Abstract
The “lipotoxic footprint” of cardiac maladaptation in diet-induced obesity is poorly defined. We investigated how manipulation of dietary lipid and carbohydrate influenced potential lipotoxic species in the failing heart. In Wistar rats, contractile dysfunction develops at 48 weeks on a high-fat/high-carbohydrate “Western” diet, but not on low-fat/high-carbohydrate or high-fat diets. Cardiac content of the lipotoxic candidates—diacylglycerol, ceramide, lipid peroxide, and long-chain acyl-CoA species—was measured at different time points by high-performance liquid chromatography and biochemical assays, as was lipogenic capacity in the heart and liver by qRT-PCR and radiometric assays. Changes in membranes fluidity were also monitored using fluorescence polarization. We report that Western feeding induced a 40% decrease in myocardial palmitoleoyl-CoA content and a similar decrease in the unsaturated-to-saturated fatty acid ratio. These changes were associated with impaired cardiac mitochondrial membrane fluidity. At the same time, hepatic lipogenic capacity was increased in animals fed Western diet (+270% fatty acid elongase activity compared with high-fat diet), while fatty acid desaturase activity decreased over time. Our findings suggest that dysregulation of lipogenesis is a significant component of heart failure in diet-induced obesity.
Keywords: heart failure, lipotoxicity, dyslipidemia, metabolic syndrome, fatty acid
Obesity, insulin resistance, and type 2 diabetes mellitus are recognized as risk factors for development of heart failure (1, 2). How do these metabolic disorders affect cardiac function? We have proposed that metabolic derangements associated with obesity and diabetes mellitus diminish the heart's capacity to convert chemical to mechanical energy, independently from hypertension and coronary artery disease (3, 4). An imbalance between myocardial substrate uptake and fatty acid oxidative capacity of the heart is thought to induce lipotoxicity, namely, an accumulation of lipid byproducts that give rise to noxious intermediates in the cardiomyocytes. However, the exact mechanism for lipotoxicity is still not known. A consistent finding is that myocardial triglycerides are increased in hearts of obese or type 2 diabetic patients (5, 6), which is associated with impaired left-ventricular diastolic function (7).
Genetically modified rodent models have been used extensively to investigate the pathophysiology of “lipotoxic cardiomyopathy” (8). Increased lipid influx in the heart (9, 10), decreased mobilization of triacylglycerol reserves (11), or increased fatty acid metabolism (12–14) all are associated with metabolic alterations that mimic those of diabetes, including hypertrophy of the heart and contractile dysfunction. These animal studies have led to the identification of the lipid molecules that are potential culprits for impaired cardiac function. Increased reliance of the heart on β-oxidation of fatty acids leads to increased reactive oxygen species generation, which promotes lipid peroxidation and alters mitochondrial function (15, 16). In addition, intracellular saturated acyl-CoA species, ceramide and diacylglycerol, are linked to changes in membrane composition, endoplasmic reticulum stress, altered lipid signaling, and ultimately, apoptosis (17, 18). These lipid species may also impair insulin signaling through increased serine phosphorylation of the insulin receptor and insulin receptor substrate 1 and/or reduced serine phosphorylation of PKB/Akt (19, 20).
However, there is controversy over whether myocardial lipids contribute to contractile dysfunction and heart failure in patients suffering from metabolic diseases. Data collected from rodent genetic models of cardiac lipotoxicity are limited as they exaggerate lipid disorders far beyond what is usually observed in patients suffering from obesity and/or diabetes. Only a few studies have investigated the effects of high-fat diets and dietary lipid composition on cardiac metabolism and function in rodents of a normal genetic background (21–26). However, most of these animal studies have been restricted to a short-term feeding protocol (usually 8–12 weeks) as a single end point (22–24, 26), and changes in the myocardial content of lipotoxic candidates were not systematically investigated. The potential role of long-chain acyl-CoA (LCACoA) esters, the obligate intermediates of fatty acid metabolism, is particularly disregarded. This is a critical point because these “active” forms of the long-chain fatty acids affect a large number of cellular systems and functions, including ion channels, ion pumps, translocators, enzymes, membrane fusion, and gene regulation (27, 28). Lastly, the toxicity of dietary fats has also been questioned because high-fat diets can either be neutral or even protective for the heart (22–24). Therefore, we wanted to know how dietary composition defines a “lipotoxic footprint” in the failing heart.
We have previously investigated the heart's capacity to adapt to different obesogenic diets by feeding Wistar rats a low-fat/high-carbohydrate diet, “Western” diet, or high-fat diet (10%, 45%, or 60% calories from fat, respectively) for up to 48 weeks. Western and high-fat diets led to a similar increase in body weight gain (+33%) when compared with the low-fat/high-carbohydrate diet. Even though ventricular weights did not differ among groups, we found that cardiac power significantly decreased (as measured in the isolated working heart) with long-term feeding of the Western diet (29). This measure of impaired contractile function was linked to a limited activation of cardiac fatty acid oxidation and fatty acid-mediated futile cycling (29). We propose that such a model of long-term, diet-induced cardiac failure may be useful to identify mechanisms of metabolic alterations relevant to humans.
The aim of the present follow-up study was to identify and to explain the cause(s) for changes in cardiac lipid composition that are associated with contractile dysfunction of the heart in our rodent model of diet-induced obesity. In particular, we decided to quantify the myocardial content of the following lipotoxic candidates throughout the feeding protocol: diacylglycerol, ceramide, lipid peroxide, and LCACoA species. Control animals fed either the high-fat diet or the low-fat/high-carbohydrate diet allowed discrimination between adaptive and maladaptive changes. Our results demonstrate that de novo synthesized fat, rather than dietary fat, may be responsible for cardiac maladaptation to diet-induced obesity.
MATERIALS AND METHODS
Animals
All the samples analyzed were recovered from the Wistar rats used in our previously published study (29). Briefly, 6-week-old male rats were housed under controlled conditions (23 ± 1°C; 12-h light/12-h dark cycle). The rats were acclimatized for 2 weeks under standard laboratory chow, containing 13.5% fat, 58% carbohydrate, and 28.5% protein as a percentage of the total energy (Laboratory Rodent Diet 5001, Labdiet®, Richmond, IN). Rats were then fed ad libitum a low-fat/high-carbohydrate diet (10% fat, 70% carbohydrate, 20% protein), Western diet (45% fat, 35% carbohydrate, 20% protein), or high-fat diet (60% fat, 20% carbohydrate, 20% protein) (Research Diets, New Brunswick, NJ; # D12450B, D12451, and D12492). The macronutrient compositions and the fatty acid profiles of the diets are given in Table 1 and Table 2, respectively. Rats were sacrificed after 1 day and 1 week (acute term; AT); 4 and 8 weeks (short term; ST); 16 and 24 weeks (intermediate term; IT); or 32 and 48 weeks (long term; LT) on the feeding protocols. Plasma was recovered at the time of sacrifice (6 ± 1.5 h into the dark phase). Hearts and livers were removed, rinsed free of blood, and freeze clamped in liquid nitrogen. All procedures were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston.
TABLE 1.
Macronutrient composition of the diets
| Contribution to Total Energy Content of the Diet (% of total) |
|||||||
|---|---|---|---|---|---|---|---|
| Fat |
Carbohydrate |
Protein |
|||||
| Diet | Soybean Oil | Lard | Corn Starch | Maltodextrin | Sucrose | Casein | Total |
| HCD | 6 | 4 | 31 | 4 | 35 | 20 | 100 |
| WD | 6 | 39 | 8 | 10 | 17 | 20 | 100 |
| HFD | 6 | 54 | — | 13 | 7 | 20 | 100 |
Diets are matched for vitamin and mineral content. Abbreviations: HCD, low-fat/high-carbohydrate diet; HFD, high-fat diet; WD, Western diet.
TABLE 2.
Fatty acid profile of the diets
| Fatty Acid Content (mmol/4057 kcal) | HCD | WD | HFD |
|---|---|---|---|
| 14:0, Myristic | 0.88 | 7.01 | 9.63 |
| 14:1, Myristoleic | 0.44 | 3.98 | 5.30 |
| 16:0, Palmitic | 28.08 | 168.47 | 228.92 |
| 16:1, Palmitoleic | 3.14 | 26.33 | 36.55 |
| 18:0, Stearic | 12.65 | 86.47 | 117.76 |
| 18:1, Oleic | 50.63 | 279.68 | 378.11 |
| 18:2, Linoleic | 53.84 | 101.98 | 122.66 |
| 18:3, Linolenic | 7.90 | 13.29 | 15.80 |
| 20:4, Arachidonic | 0.98 | 9.85 | 13.79 |
| Molar Ratio | |||
| 16:1/16:0a | 0.11 | 0.16 | 0.16 |
| 18:1/18:0b | 4.00 | 3.23 | 3.21 |
Abbreviations: HCD, low-fat high/carbohydrate diet; HFD, high-fat diet; WD, Western diet.
The molar ratio of palmitoleate to palmitate.
The molar ratio of oleate to stearate.
Analysis of plasma parameters
Fed insulin and leptin levels were determined with rat-specific radioimmunossay kits (Millipore, Billerica, MA). Within and between assay variation was 3.76% and 10.55% for insulin, and 3.54% and 5.07% for leptin, respectively. Plasma triglycerides were quantified with L-Type TG H assay (Wako Chemicals, Richmond, VA). Plasma total cholesterol levels were determined with a cholesterol assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. Fed blood glucose levels, fed plasma nonesterified fatty acid levels, and body weight curves have been reported previously (29).
