Abstract
Fast migrating cerebrosides (FMC) are derivatives of galactosylceramide (GalCer). The structures of the most hydrophobic FMC-5, FMC-6, and FMC-7 were determined by electrospray ionization linear ion-trap mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy complementing previous NMR spectroscopy and gas chromatography-mass spectrometry to be 3-O-acetyl-sphingosine-GalCer derivatives with galactose O-acetyl modifications. FMC-5 and FMC-6 are 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer with nonhydroxy and hydroxy-N-fatty-acids, while FMC-7 has an additional O-acetylation of the 2-hydroxy-fatty acid. The immuno-reactivity in human cerebrospinal fluid (CSF) to these acetylated glycolipids was examined in central nervous system (CNS) infectious disease, noninflammatory disorders, and multiple sclerosis (MS). Screening for lipid binding in MS and other neurological disease groups revealed that the greatest anti-hydrophobic FMC reactivity was observed in the inflammatory CNS diseases (meningitis, meningo-encephalitis, and subacute sclerosing panencephalitis). Some MS patients had increased reactivity with the hydrophobic FMCs and with glycoglycerophospholipid MfGL-II from Mycoplasma fermentans. The cross-reactivity of highly acetylated GalCer with microbial acyl-glycolipid raises the possibility that myelin-O-acetyl-cerebrosides, bacterial infection, and neurological disease are linked.
Keywords: acetyl-cerebroside, cross-reactivity, electrospray ionization, Escherichia coli, fast migrating cerebrosides, glycosphingolipids, inflammation, lipopolysaccharide, multiple sclerosis, multistage mass spectrometry, Mycoplasma fermentans, neurological disease
We have found a series of myelin glycolipids that are derivatives of galactosylceramide (GalCer) with higher TLC-Rf, and we have designated them as fast migrating cerebrosides (FMC) (1). The simplest two compounds of this series (FMC-1 and FMC-2) are GalCer derivatives with 3-O-acetylation of the sphingosyl moiety that differ by incorporation of nonhydroxy and 2-hydroxy fatty-N-acylation, respectively (1). The next two (FMC-3 and FMC-4) are analogs of FMC-1 and FMC-2 with additional O-acetylation at the galactose C6 hydroxyl (2). Previously, we have examined these cerebroside (galactosylceramide) derivatives in rat, bovine, and human brain and found them present in all three species, varying in species from 15–35% of tissue galactosylceramide content (1) although only FMC components from rat brain have been completely characterized with respect to molecular structure (1, 2).
In the current study, the structural characterization was extended, and chemical acetylation of galactosylceramide from bovine brain was also employed. We report the purification and characterization of the most hydrophobic of the 3-O-acetyl-sphingosine-GalCer series that are the most complex and include FMC-5, FMC-6, and FMC-7. We focused on the structural characterization of FMC-7 that was characterized and found to be identical in the brain tissue of two species, rat and bovine. The penta- and hexa-acetylcerebrosides resemble acyl- and/or acetyl-carbohydrates of lipopolysaccharide (LPS) and/or glycolipids (GL) found in many bacteria (3–10) and are candidate lipid antigen mimics targeted by immune responses initially directed at bacterial acyl- or acetyl-conjugated glycosides and subsequently inducing autoimmune inflammation in CNS.
To explore this, we prepared polyclonal antibody of purified FMC-7 that reacts with the tetra-acetyl-galactose moiety shared by FMC-5/-6/-7, similar to that previously reported (11), which turned out to be a mixture of FMC-5/-6/-7 that resolved into the individual components FMC-5, FMC-6, and FMC-7. Reactivities to glycoconjugates (GL and LPS) were examined, and the anti–FMC-7 antibody reacted best with IgG in CSF from patients with encephalitis, meningitis, and subacute sclerosing panencephalitis (SSPE). Low affinity reactivity with surface lipoglycans from Mycoplasma fermentans (with a purified glycoglycerophospholipid MfGL-II) was observed in some MS patients. We also observed binding of MS CSF to LPS from Escherichia coli J5 though not to LPS of other bacterial species. A detailed analysis was made of 38 CSF samples: 29 MS and 9 from other neurological diseases (OND), including inflammatory OND (I-OND) and noninflammatory OND (NI-OND) controls. The purified lipid antigens examined by enzyme-linked immunosorbent assay (ELISA) included MfGL-II from M. fermentans, E. coli J5 LPS, FMC-7, cerebroside (GalCer), and sulfatide (3-O-sulfo-GalCer).
