriptional program regulated by PPARδ enhanced GSIS in both primary rat islets and INS-1E cells without affecting basal insulin secretion. Most likely, this effect corresponds to increased mitochondrial activity and possibly increased anaplerotic glucose flow. As previously reported for PPARα (7), activation of PPARδ by either natural or synthetic agonists increased PDK4 mRNA expression in both rat islets and INS-1E cells (supplementary Figure II). By inhibiting the pyruvate dehydrogenase complex, PDK4 is believed to increase the anaplerotic carbon flow through pyruvate carboxylase and sensitize the β-cells to glucose (42, 43).
It is recognized that the adverse chronic effects of fatty acids on β-cell function is counteracted by conditions increasing mitochondrial fatty acid oxidation. Stimulation of β-cells with leptin, AICAR, and troglitazone increases fatty acid oxidation and protects against the lipotoxic effects of fatty acids (44–46). Likewise, glucose is known to direct fatty acids from mitochondrial oxidation to lipid accumulation and thereby aggravate the toxicity of fatty acids (23, 47). Moreover, unmetabolized fatty acids, such as 2-bromopalmitate, are generally more lipotoxic than their metabolizable analogs (48, 49). We therefore wanted to investigate whether PPARδ activity would counteract alterations of the insulin response to glucose by fatty acids. Exposure of the cells to 0.4 mM oleic acid for 24 h increased basal insulin secretion (P < 0.001) and blunted the glucose response. Similar effects of oleic acid have recently been described in other studies (25, 35). In the presence of oleic acid, PPARδ is presumably already active, and addition of synthetic ligands would not further increase PPARδ activity. We therefore employed shRNA-mediated knockdown to evaluate if PPARδ preserves insulin secretion during exposure to elevated fatty acid levels. Importantly, the effect of oleic acid on insulin secretion was much more pronounced in PPARδ-depleted cells than in control infected cells. Basal insulin secretion was further increased, and GSIS was totally ablated. In the absence of PPARδ, fatty acids and their derivatives would accumulate to higher levels and override the tight coupling between glucose stimulation and fatty acid turnover known to be important for the normal glucose response (50). Activated PPARδ may hence protect against the adverse effects of fatty acids on glucose responsiveness by adjusting the mitochondrial capacity to the increased load of fatty acid. A β-cell protective role is in keeping with previous studies showing that loss of PPARδ in cardiomyocytes caused lipid accumulation and lipotoxic cardiomyopathy (12). A recent study showed that feeding mice a high-fat diet rich in oleic acid increased islet mitochondrial metabolism (51). The observed increase in mitochondrial capacity is reminiscent of PPARδ activation in other tissues (10, 11), but whether this adaptation of the islet cells in vivo to increased fatty acid loads is dependent on PPARδ remains to be determined.
In conclusion, we show that PPARδ is abundant in pancreatic β-cells and plays a central role as effector of unsaturated fatty acids in the regulation of fatty acid metabolism and β-cell function. The transcriptional activity of PPARδ is synergistically potentiated by simultaneous activation of RXR by agonists, and co-stimulation of PPARδ and RXR leads to a pronounced increase of the oxidative potential of the insulin secreting cells. Our results indicate that this increase in oxidative potential may counteract the harmful actions of fatty acids and therefore may represent an adaptive mechanism for protection of β-cell function and a promising target for pharmacological prevention of lipid induced β-cell dysfunction.
Supplementary Material
Acknowledgments
The authors are thankful to Gaelle Chaffard for expert technical assistance and to Novo Nordisk and Merck Research Laboratories for kindly providing PPAR and RXRα agonists.
Footnotes
Abbreviations:
- CPT-1b
- carnitine palmitoyltransferase 1b
- GSIS
- glucose-stimulated insulin secretion
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- peroxisome proliferator response element
- RXR
- retinoid-X-receptor
- UCP2
- uncoupling protein 2
This study was supported by the Danish Health Science Research Council, the Danish Diabetes Foundation (S. M.), and the Swiss National Science Foundation (P. M.).