Cardiac lipid extraction and analysis of lipid byproducts
Total lipids were extracted from heart tissue according to the method of Bligh and Dyer (30). The extraction was repeated three times to ensure the complete recovery of lipids. Myocardial triacylglycerol content was quantified using the L-Type TG H assay (Wako Chemicals). Cholesterol content of tissue lipid extracts was determined with a cholesterol assay kit (Cayman Chemical).
High-performance liquid chromatography for quantification of ceramide and diacylglycerol
Ceramide and diacylglycerol levels were determined by HPLC according to the method of Previati et al. (31) with slight modifications. Chromatography was carried out on a LC-10ATVP instrument (Shimadzu Scientific Instruments, Columbia, MD) equipped with a Econosphere CN 5U, 250 × 4.6 mm column (Grace, Deerfield, IL) and a Opti-Guard® 1 mm guard column (Optimize Technologies, Oregon City, OR). UV absorption was determined at 230 nm. The mobile phase was delivered at the flow rate of 1 ml/min. The solvents used for ceramide assay were (A) hexane and (B) isopropanol. After equilibration of the column with 100% A, 0% B, the separation was accomplished with a linear gradient to 6 min to 95% A, 5% B, followed by isocratic conditions to 16 min. Peak area was used to estimate the amount of material based on a standard curve realized with authentic bovine brain ceramides. Within and between assay variation was 1.89% and 4.80%, respectively. The solvents used for diacylglycerol assay were (A) hexane and (B) 10% isopropanol in hexane. The separation was achieved by 2 min of isocratic run with 97.5% A, 2.5% B, a linear gradient to 8 min to 50% A, 50% B, followed by isocratic conditions to 12 min. Peak area was used for quantification based on a standard curve realized with 1,2-Dioleoyl-rac-glycerol. Within and between assay variation was 3.19% and 3.90%, respectively.
Quantitative and qualitative analyses of cardiac long-chain acyl-CoA species and of plasma fatty acid species
LCACoAs were extracted and quantified according to the method developed by Mangino et al. (32). Separation was carried out using an Ultrasphere C18 ODS, 250 × 4.6 mm column (Beckman Coulter, Fullerton, CA). UV absorption was determined at 254 nm. The following solvents were delivered at a flow rate of 1 ml/min: (A) acetonitrile and (B) 10 mM KH2PO4, pH 5.3. The program was started with 24% A, 76% B with linear gradient to 4 min to 34% A, 66% B; a linear gradient to 14 min to 45% A, 55% B; a linear gradient to 23 min to 57% A, 43% B; and a isocratic run to 70% A, 30% B. After 25 min, the system was reequilibrated to the initial time zero solvent composition for 10 min before injecting another sample. The ratio of the peak areas of LCACoA species to the area obtained from the internal standard n-heptadecanoyl-CoA was used for quantification based on curves realized with authentic standards (All from Sigma-Aldrich, St. Louis, MO). Total plasma fatty acids were analyzed by gas chromatography using their methyl ester derivatives (FAME) at the Lipidomic Platform facility of Toulouse, INSERM IFR 30, Toulouse, France.
Lipid peroxidation assay
Lipid peroxidation was assessed using the thiobarbituric acid reactive substances (TBARS) assay (33). Briefly, samples extracted in presence of 100 µg/ml butylated hydroxytoluene were incubated in TBARS reagent (0.4% 2-thiobarbituric acid, 0.5% SDS, 10% acetic acid, pH 3.5) for 60 min at 95°C. The pink chromogene was then purified with chloroform extraction, and the absorbance was read at 532 nm. The amount of TBARS was quantified using a standard curve of malondialdehyde.
Glycogen assay
Glycogen was extracted from ethanol precipitation of heart tissue digested in hot 30% potassium hydroxide and measured as glucose after digestion with amyloglucosidase (34).
RNA extraction and transcript analysis
Total RNA was extracted with TRI Reagent® (Molecular Research Center, Cincinnati, OH) and purified on PureLinkTM RNA columns (Invitrogen, Carlsbad, CA). Samples were treated for genomic DNA contamination with DNA-freeTM (Applied Biosystems/Ambion, Austin, TX). RNA concentration was measured with a NanoDrop 1000 apparatus. Absolute quantification of transcripts was based on known amounts of synthetic DNA standard (Integrated DNA Technologies, Coralville, IA). The primers and probes sequences are given in supplementary Table I.
Preparation of crude membrane and inner mitochondrial membrane fractions
Heart tissue (∼50 mg) was homogenized in 10 vols of ice-cold 23% sucrose (w/w), 20 mM Hepes, and 1 mM EDTA buffer (pH 7.7) supplemented with proteases and phosphatases inhibitors. Mitochondria and the microsomal fraction were isolated by differential centrifugation performed at 4°C. Briefly, the tissue homogenate was centrifuged twice at 1,000 g for 15 min, and the supernatant fractions were further centrifuged at 6,000 g for 15 min. The mitochondria pellet was recovered, and the supernatant was centrifuged at 100,000 g for 25 min in a Beckman OptimaTM TL micro-ultracentrifuge. The microsome pellet was washed with 23% sucrose (w/w), 20 mM Hepes, and 1 mM EDTA buffer (pH 7.7) and then resuspended in 400 µl of the same buffer. Mitochondria were deprived of their outer membrane by a 15 min hypotonic treatment in 10 mM sucrose, 5 mM Tris-HCl (pH 7.2) (35). After centrifugation at 9,000 g for 20 min, the pellet was suspended in 0.3 M sucrose, 5 mM Tris-HCl (pH 7.2). The protein concentration was determined with the Bradford method. The samples were snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Enzyme activities
Specific delta-9 desaturase activity was determined from the production of 3H2O using [9,10-3H]stearoyl-CoA (Perkin-Elmer, Waltham, MA) (36, 37). After 20 min incubation at 25°C, the samples were loaded on a 2 ml column of AG® 1-X8 resin 100–200 mesh, hydroxide form (Bio-Rad, Hercules, CA). The 3H2O was eluted in a scintillation vial with 3.5 ml of double-distilled H2O mixed with 10 ml of Ultima GoldTM scintillation cocktail (Perkin-Elmer), and 3H radioactivity was quantified by β-scintillation counting. In vitro fatty acid elongation assay was performed using palmitoyl-CoA as a substrate according to the procedures described previously (38, 39).
Fluorescence polarization
Membrane fluidity was assessed by fluorescence polarization using the lipid fluorophore 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine (NBD C6-HPC; Invitrogen). Crude membrane and inner mitochondrial membrane aliquots diluted at 50 μg protein/ml in 50 mM Tris-HCl buffer (pH 7.6) were incubated for 60 min at 37°C with 5 μg/ml NBD C6-HPC (final concentration = 6.5 μM). Next, the membranes were washed with 3 vols of 50 mM Tris-HCl buffer. Steady-state fluorescence polarization studies were performed at 25°C using a fluorescence spectrophotometer (POLARstar OPTIMA, BMG Labtech Inc., Durham, NC) at 485/520 (Ex/Em) wavelengths. The degree of fluorescence polarization (in mP units) was calculated from the equation P = (Ivv−kIvh)/(Ivv+kIvh), where P = polarization, I is the fluorescence intensity, the first and second subscripts refer to the plane of polarization of the excitation and emission beams, respectively (v = vertical, h = horizontal). The factor k = Ihv/Ihh compensates for slightly unequal horizontal and vertical excitation intensities. We corrected for intrinsic fluorescence and light-scattering from the membrane suspension by subtracting the values obtained with unlabeled samples.
Data analysis
Data are shown as means ± SE. Analyses were performed by grouping similar time points into a “term” of feeding. The groups were: 1 day and 1 week (acute term), 4 and 8 weeks (short term), 16 and 24 weeks (intermediate term), and 32 and 48 weeks (long term). Pairwise comparisons were performed using Student's t-test. Multiple pairwise comparisons were performed using one-way or two-way ANOVA with post hoc Student-Newman-Keuls's test. Data were analyzed with SigmaStat v3.0.1 (SPSS, Chicago, IL). Significance was considered P < 0.05.
RESULTS
Plasma parameters
Leptin levels rose quickly with the three diets during the first 4 months of the feeding protocol and started to reach a plateau in the intermediate term (Table 3). The intermediate term is also the only time point where a slight, but significantly higher, increase in leptin levels occurred with high-fat and Western diets when compared with low-fat/high-carbohydrate diet (Table 3). Leptin levels were highly correlated to body weight (r2 = 0.46, P < 0.0001 for low-fat/high-carbohydrate diet; r2 = 0.72, P < 0.0001 for high-fat diet; r2 = 0.71, P < 0.0001 for Western diet) and to mesenteric fat mass (r2 = 0.46, P < 0.0001 for low-fat/high-carbohydrate diet; r2 = 0.66, P < 0.0001 for high-fat diet; r2 = 0.68, P < 0.0001 for Western diet) throughout the study. Insulin levels were also increased with all three diets and at all time points investigated. However, the increase was gradual between the diets, with the high-fat diet inducing the lowest increase and low-fat/high-carbohydrate diet inducing the highest raise (Table 3). Triacylglycerol levels followed insulin levels: Plasma triacylglycerol was higher with the low-fat/high-carbohydrate diet, and the Western diet induced an intermediate raise that became significantly higher than high-fat diet in the long term (Table 3). The increase in total plasma cholesterol was also greater in rats fed the low-fat/high-carbohydrate diet (Table 3).
TABLE 3.
Blood parameters
| Baseline | Diet | Acute Term | Short Term | Intermediate Term | Long Term | |
|---|---|---|---|---|---|---|
| Leptin (ng/ml) | 3.78 ± 0.50 | HFD | 17.74 ± 1.03 | 26.38 ± 0.73 | 34.16 ± 0.82b | 34.79 ± 0.91 |
| WD | 14.87 ± 1.04 | 26.25 ± 0.98 | 32.97 ± 0.73a | 36.14 ± 0.77 | ||
| HCD | 14.32 ± 1.37 | 26.80 ± 1.04 | 30.74 ± 0.75 | 33.78 ± 1.29 | ||
| Insulin (ng/ml) | 0.23 ± 0.02 | HFD | 1.60 ± 0.25 | 1.94 ± 0.27 | 1.72 ± 0.40 | 1.74 ± 0.29 |
| WD | 1.86 ± 0.31 | 2.57 ± 0.26 | 2.02 ± 0.24 | 2.46 ± 0.40 | ||
| HCD | 3.37 ± 0.48dg | 5.38 ± 0.67eh | 3.01 ± 0.31cf | 3.34 ± 0.37g | ||
| TG (mg/dl) | 82 ± 12 | HFD | 143 ± 11 | 130 ± 13 | 133 ± 11 | 131 ± 18 |
| WD | 128 ± 7 | 158 ± 15 | 150 ± 13 | 195 ± 31f | ||
| HCD | 202 ± 21cf | 212 ± 24cg | 168 ± 12 | 234 ± 27h | ||
| CHO (mmol/l) | HFD | 1.57 ± 0.14 | 1.15 ± 0.08 | 1.55 ± 0.13 | 1.93 ± 0.17 | |
| 1.28 ± 0.08 | WD | 1.45 ± 0.13 | 1.30 ± 0.34 | 1.68 ± 0.09 | 2.34 ± 0.18 | |
| HCD | 1.45 ± 0.09 | 1.57 ± 0.14 | 1.87 ± 0.13 | 3.10 ± 0.43f |
Plasma parameters were assayed for 8-week-old rats fed standard rodent chow for 2 weeks (Baseline). The same parameters were assayed in animals fed either low-fat/high-carbohydrate diet (HCD), high-fat diet (HFD), or Western diet (WD) for 1 day or 1 week (acute term), 4 or 8 weeks (short term), 16 or 24 weeks (intermediate term), and 32 or 48 weeks (long term). Data are means ± SE of n = 14–18 animals per group per time point (except baseline; n = 6).
Abbreviations: CHO, cholesterol; TG, triacylglycerol.
P < 0.05 vs. HCD at same age.
P < 0.01 vs. HCD at same age.
P < 0.05 vs. Western diet at same age.
P < 0.01 vs. Western diet at same age.
P < 0.001 vs. Western diet at same age.
P < 0.05 vs. high-fat diet at same age.
P < 0.01 vs. high-fat diet at same age.
P < 0.001 vs. high-fat diet at same age.
Changes in cardiac lipid content
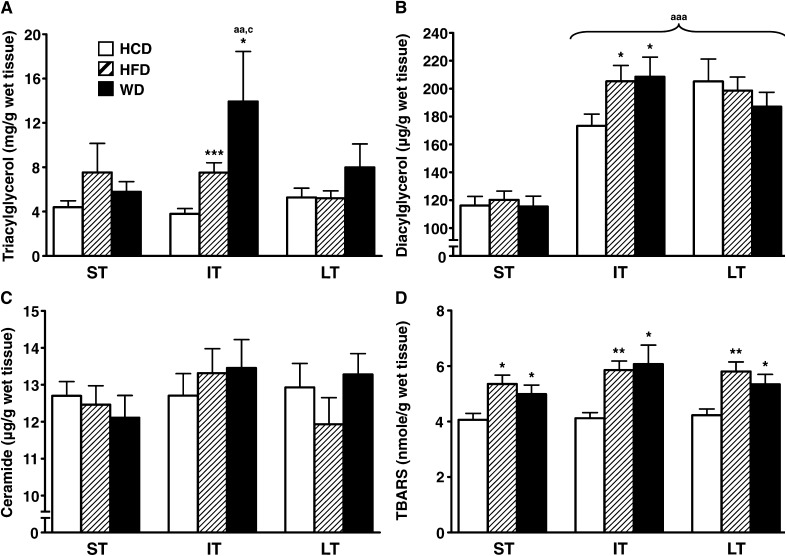
Cardiac triacylglycerol content was transiently higher with both high-fat and Western feeding after 16–24 weeks on the diet compared with animals on the low-fat/high-carbohydrate diet (Fig. 1A). However, there was no significant difference between the high-fat and Western diets. Cardiac diacylglycerol content was also higher with the high-fat and Western diets in the intermediate term (Fig. 1B). Ceramide content was unchanged among the three diets from the short- to long-term feeding periods (Fig. 1C). Lipid peroxidation was increased with the high-fat and Western diets throughout the feeding protocol (Fig. 1D). Cardiac cholesterol and glycogen contents remained unaffected by all three diets over time (data not shown).
Fig. 1.
High-fat diet and Western diet induce similar changes in myocardial lipid byproducts content. Myocardial triacylglycerol (A), diacylglycerol (B), ceramide (C), and TBARS (D), the latter taken as an index of lipid peroxidation, were quantified in the heart of male Wistar rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for short term (ST), intermediate term (IT), or long term (LT). Data are means ± SE of n = 13–18 animals per group per time point. One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively. * = vs. HCD at same age; a = vs. short term; b = vs. intermediate term; c = vs. long term. TBARS, thiobarbituric acid reactive substances.
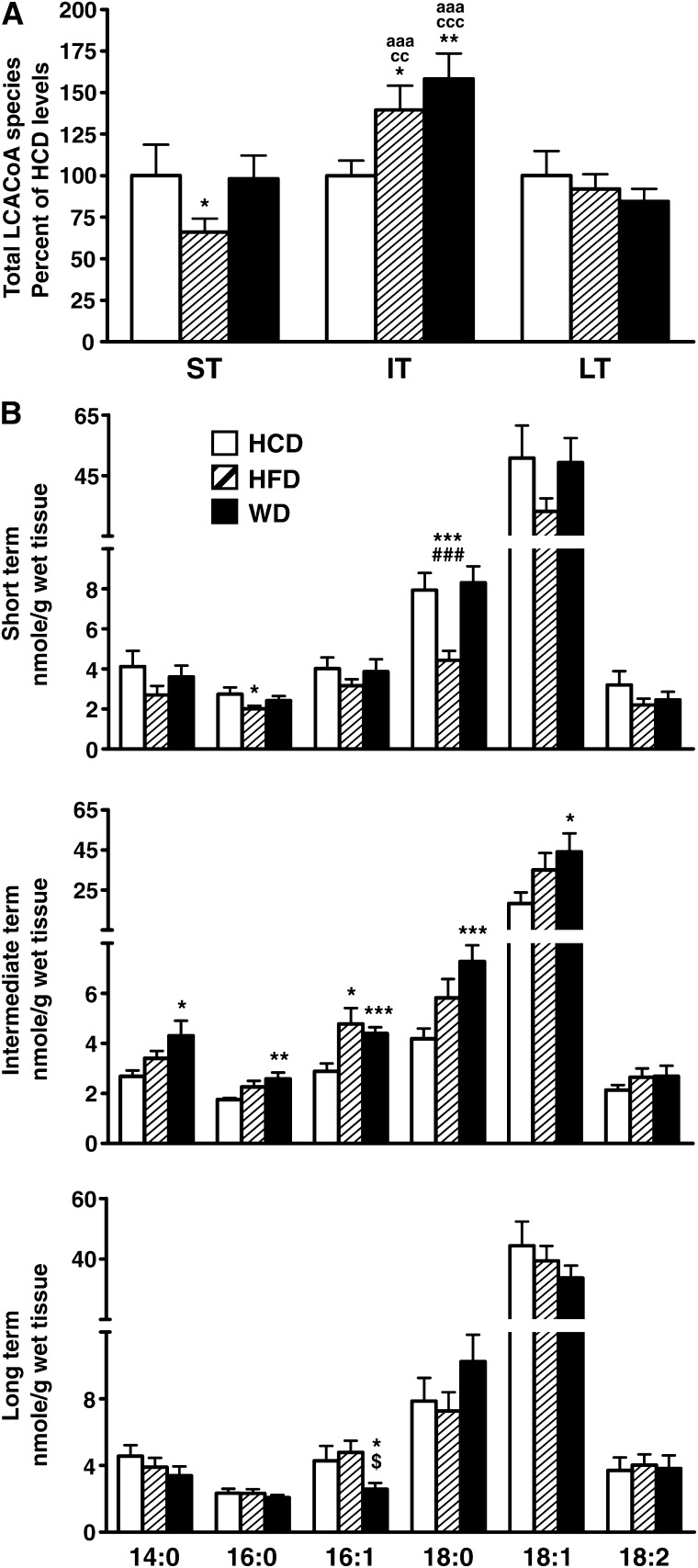
Changes in cardiac long-chain acyl-CoA content
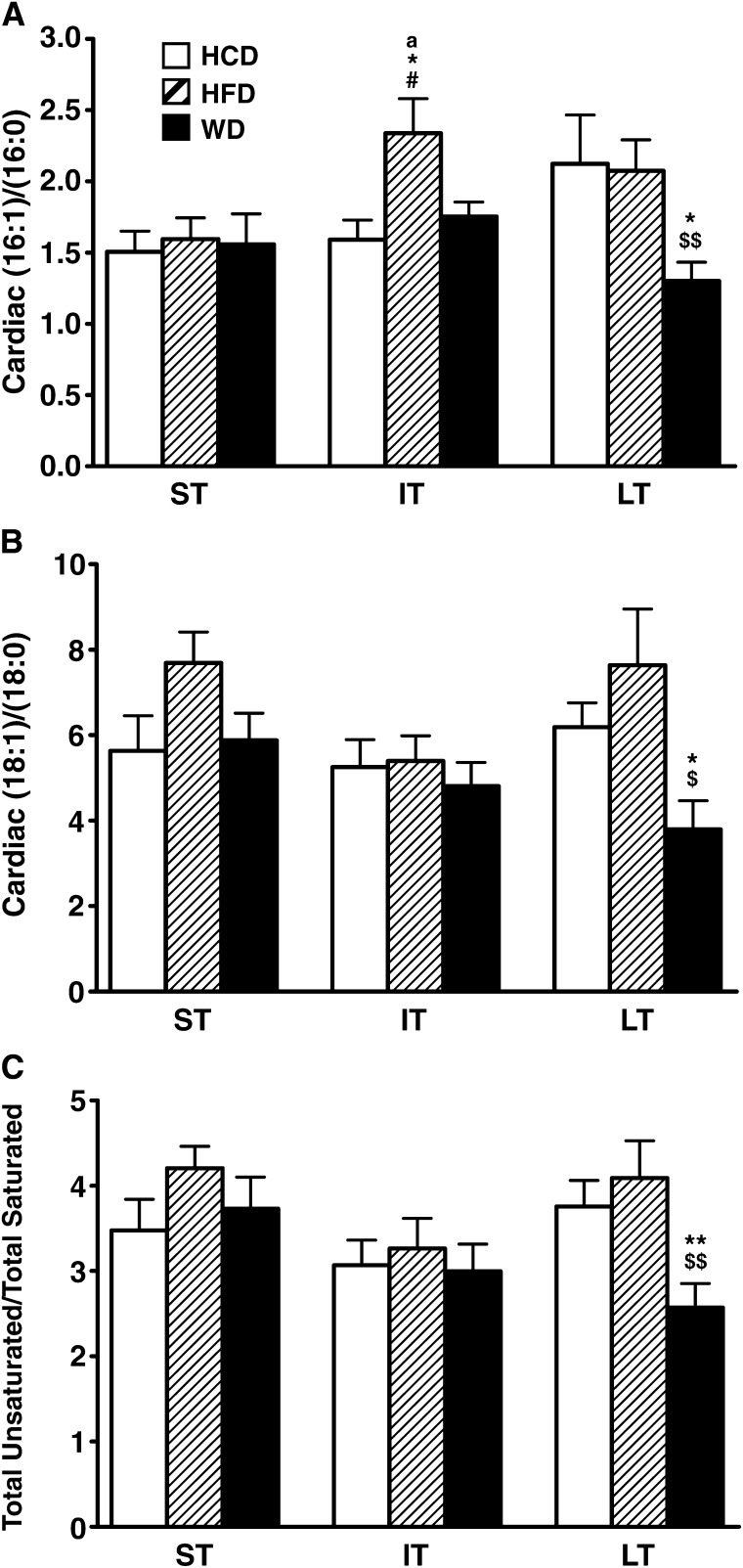
Total LCACoA cardiac content was decreased with the high-fat diet in the short term (Fig. 2A), which is mainly explained by the decrease of the most abundant, 18-carbon chain length species stearoyl-CoA and oleoyl-CoA (Fig. 2B). Conversely, LCACoAs accumulated with both the high-fat and Western diets in the intermediate term, therefore mirroring the transient increase observed for the glycerolipid species triacylglycerol and diacylglycerol (Fig. 2A). This was due to an increase of all the saturated and monounsaturated species (Fig. 2B). As observed for triacylglycerol and diacylglycerol, total LCACoA content normalized among groups on the long-term feeding (Fig. 2A). However, a closer analysis of LCACoA species revealed striking variations for the Western diet when compared with the other diets. Stearoyl-CoA content tended to increase at the expense of oleoyl-CoA, and there was a 40% drop of the monounsaturated species palmitoleoyl-CoA (Fig. 2B). Despite significant quantitative changes, cardiac unsaturated-to-saturated LCACoA ratios were conserved among the three diets in the short term and intermediate term (Fig. 3). This was the case not only for the monounsaturated-to-saturated ratios of 16-carbon and 18-carbon chain length species but also when considering all the LCACoA species together. However, all ratios were dramatically decreased (by 32–50%) on the Western diet in the long term (Fig. 3), thus confirming the loss of monounsaturated species and a subsequent enrichment in saturated fat, which could potentially contribute to cardiac dysfunction in these animals.