REFERENCES
- 1.Evans R. M., Barish G. D., Wang Y. X. 2004. PPARs and the complex journey to obesity. Nat. Med. 10: 355–361. [DOI] [PubMed] [Google Scholar]
- 2.Brun R. P., Tontonoz P., Forman B. M., Ellis R., Chen J., Evans R. M., Spiegelman B. M. 1996. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 10: 974–984. [DOI] [PubMed] [Google Scholar]
- 3.Tachibana K., Kobayashi Y., Tanaka T., Tagami M., Sugiyama A., Katayama T., Ueda C., Yamasaki D., Ishimoto K., Sumitomo M., et al. 2005. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nucl. Recept. 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen R., Grontved L., Stunnenberg H. G., Mandrup S. 2006. Peroxisome proliferator-activated receptor subtype- and cell-type-specific activation of genomic target genes upon adenoviral transgene delivery. Mol. Cell. Biol. 26: 5698–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon J. S., Yaney G. C., Zhou Y., Voilley N., Bowen S., Chipkin S., Bliss C. R., Schultz V., Schuit F. C., Prentki M., et al. 2000. Dehydroepiandrosterone sulfate and beta-cell function: enhanced glucose-induced insulin secretion and altered gene expression in rodent pancreatic beta-cells. Diabetes. 49: 2012–2020. [DOI] [PubMed] [Google Scholar]
- 6.Laybutt D. R., Sharma A., Sgroi D. C., Gaudet J., Bonner-Weir S., Weir G. C. 2002. Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. J. Biol. Chem. 277: 10912–10921. [DOI] [PubMed] [Google Scholar]
- 7.Ravnskjaer K., Boergesen M., Rubi B., Larsen J. K., Nielsen T., Fridriksson J., Maechler P., Mandrup S. 2005. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology. 146: 3266–3276. [DOI] [PubMed] [Google Scholar]
- 8.Ravnskjaer K., Boergesen M., Dalgaard L. T., Mandrup S. 2006. Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J. Mol. Endocrinol. 36: 289–299. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y. T., Shimabukuro M., Wang M. Y., Lee Y., Higa M., Milburn J. L., Newgard C. B., Unger R. H. 1998. Role of peroxisome proliferator-activated receptor alpha in disease of pancreatic beta cells. Proc. Natl. Acad. Sci. USA. 95: 8898–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T., Yamamoto J., Iwasaki S., Asaba H., Hamura H., Ikeda Y., Watanabe M., Magoori K., Ioka R. X., Tachibana K., et al. 2003. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA. 100: 15924–15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y. X., Lee C. H., Tiep S., Yu R. T., Ham J., Kang H., Evans R. M. 2003. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 113: 159–170. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L., Ding G., Qin Q., Huang Y., Lewis W., He N., Evans R. M., Schneider M. D., Brako F. A., Xiao Y., et al. 2004. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 10: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 13.Forman B. M., Chen J., Evans R. M. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevillotte E., Rieusset J., Roques M., Desage M., Vidal H. 2001. The regulation of uncoupling protein-2 gene expression by omega-6 polyunsaturated fatty acids in human skeletal muscle cells involves multiple pathways, including the nuclear receptor peroxisome proliferator-activated receptor beta. J. Biol. Chem. 276: 10853–10860. [DOI] [PubMed] [Google Scholar]
- 15.Chuang J. C., Cha J. Y., Garmey J. C., Mirmira R. G., Repa J. J. 2008. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol. Endocrinol. 22: 2353–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roduit R., Nolan C., Alarcon C., Moore P., Barbeau A., Delghingaro-Augusto V., Przybykowski E., Morin J., Masse F., Massie B., et al. 2004. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 53: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 17.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., et al. 2003. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y., Hirose H., Ohneda M., Johnson J. H., McGarry J. D., Unger R. H. 1994. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA. 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. 1998. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. USA. 95: 2498–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frigerio F., Chaffard G., Berwaer M., Maechler P. 2006. The antiepileptic drug topiramate preserves metabolism-secretion coupling in insulin secreting cells chronically exposed to the fatty acid oleate. Biochem. Pharmacol. 72: 965–973. [DOI] [PubMed] [Google Scholar]
- 21.Stein D. T., Stevenson B. E., Chester M. W., Basit M., Daniels M. B., Turley S. D., McGarry J. D. 1997. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 100: 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maedler K., Spinas G. A., Dyntar D., Moritz W., Kaiser N., Donath M. Y. 2001. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 50: 69–76. [DOI] [PubMed] [Google Scholar]
- 23.El Assaad W., Buteau J., Peyot M. L., Nolan C., Roduit R., Hardy S., Joly E., Dbaibo G., Rosenberg L., Prentki M. 2003. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 144: 4154–4163. [DOI] [PubMed] [Google Scholar]
- 24.Hagman D. K., Hays L. B., Parazzoli S. D., Poitout V. 2005. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J. Biol. Chem. 280: 32413–32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frigerio F., Brun T., Bartley C., Usardi A., Bosco D., Ravnskjaer K., Mandrup S., Maechler P. 2009. Peroxisome proliferator-activated receptor alpha (PPARalpha) protects against oleate-induced INS-1E beta cell dysfunction by preserving carbohydrate metabolism. Diabetologia. 53: 331–340 [DOI] [PubMed] [Google Scholar]