Fig. 2.
Myocardial long-chain acyl-CoA profile is more profoundly altered by Western diet. A: Total myocardial long-chain acyl-CoA content was determined for male Wistar rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for short term (ST), intermediate term (IT), or long term (LT). Results are expressed in percentage of long-chain acyl-CoA levels determined for animals fed with low-fat/high-carbohydrate diet. B: Myocardial levels of myristoyl-CoA (14:0), palmitoyl-CoA (16:0), palmitoleoyl-CoA (16:1), stearoyl-CoA (18:0), oleoyl-CoA (18:1), and linoleoyl-CoA (18:2) were also quantified separately in the same animals. Data are means ± SE of n = 13–17 animals per group per time point. One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively. * = vs. HCD at same age; # = vs. WD at same age; $ = vs. HFD at same age; a = vs. short term; c = vs. long term.
Fig. 3.
Western feeding induces a dramatic decrease in myocardial unsaturated-to-saturated fatty acid ratio. Myocardial palmitoleoyl-CoA–to–palmitoyl-CoA ratio (16:1)/(16:0) (A), oleoyl-CoA–to–stearoyl-CoA ratio (18:1)/(18:0) (B), and total unsaturated-to-saturated long-chain acyl-CoA species ratio (C) are given for rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for short term (ST), intermediate term (IT), or long term (LT). Data are means ± SE of n = 13–17 animals per group per time point. * = P < 0.05; ** = P < 0.01 vs. HCD at same age; # = P < 0.05 vs. WD at same age; $ = P < 0.05; $$ = P < 0.01 vs. HFD at same age; a = P < 0.05 vs. short term.
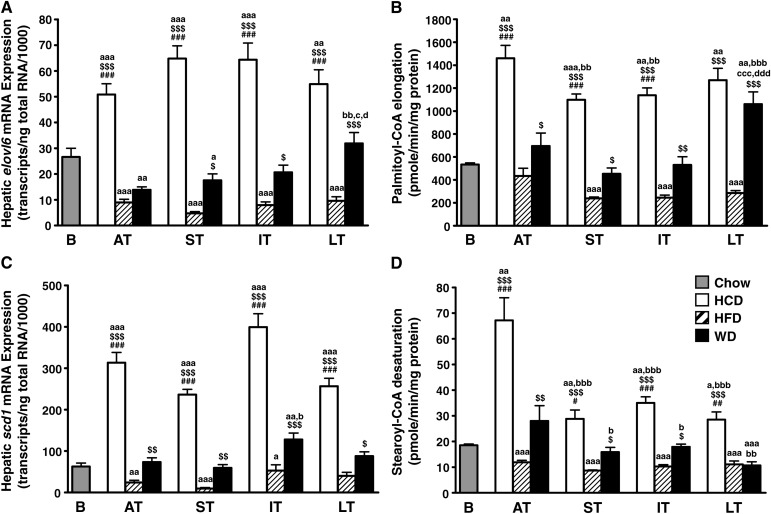
Changes in hepatic gene expression and activity of lipogenic factors and enzymes
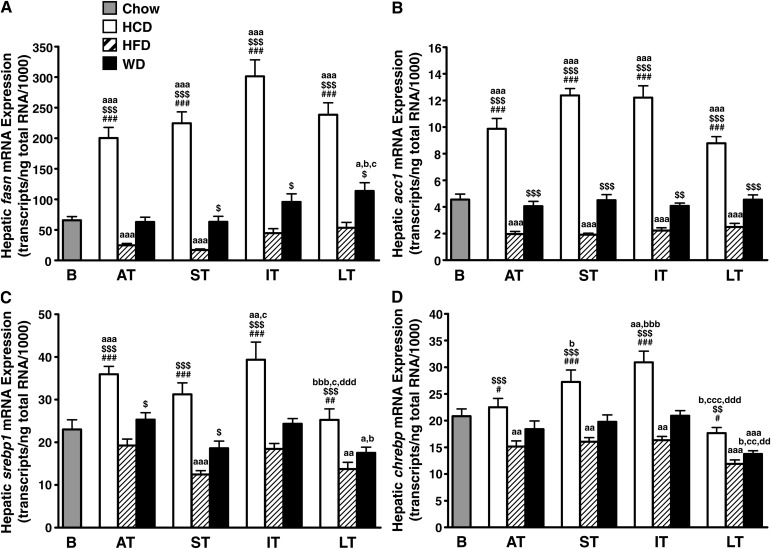
The fact that neither the high-fat nor low-fat/high-carbohydrate diet modified the cardiac fat ratios implies not only an excess in dietary fat but also an excess in dietary sugars. Therefore, we decided to investigate for a possible involvement of hepatic lipogenesis. The low-fat/high-carbohydrate diet induced a massive and sustained upregulation of genes that encode for enzymes involved in de novo lipogenesis, including fatty acid synthase (fasn; Fig. 4A), acetyl-CoA carboxylase 1 (acc1; Fig. 4B), the elongase that catalyzes the conversion of palmitate to stearate (elovl6; Fig. 5A), and stearoyl-CoA desaturase 1 (scd1; Fig. 5C). Conversely, the high-fat diet induced a sustained repression of the same genes. In addition, the low-fat/high-carbohydrate diet upregulated, while the high-fat diet downregulated the expression of the key lipogenic transcription factors sterol-regulatory element binding protein 1 (srebp1; Fig. 4C) and carbohydrate-response element binding protein (chrebp; Fig. 4D). The effect of Western diet varied, depending on the gene and the time point considered. Hepatic acc1 mRNA expression remained stable and close to baseline levels from the acute term to the long term (Fig. 4B). Fatty acid synthase mRNA levels were also unchanged in the acute and short terms but then started to increase to be significantly higher in the long term (Fig. 4A). elovl6 expression was downregulated in the acute and short terms, but thereafter became upregulated in the long term (Fig. 5A). Surprisingly the expression of the delta-9 desaturase SCD1, which catalyzes the monounsaturation of palmitate and stearate, was not upregulated in the long term (Fig. 5C). The changes in transcript levels matched the activity of lipogenic enzymes. As expected, acyl-CoA elongation and delta-9 desaturation processes were more active in the liver of animals fed the low-fat/high-carbohydrate diet (Fig. 5B, D). Hepatic lipogenic capacity was also high in animals fed the Western diet, with a fatty acid elongase activity increased by 270% in the long term compared with the high-fat diet (Fig. 5B). Conversely, delta-9 desaturase activity dropped to the high-fat diet levels in the long term (Fig. 5D).
Fig. 4.
Hepatic de novo lipogenesis is regulated by diet composition at the transcriptional level. Transcript levels of fatty acid synthase (fasn; A), acetyl-CoA carboxylase 1 (acc1; B), sterol regulatory element-binding proteins 1 (srebp1; C), and carbohydrate response element binding protein (chrebp; D) were quantified by real-time PCR in the liver of Wistar rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for acute term (AT), short term (ST), intermediate term (IT), or long term (LT). Baseline mRNA levels (B; gray bar) were determined from 8-week-old rats fed standard rodent chow for 2 weeks before the beginning of the feeding protocol. Data are means ± SE of n = 13–17 animals per group per time point (except baseline; n = 7). One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively. # = vs. WD at same age; $ = vs. HFD at same age; a = vs. baseline; b = vs. acute term; c = vs. short term; d = vs. intermediate term.
Fig. 5.
The activity of lipogenic enzymes is differentially regulated with long-term Western feeding. Transcript levels of the long chain fatty acid elongase ELOVL family member 6 (elovl6; A) and stearoyl-CoA desaturase 1 (scd1; C) were quantified by real-time PCR in the liver of Wistar rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for acute term (AT), short term (ST), intermediate term (IT), or long term (LT). Baseline mRNA levels (B; gray bar) were determined from 8-week-old rats fed standard rodent chow for 2 weeks before the beginning of the feeding protocol. Data are means ± SE of n = 13–17 animals per group per time point (except baseline; n = 7). To confirm the variations observed at the mRNA level, hepatic acyl-CoA elongase (B) and delta-9 desaturase (D) activities were also assessed over time. Data are means ± SE of n = 6 animals per group per time point. One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively. # = vs. WD at same age; $ = vs. HFD at same age; a = vs. baseline; b = vs. acute term; c = vs. short term; d = vs. intermediate term.
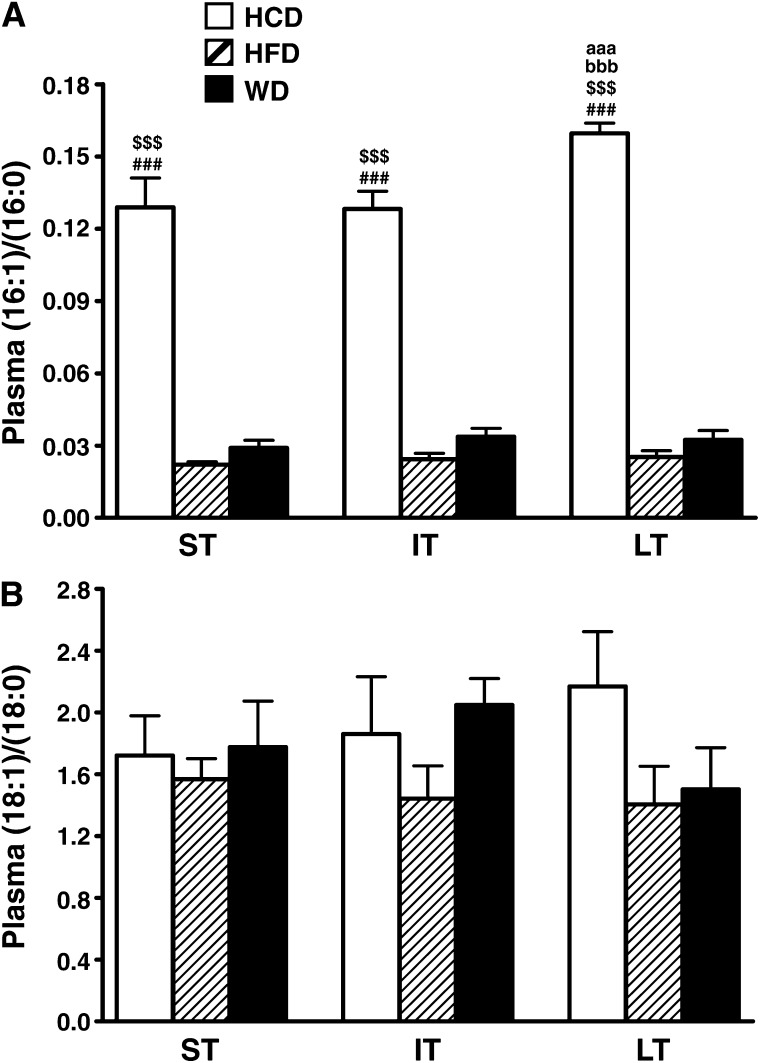
Changes in plasma fatty acid composition
Plasma palmitate levels were higher for rats fed the low-fat/high-carbohydrate diet (see supplementary Fig. I). In accordance with enhanced hepatic delta-9 desaturase activity, there was also a 6 to 14-fold increase in palmitoleate levels associated to low-fat/high-carbohydrate diet consumption at all time points examined (see supplementary Fig. I). This led to a much higher palmitoleate-to-palmitate ratio compared with animals fed either high-fat or Western diets (Fig. 6A). Similarly, the oleate-to-stearate ratio tended to increase over time with low-fat/high-carbohydrate diet. Interestingly, while the oleate-to-stearate ratio rose with Western diet in the intermediate term, the increase was not sustained in the long term, and the ratio dropped back to high-fat diet levels (Fig. 6B).
Fig. 6.
Plasma monounsaturated-to-saturated fatty acid ratios are differentially altered by the diets. Plasma palmitoleate-to-palmitate (16:1)/(16:0) (A) and oleate-to-stearate (18:1)/(18:0) (B) ratios are given for rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for short term (ST), intermediate term (IT), or long term (LT). Data are means ± SE of n = 9 animals per group per time point. ### = P < 0.001 vs. WD at same age; $$$ = P < 0.001 vs. HFD at same age; aaa = P < 0.001 vs. short term; bbb = P < 0.001 vs. intermediate term.
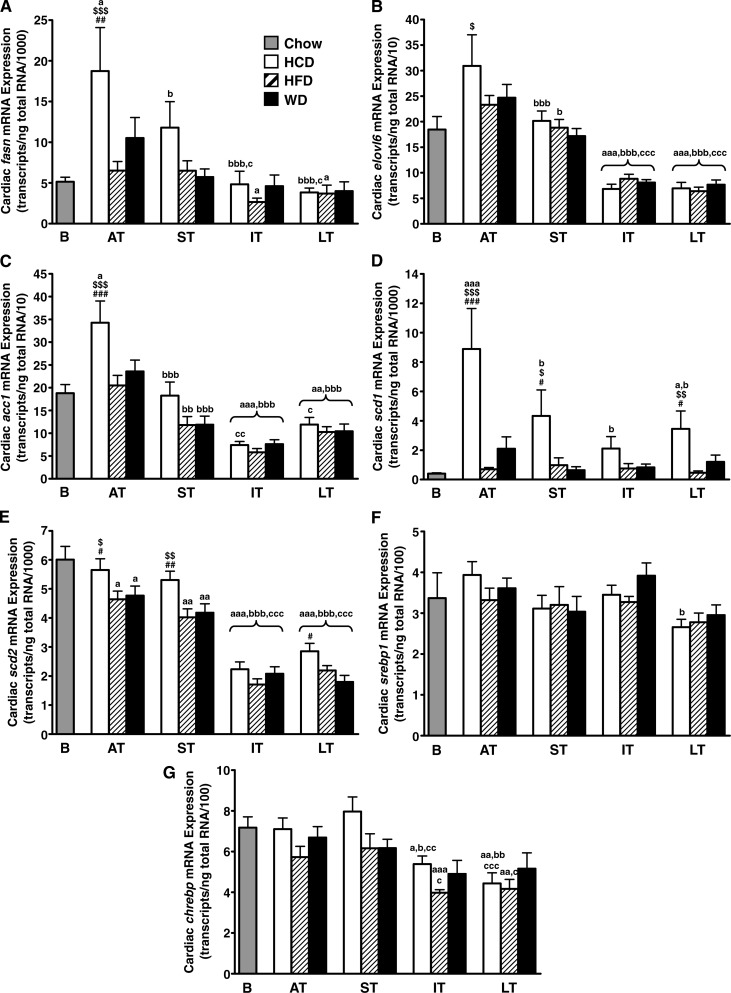
Changes of delta-9 desaturase expression in the heart
The expression of fasn, acc1 and elovl6 was increased with low-fat/high-carbohydrate diet only in the early phase (Fig. 7A–C), which precludes any major role for cardiac de novo fatty acid synthesis in the long term. In addition, the expression of srebp1 or chrebp did not significantly change among the three diets (Fig. 7F, G). However, scd1 mRNA levels were significantly increased from acute term to long term with low-fat/high-carbohydrate diet (Fig. 7D). scd2 mRNA levels decreased steadily over time with the three diets, still they remain higher with low-fat/high-carbohydrate diet when compared with high-fat diet or Western diet (Fig. 7E).