- 26.Merglen A., Theander S., Rubi B., Chaffard G., Wollheim C. B., Maechler P. 2004. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 145: 667–678. [DOI] [PubMed] [Google Scholar]
- 27.Carobbio S., Maechler P. 2004. Sustained glucose-stimulated insulin secretion in mouse islets is not culture-dependent. Diabetologia. 47: 1856–1857. [DOI] [PubMed] [Google Scholar]
- 28.Willson T. M., Brown P. J., Sternbach D. D., Henke B. R. 2000. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 43: 527–550. [DOI] [PubMed] [Google Scholar]
- 29.Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. 1992. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 358: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brummelkamp T. R., Bernards R., Agami R. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science. 296: 550–553. [DOI] [PubMed] [Google Scholar]
- 31.Roduit R., Morin J., Masse F., Segall L., Roche E., Newgard C. B., Assimacopoulos-Jeannet F., Prentki M. 2000. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-alpha gene in the pancreatic beta-cell. J. Biol. Chem. 275: 35799–35806. [DOI] [PubMed] [Google Scholar]
- 32.Berge K., Tronstad K. J., Bohov P., Madsen L., Berge R. K. 2003. Impact of mitochondrial beta-oxidation in fatty acid-mediated inhibition of glioma cell proliferation. J. Lipid Res. 44: 118–127. [DOI] [PubMed] [Google Scholar]
- 33.Larsen L. K., Amri E. Z., Mandrup S., Pacot C., Kristiansen K. 2002. Genomic organization of the mouse peroxisome proliferator-activated receptor beta/delta gene: alternative promoter usage and splicing yield transcripts exhibiting differential translational efficiency. Biochem. J. 366: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krey G., Braissant O., L'Horset F., Kalkhoven E., Perroud M., Parker M. G., Wahli W. 1997. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 11: 779–791. [DOI] [PubMed] [Google Scholar]
- 35.Koshkin V., Wang X., Scherer P. E., Chan C. B., Wheeler M. B. 2003. Mitochondrial functional state in clonal pancreatic beta-cells exposed to free fatty acids. J. Biol. Chem. 278: 19709–19715. [DOI] [PubMed] [Google Scholar]
- 36.Chawla A., Lee C. H., Barak Y., He W., Rosenfeld J., Liao D., Han J., Kang H., Evans R. M. 2003. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc. Natl. Acad. Sci. USA. 100: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H. E., Lambert M. H., Montana V. G., Parks D. J., Blanchard S. G., Brown P. J., Sternbach D. D., Lehmann J. M., Wisely G. B., Willson T. M., et al. 1999. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell. 3: 397–403. [DOI] [PubMed] [Google Scholar]
- 38.Fyffe S. A., Alphey M. S., Buetow L., Smith T. K., Ferguson M. A., Sorensen M. D., Bjorkling F., Hunter W. N. 2006. Recombinant human PPAR-beta/delta ligand-binding domain is locked in an activated conformation by endogenous fatty acids. J. Mol. Biol. 356: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 39.Rocchi S., Picard F., Vamecq J., Gelman L., Potier N., Zeyer D., Dubuquoy L., Bac P., Champy M. F., Plunket K. D., et al. 2001. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol. Cell. 8: 737–747. [DOI] [PubMed] [Google Scholar]
- 40.Matsusue K., Peters J. M., Gonzalez F. J. 2004. PPARbeta/delta potentiates PPARgamma-stimulated adipocyte differentiation. FASEB J. 18: 1477–1479. [DOI] [PubMed] [Google Scholar]
- 41.Park B. H., Vogelstein B., Kinzler K. W. 2001. Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc. Natl. Acad. Sci. USA. 98: 2598–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y. Q., Jetton T. L., Leahy J. L. 2002. Beta-cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J. Biol. Chem. 277: 39163–39168. [DOI] [PubMed] [Google Scholar]
- 43.Sugden M. C., Bulmer K., Augustine D., Holness M. J. 2001. Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-alpha: implications for glucose-stimulated insulin secretion. Diabetes. 50: 2729–2736. [DOI] [PubMed] [Google Scholar]
- 44.Shimabukuro M., Koyama K., Chen G., Wang M. Y., Trieu F., Lee Y., Newgard C. B., Unger R. H. 1997. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc. Natl. Acad. Sci. USA. 94: 4637–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diraison F., Parton L., Ferre P., Foufelle F., Briscoe C. P., Leclerc I., Rutter G. A. 2004. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Biochem. J. 378: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimabukuro M., Zhou Y. T., Lee Y., Unger R. H. 1998. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J. Biol. Chem. 273: 3547–3550. [DOI] [PubMed] [Google Scholar]
- 47.Roche E., Farfari S., Witters L. A., Assimacopoulos-Jeannet F., Thumelin S., Brun T., Corkey B. E., Saha A. K., Prentki M. 1998. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 47: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 48.Briaud I., Harmon J. S., Kelpe C. L., Segu V. B., Poitout V. 2001. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 50: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cnop M., Hannaert J. C., Hoorens A., Eizirik D. L., Pipeleers D. G. 2001. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 50: 1771–1777. [DOI] [PubMed] [Google Scholar]
- 50.Nolan C. J., Madiraju M. S., Delghingaro-Augusto V., Peyot M. L., Prentki M. 2006. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 55 (Suppl. 2): S16–S23. [DOI] [PubMed] [Google Scholar]
- 51.Fex M., Nitert M. D., Wierup N., Sundler F., Ling C., Mulder H. 2007. Enhanced mitochondrial metabolism may account for the adaptation to insulin resistance in islets from C57BL/6J mice fed a high-fat diet. Diabetologia. 50: 74–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.