Fig. 7.
Low-fat/high-carbohydrate diet, but not Western diet, upregulates cardiac delta-9 desaturase gene expression. Transcript levels of fatty acid synthase (fasn; A), the long chain fatty acid elongase ELOVL family member 6 (elovl6; B), acetyl-CoA carboxylase 1 (acc1; C), stearoyl-CoA desaturase isoforms 1 and 2 (scd1 and scd2; D and E, respectively), sterol regulatory element-binding proteins 1 (srebp1; F), and carbohydrate response element binding protein (chrebp; G) were quantified by real-time PCR in the heart of Wistar rats fed either low-fat/high-carbohydrate diet (HCD; open bars), high-fat diet (HFD; hatched bars), or Western diet (WD; black bars) for acute term (AT), short term (ST), intermediate term (IT), or long term (LT). Baseline mRNA levels (B; gray bar) were determined from 8-week-old rats fed standard rodent chow for 2 weeks before the beginning of the feeding protocol. Data are means ± SE of n = 13–18 animals per group per time point (except baseline; n = 6). One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively. # = vs. Western diet at same age; $ = vs. high-fat diet at same age; a = vs. baseline; b = vs. acute term; c = vs. short term.
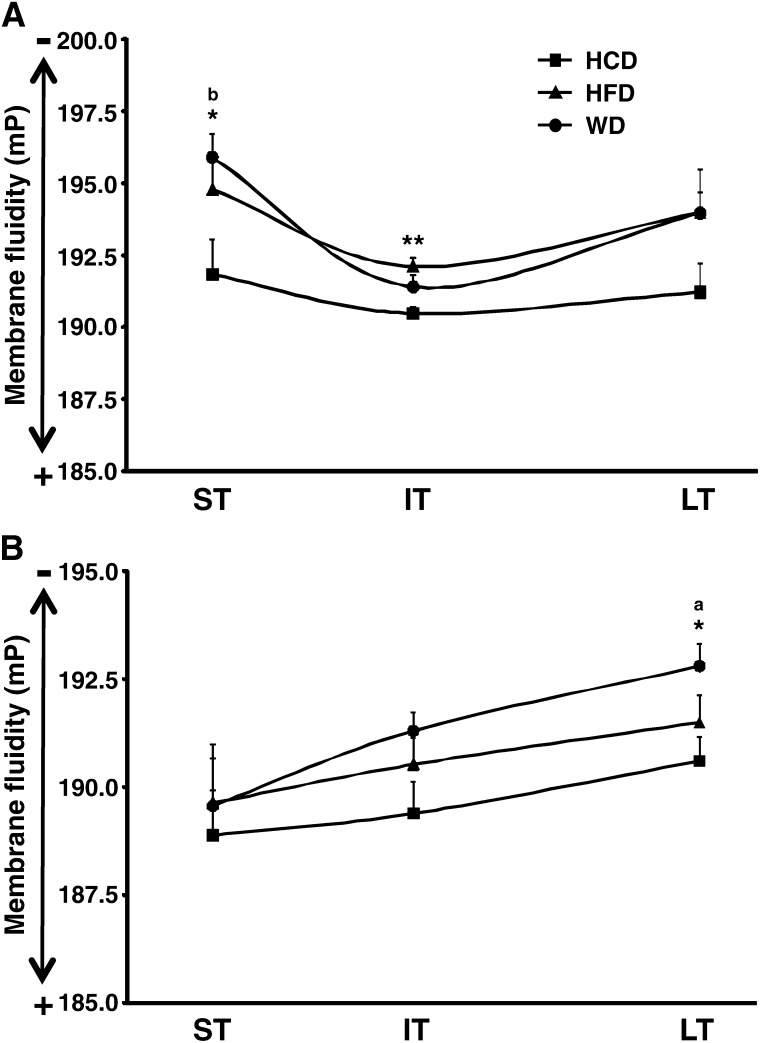
Impact of diets on membrane fluidity in the heart
Crude membrane fraction fluidity was decreased similarly from short-term to long-term feeding with the high-fat and Western diets compared with the low-fat/high-carbohydrate diet (Fig. 8A). The fluidity of the inner mitochondrial membrane was significantly impaired only with the Western diet after long-term feeding (Fig. 8B). It is noteworthy that mitochondrial membrane fluidity decreased concomitantly to the alteration in the cardiac LCACoA profile and the development of cardiac dysfunction (29).
Fig. 8.
High-fat diet and Western diet induce time-dependent, organelle-specific impairment in membrane fluidity. Microsomal (A) and inner mitochondrial (B) membranes fluidity were assessed for male Wistar rats fed either low-fat high/carbohydrate diet (HCD; black squares), high-fat diet (HFD; black triangles), or Western diet (WD; black circles) for short term (ST), intermediate term (IT), or long term (LT). Results are expressed in millipolarization (mP) units. An increase in mP units means a decrease in membrane fluidity. Data are means ± SE of n = 6 (A) or n = 5 to 6 (B) animals per group per time point. * = P < 0.05; **= P < 0.01 vs. low-fat/high-carbohydrate diet at same age; a = P < 0.05 vs. short term; b = P < 0.05 vs. intermediate term.
DISCUSSION
The main findings are that the Western diet induces robust changes in the fatty acid profile of failing rat hearts, including a loss in monounsaturates and a subsequent enrichment in saturated species. The changes in cardiac LCACoA composition likely result from abnormally high hepatic de novo lipogenesis coupled to insufficient delta-9 desaturase activity. Among all the commonly described lipotoxic molecules, we identified LCACoA species as the main potential contributor for contractile dysfunction in our rat models of diet-induced obesity. These changes in cardiac lipid composition were associated with a decrease in inner mitochondrial membrane fluidity.
Obese and diabetic individuals frequently present with impaired cardiac function that might be due to toxic effects of lipid metabolites in the myocardium (6, 7). Potential lipotoxic molecules were quantified in the heart of our Western diet–fed rats. While glycerolipid and lipid peroxide contents increased compared with animals fed the low-fat/high-carbohydrate diet, the same trend was observed for rats fed the high-fat diet. Moreover, cardiac ceramide content, which is increased in several genetic models of lipotoxic cardiomyopathy (12, 13, 40), was unchanged in all groups. These observations rule out a putative involvement of these lipid species in the development of cardiac dysfunction in our model. Unlike mice with genetically increased cardiac lipid uptake (40), cardiac glycogen levels were unaltered despite the dramatic switches in cardiac substrate utilization (29). It is also of note that most of the changes in lipid metabolism induced by the high-fat and Western diets in the intermediate term, namely the myocardial accumulation of triacylglycerol, diacylglycerol and LCACoAs, which are concomitant with higher blood leptin levels, were comparable to the low-fat/high-carbohydrate diet in the long term. Altogether these results demonstrate the remarkable ability of the normal heart to maintain a near-constant metabolic environment even with excess substrate availability.
However, it is unlikely that the evaluation of total fatty acid levels is sufficiently informative, as the final outcome may be influenced by the ratio of unsaturated-to-saturated species and by the cumulative response to these species over time. While the Western diet did not exclusively induce quantitative changes in lipid species, it did modify the nature of LCACoAs present in the heart. More specifically, we found that the Western feeding induced a 40% drop in the myocardial palmitoleoyl-CoA content and a similar decrease in the ratio of cardiac unsaturated-to- saturated fat. This could not be solely explained by the composition of the diet, because the molar ratios of monounsaturated-to-saturated fatty acid species provided by WD and HFD are identical (Table 2). The fact that neither the high-fat nor the low-fat/high-carbohydrate diet modified cardiac fat ratios implies not only an excess in dietary fat but also an excess in dietary sugars. Therefore, we decided to investigate for a possible involvement of de novo lipogenesis. The liver is the major site for de novo synthesis of long chain fatty acids that are released in the form of VLDL-bound triglyceride (41). Hepatic de novo lipogenesis, a process highly regulated at the transcriptional level, is activated by high levels of insulin and carbohydrate and repressed by dietary fat (42). Consequently, blood levels of insulin and triacylglycerol are higher with the low-fat/high-carbohydrate diet, while they remain lower with the high-fat diet. Gene expression analysis confirmed a sustained enhancement in the transcription of genes involved in de novo lipogenesis in the liver of rats fed the low-fat/high-carbohydrate diet, which was associated with high plasma levels of palmitate and, mostly, palmitoleate. Conversely, and as expected, the high-fat diet led to a repression of these genes. In a typical Western diet there is usually sufficient fat to suppress de novo lipogenesis (43). However, the expression pattern of FAS suggests higher lipogenic capacity with the Western diet in the long term. This is further supported by the finding that hepatic fatty acid elongase activity was greatly enhanced with the Western diet at the same time point. Increased lipogenesis will explain the concomitant rise in circulating triacylglycerol. The loss of the repressive effect of the Western diet on hepatic de novo lipogenesis over time could be related to the development of obesity and insulin resistance. Indeed, obese hyperinsulinemic patients consuming a Western diet have a 3- to 4-fold higher lipogenic activity in the liver compared with lean or obese normoinsulinemic subjects (44). Interestingly, recent investigations performed in overweight or obese individuals concluded that consumption of fructose-sweetened beverages promotes dyslipidemia, impairs insulin sensitivity, and increases visceral adiposity through increased de novo lipid synthesis (45). All together, these findings point out that de novo synthesized fat, rather than dietary fat per se, may be responsible for cardiac maladaptation to diet-induced obesity.
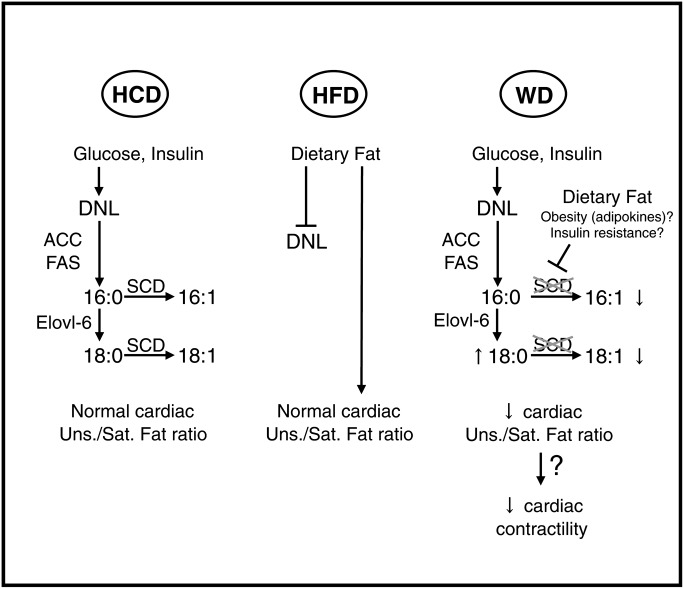
De novo synthesis of fatty acids is principally a polymerization of acetate to form palmitate. Palmitate then enters in an elongation/desaturation process to form very long-chain fatty acids (46). The fatty acid elongase Elovl-6 catalyzes the elongation of palmitate to stearate, and the delta-9 desaturase SCD1 (and SCD2 to a lesser extent for the heart) introduces a double bond between carbons 9 and 10 to form palmitoleate and oleate (46). Therefore, SCDs have a fundamental role in regulating the balance between de novo synthesized saturated and monounsaturated fat. While both saturated and monounsaturated fat are from dietary origin with a high-fat diet, scd expression has to be higher in animals fed a low-fat/high-carbohydrate diet to maintain a normal monounsaturated-to-saturated fat ratio. Concerning Western diet, the long-term increase of hepatic lipogenic capacity was not accompanied by scd upregulation, neither in the liver nor in the heart. In addition, delta-9 desaturase activity was even decreased in the liver of rats fed the Western diet for 32–48 weeks, a decrease that coincided with a drop in the plasma oleate-to-stearate ratio. This discrepancy is likely responsible for the relative enrichment in cardiac-saturated LCACoAs. The regulation of scd expression by nutritional environment is controlled by a very complex network of cofactors and transcription factors including SREBPs, ChREBP, liver X receptor (LXR), and peroxisome proliferator-activated receptor α (PPARα) (46, 47). A modification in the activity of one or more of these factors may be respon sible for the deregulation of scd expression (Fig. 9).
Fig. 9.
Model for the role of hepatic de novo lipogenesis in Western diet–induced alteration of cardiac LCACoA profile. The consumption of a low-fat/high-carbohydrate diet (HCD; left panel) and the resulting high plasma carbohydrate and insulin levels induce the transcriptional activation of de novo lipogenesis (DNL) in the liver. Delta-9 desaturase expression/activity is increased to supply the heart with a normal amount of monounsaturated fat. Conversely, dietary fat is the essential provider of saturated and monounsaturated fatty acids under high-fat diet (HFD; middle panel) consumption, which consequently, inhibits hepatic de novo lipogenesis. Western diet (WD; right panel) induces de novo lipogenesis in the long term, but significant amounts of dietary fat may combine to obesity and/or insulin resistance to avoid stearoyl-CoA desaturases upregulation both in the liver and in the heart. Insufficient desaturation of de novo synthesized fat thus induces a decrease in the cardiac monounsaturated-to-saturated fat ratio, which could indirectly impair the contractility of the heart. Bar heads show inhibition of target. 16:0, palmitate; 16:1, palmitoleate; 18:0, stearate; 18:1, oleate; ACC, acetyl-CoA carboxylase; Elovl-6, long chain fatty acid elongase ELOVL family member 6; FAS, fatty acid synthase; SCD, stearoyl-CoA desaturase.
Such alteration in cardiac fatty acid profile can have dire consequences on cardiomyocyte survival, cardiac insulin sensitivity, and even cardiac metabolic adaptation to a high-fat diet. Saturated fatty acids are known to promote apoptosis in a number of cell types, including the cardiomyocyte, whereas monounsaturated fat is more protective: Oleate rescues palmitate-induced apoptosis by channeling palmitate into triacylglycerol pools (48), and palmitoleate antagonizes palmitate-induced activation of endoplasmic reticulum stress (49). Palmitoleate acts in vivo as an endocrine signal, improving muscle insulin sensitivity and decreasing hepatosteatosis (50). We could not detect any change in the expression of endoplasmic reticulum stress markers in the heart of these animals (see supplementary Fig. II). However, we currently have strong evidence for increased cardiac apoptosis following long-term feeding with the Western diet (K. Ballal and H. Taegtmeyer, unpublished observations). Physiological concentrations of acyl-CoA can regulate the activity of acetyl-CoA carboxylase, AMP-activated kinase, and several other proteins involved in energy metabolism and Ca2+ homeostasis at higher concentrations (27). LCACoAs are also located in the nuclei where they can regulate key nuclear receptors for lipid homeostasis like PPARα and hepatocyte nuclear factor 4α (HNF4α) (28). A dysregulation of PPARα activity with a Western diet is consistent with our report on inadequate induction of a cassette of fatty acid–responsive genes and impaired activation of fatty acid oxidation (29). It is, however, challenging to address how different compositions of Acyl-CoA can affect metabolic output and cardiac contractility.
Impaired membrane fluidity could be related to the deleterious effect of a Western diet on cardiac function. Monounsaturated fatty acids are a critical component of lipids in membranes, which are themselves involved in the regulation of fundamental mechanisms like receptor/ enzyme activity and membrane fusion/fission (51). In that sense, decreased fluidity of the inner mitochondrial membrane negatively affects the activity of lipid-dependent enzymes and can contribute to an impairment of mitochondrial function. Changes in inner mitochondrial membrane composition, characterized by a decrease in unsaturated fatty acyls with a reciprocal increase in saturated acyls, have been proposed as a possible mechanism for heart failure in the aging myocardium (52). Defining a possible cause-effect relationship among the changes in cardiac fatty acid composition, mitochondrial membrane fluidity, and cardiac function deserves further investigation.
A large study like ours has limitations. First, we did not include age-matched rats fed on standard rodent chow for all time points examined. This group would have been useful to discriminate between diet- and age-induced changes. Second, insulin sensitivity of the different organs was not assessed. We previously reported that fed blood glucose levels did not change among the groups over time, which precludes the development of frank diabetes with the long-term Western or high-fat feeding (29). However, the fact that plasma insulin and plasma lipid levels dramatically increased with the long-term Western feeding might indicate that the rats were suffering from selective insulin resistance, a state known to stimulate lipogenesis and to predispose to cardiovascular diseases and diabetes (53). It is noteworthy that the failing heart of patients with metabolic syndrome, but free of diabetes, displays increased lipid deposition and higher levels of SREBP-1c (54).
In conclusion, our findings suggest a critical role for alterations in the pathway of de novo lipogenesis that cause cardiac maladaptation to diet-induced obesity. We speculate that insufficient fatty acid desaturase activity is a critical determinant of cardiac failure in diet-induced obesity. Further experiments are needed to determine whether obesity and/or insulin resistance are involved in the activation and dysregulation of the lipogenic program. The exact mechanism by which the observed changes in the cardiac LCACoA profile impair contractility also still remains to be established.
Supplementary Material
Acknowledgments
The authors thank Dr. Fatima Smih and Dr. Philippe Rouet (INSERM U858, Toulouse, France) for their assistance in the analysis of plasma fatty acids. We thank Mohamed F. Algahim and Genna Lubrano for technical assistance. We are indebted to Dr. Martin E. Young for his critical review of the manuscript. We also thank Roxy A. Tate and Rebecca Salazar for editorial assistance.
Footnotes
Abbreviations:
- ChREBP
- carbohydrate response element binding protein
- FAME
- fatty acid methyl ester
- LCACoA
- long-chain acyl-coenzyme A
- NBD C6-HPC
- 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine
- PPARα
- peroxisome proliferator-activated receptor α
- SCD
- stearoyl-CoA desaturase
- SREBP
- sterol-regulatory element binding protein
- TBARS
- thiobarbituric acid reactive substances
This work was supported in part by a grant from the National Heart, Lung and Blood Institute (R01-HL073162). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Kenchaiah S., Evans J. C., Levy D., Wilson P. W., Benjamin E. J., Larson M. G., Kannel W. B., Vasan R. 2002. Obesity and the risk of heart failure. N. Engl. J. Med. 347: 305–313. [DOI] [PubMed] [Google Scholar]
- 2.Smith W. M. 1985. Epidemiology of congestive heart failure. Am. J. Cardiol. 55: 3A–8A. [DOI] [PubMed] [Google Scholar]
- 3.Harmancey R., Wilson C. R., Taegtmeyer H. 2008. Adaptation and maladaptation of the heart in obesity. Hypertension. 52: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taegtmeyer H., McNulty P., Young M. E. 2002. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 105: 1727–1733. [DOI] [PubMed] [Google Scholar]
- 5.McGavock J. M., Lingvay I., Zib I., Tillery T., Salas N., Unger R., Levine B. D., Raskin P., Victor R. G., Szczepaniak L. S. 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S., Adrogue J. V., Golfman L., Uray I., Lemm J., Youker K., Noon G. P., Frazier O. H., Taegtmeyer H. 2004. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 18: 1692–1700. [DOI] [PubMed] [Google Scholar]
- 7.Rijzewijk L. J., van der Meer R. W., Smit J. W., Diamant M., Bax J. J., Hammer S., Romijn J. A., de Roos A., Lamb H. J. 2008. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 52: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh W., Abel E. D., Breslow J. L., Maeda N., Davis R. C., Fisher E. A., Dansky H., McClain D. A., McIndoe R., Wassef M. K., et al. 2007. Recipes for creating animal models of diabetic cardiovascular disease. Circ. Res. 100: 1415–1427. [DOI] [PubMed] [Google Scholar]
- 9.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., et al. 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96: 225–233. [DOI] [PubMed] [Google Scholar]
- 10.Yagyu H., Chen G., Yokoyama M., Hirata K., Augustus A., Kako Y., Seo T., Hu Y., Lutz E. P., Merkel M., et al. 2003. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 12.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finck B. N., Han X., Courtois M., Aimond F., Nerbonne J. M., Kovacs A., Gross R. W., Kelly D. P. 2003. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc. Natl. Acad. Sci. USA. 100: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., et al. 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnard C., Durand A., Peyrol S., Chanseaume E., Chauvin M. A., Morio B., Vidal H., Rieusset J. 2008. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 118: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudina S., Sena S., Theobald H., Sheng X., Wright J. J., Hu X. X., Aziz S., Johnson J. I., Bugger H., Zaha V. G., et al. 2007. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 56: 2457–2466. [DOI] [PubMed] [Google Scholar]
- 17.Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., Schaffer J. E. 2006. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47: 2726–2737. [DOI] [PubMed] [Google Scholar]
- 18.Summers S. A. 2006. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 45: 42–72. [DOI] [PubMed] [Google Scholar]
- 19.Morino K., Petersen K. F., Shulman G. I. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 55(Suppl 2): S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers S. A., Nelson D. H. 2005. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing's syndrome. Diabetes. 54: 591–602. [DOI] [PubMed] [Google Scholar]
- 21.Aguila M. B., Mandarim-de-Lacerda C. A. 2003. Heart and blood pressure adaptations in Wistar rats fed with different high-fat diets for 18 months. Nutrition. 19: 347–352. [DOI] [PubMed] [Google Scholar]
- 22.Carroll J. F., Zenebe W. J., Strange T. B. 2006. Cardiovascular function in a rat model of diet-induced obesity. Hypertension. 48: 65–72. [DOI] [PubMed] [Google Scholar]
- 23.Okere I. C., Chandler M. P., McElfresh T. A., Rennison J. H., Kung T. A., Hoit B. D., Ernsberger P., Young M. E., Stanley W. C. 2007. Carnitine palmitoyl transferase-I inhibition is not associated with cardiac hypertrophy in rats fed a high-fat diet. Clin. Exp. Pharmacol. Physiol. 34: 113–119. [DOI] [PubMed] [Google Scholar]
- 24.Okere I. C., Chandler M. P., McElfresh T. A., Rennison J. H., Sharov V., Sabbah H. N., Tserng K. Y., Hoit B. D., Ernsberger P., Young M. E., et al. 2006. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am. J. Physiol. Heart Circ. Physiol. 291: H38–H44. [DOI] [PubMed] [Google Scholar]
- 25.Park S. Y., Cho Y. R., Kim H. J., Higashimori T., Danton C., Lee M. K., Dey A., Rothermel B., Kim Y. B., Kalinowski A., et al. 2005. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 54: 3530–3540. [DOI] [PubMed] [Google Scholar]
- 26.Relling D. P., Esberg L. B., Fang C. X., Johnson W. T., Murphy E. J., Carlson E. C., Saari J. T., Ren J. 2006. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J. Hypertens. 24: 549–561. [DOI] [PubMed] [Google Scholar]
- 27.Faergeman N. J., Knudsen J. 1997. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder F., Petrescu A. D., Huang H., Atshaves B. P., McIntosh A. L., Martin G. G., Hostetler H. A., Vespa A., Landrock D., Landrock K. K., et al. 2008. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 43: 1–17. [DOI] [PubMed] [Google Scholar]
- 29.Wilson C. R., Tran M. K., Salazar K. L., Young M. E., Taegtmeyer H. 2007. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem. J. 406: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 31.Previati M., Bertolaso L., Tramarin M., Bertagnolo V., Capitani S. 1996. Low nanogram range quantitation of diglycerides and ceramide by high-performance liquid chromatography. Anal. Biochem. 233: 108–114. [DOI] [PubMed] [Google Scholar]
- 32.Mangino M. J., Zografakis J., Murphy M. K., Anderson C. B. 1992. Improved and simplified tissue extraction method for quantitating long-chain acyl-coenzyme A thioesters with picomolar detection using high-performance liquid chromatography. J. Chromatogr. B. 577: 157–162. [DOI] [PubMed] [Google Scholar]
- 33.Ohkawa H., Ohishi N., Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 34.Walaas O., Walaas E. 1950. Effect of epinephrine on rat diaphragm. J. Biol. Chem. 187: 769–776. [PubMed] [Google Scholar]
- 35.Douce R., Mannella C. A., Bonner W. D., Jr. 1973. The external NADH dehydrogenases of intact plant mitochondria. Biochim. Biophys. Acta. 292: 105–116. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki M., Gomez F. E., Ntambi J. M. 2002. Lack of stearoyl-CoA desaturase-1 function induces a palmitoyl-CoA Delta6 desaturase and represses the stearoyl-CoA desaturase-3 gene in the preputial glands of the mouse. J. Lipid Res. 43: 2146–2154. [DOI] [PubMed] [Google Scholar]
- 37.Talamo B. R., Bloch K. 1969. A new assay for fatty acid desaturation. Anal. Biochem. 29: 300–304. [DOI] [PubMed] [Google Scholar]
- 38.Moon Y. A., Shah N. A., Mohapatra S., Warrington J. A., Horton J. D. 2001. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 276: 45358–45366. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Botolin D., Christian B., Busik J., Xu J., Jump D. B. 2005. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 46: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aarsland A., Wolfe R. R. 1998. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J. Lipid Res. 39: 1280–1286. [PubMed] [Google Scholar]
- 42.Sul H. S., Smith S. 2008. Fatty acid synthesis in eukaryotes. Biochemistry of Lipids, Lipoproteins and Membranes. 5th ed Vance D. E., Vance J. E., Elsevier, Amsterdam, The Netherlands: 155–190. [Google Scholar]
- 43.Jeffcoat R. 2007. Obesity— a perspective based on the biochemical interrelationship of lipids and carbohydrates. Med. Hypotheses. 68: 1159–1171. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz J. M., Linfoot P., Dare D., Aghajanian K. 2003. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 77: 43–50. [DOI] [PubMed] [Google Scholar]
- 45.Stanhope K. L., Schwarz J. M., Keim N. L., Griffen S. C., Bremer A. A., Graham J. L., Hatcher B., Cox C. L., Dyachenko A., Zhang W., et al. 2009. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 119: 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazaki M., Ntambi J. M. 2008. Fatty acid desaturation and chain elongation in mammals. Biochemistry of Lipids, Lipoproteins and Membranes. 5th ed. Vance D. E., Vance J. E., Elsevier, Amsterdam, The Netherlands: 191–211. [Google Scholar]
- 47.Wang Y., Botolin D., Xu J., Christian B., Mitchell E., Jayaprakasam B., Nair M. G., Peters J. M., Busik J. V., Olson L. K., et al. 2006. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 47: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diakogiannaki E., Welters H. J., Morgan N. G. 2008. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J. Endocrinol. 197: 553–563. [DOI] [PubMed] [Google Scholar]
- 50.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowhan W., Bogdanov M., Mileykovskaya E. 2008. Functional roles of lipids in membranes. Biochemistry of Lipids, Lipoproteins and Membranes. 5th ed. Vance D. E., Vance J. E., Elsevier, Amsterdam, The Netherlands: 1–37. [Google Scholar]
- 52.Odiet J. A., Wei J. Y. 1996. Heart failure and the aging myocardium: possible role of cardiac mitochondria. Heart Fail. Rev. 1: 139–149. [Google Scholar]
- 53.Chavez J. A., Summers S. A. 2010. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim. Biophys. Acta. 1801: 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marfella R., Di Filippo C., Portoghese M., Barbieri M., Ferraraccio F., Siniscalchi M., Cacciapuoti F., Rossi F., D'Amico M., Paolisso G. 2009. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J. Lipid Res. 50: 2314–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.