METHODS
Purification and preparation of brain FMC
Previously, we had purified five fractions of FMCs from developing rat brain; four of those purified components have been characterized. The FMC-1 and FMC-2 fractions were determined to be 3-O-acetyl-sphingosine-GalCer components (1), whereas the FMC-3 and FMC-4 fractions were determined to be 6-O-acetyl Gal derivatives of FMC-1 and FMC-2, respectively (2). The carbohydrate content of “FMC-5” had been characterized as 2,3,4,6-tetra-O-acetyl Gal by GC-MS after methylation procedure under neutral conditions (data not shown). No partially methylated derivative was identified, indicating that all four hydroxyls of Gal were substituted. The initial NMR spectroscopy suggested that “FMC-5” was composed of three different components, representing penta-/hexa-O-acetyl analogs of FMC-1 and FMC-2 (1). Further purification was carried out to facilitate precise structural characterization. We now report the separation and purification of the “FMC-5” mixture into three individual components.
Individual components of the FMC mixture were purified using silicic acid column (62.5 cm × 0.5 cm) chromatography. Briefly, the FMC mixture was applied to the column in chloroform:hexane (3:2; v/v), and the column was eluted with chloroform:hexane:glacial acetic acid (60:40:0.26; v/v/v) as initial solvent, and then the concentration of glacial acetic acid was increased (linear gradient) to proportions of 40:60:0.30 (v/v/v). Approximately 1–2 ml was collected in each tube in a fraction collector, and 10 μl from each fraction was applied to a TLC-plate (20 × 20 cm, E. Merck, Darmstadt, Germany). The bands were resolved using chloroform:hexane:methanol:glacial acetic acid (75:20:1.5:1.0; v/v/v/v) and visualized with DPA spray (12). Pure fractions were pooled, dried down, and dissolved in a defined volume of solvent. The purity of each pooled fraction was assessed by TLC using two different solvent systems, chloroform:hexane:methanol:glacial acetic acid (75:18:1.2:0.6; v/v/v/v) and chloroform:hexane:methanol (75:18:1.2; v/v/v), followed by visualization of bands by DPA spray. We obtained three homogeneous FMC subfractions and designated them as FMC-5, FMC-6, and FMC-7, respectively, according to their TLC-Rf migration, the FMC-5 being the slowest migrating band. Each homogeneous FMC fraction was stored at 4°C until use. The structure of each purified FMC was determined by mass spectrometry as described below.
Non-polar FMCs were tentatively characterized (by TLC) in vertebrate CNS and PNS myelin of rat, bovine, and human (1). FMC-5 through FMC-7 are more hydrophobic than galactosylceramide. The specific polyclonal antibody prepared against the mixture of FMC-5/-6/-7 stained myelin and primary oligodendrocytes in culture (11). The initial structural elucidation by GC-MS (Hewlett Packard, Agilent Technologies) and NMR guided the preparation of large amounts of FMC-5 and FMC-7 by O-acetylation of GalCer purified from bovine brain, and its structure was chemically characterized. Briefly, GalCer (15 mg) was dissolved in a mixture of pyridine:acetic anhydride (3:2, v/v) and stirred for 18 h in a closed-cap tube (13). The reaction mixture was concentrated using a flash evaporator by repeated addition of toluene. Further resolution of the acetyl-GalCer was achieved by TLC, developed with chloroform:hexane:methanol:glacial acetic acid (75:18:1.2:1.2; v/v/v/v), and individual bands were visualized by DPA spray. This revealed three bands (two major and one minor), and as was subsequently confirmed, FMC-7 had the highest Rf value, and FMC-5 the lowest. The structure of each FMC was confirmed by GC-MS, each containing 2,3,4,6-tetra-O-acetyl Gal as the only carbohydrate moiety. The individual components of FMCs from the mixture were further purified using column chromatography as described above.
Positive ion-mode electrospray ionization mass spectrometry
Electrospray ionization mass spectrometry (ESI-MS) was performed in the positive ion-mode on lithium-adducted samples dissolved in pure methanol as described previously (2), except for use of an linear ion-trap (LTQ) module of a ThermoFisher LTQ-Orbitrap XL (Bremen, Germany), so that fragmentation by collision-induced dissociation (CID) was achieved through multi-stage ion-trap MSn rather than tandem MS/CID-MS. In addition, sample introduction by direct infusion was carried out at low nl/min flow rates using an Advion BioSystems TriVersa NanoMate ESI chip interface. The NanoMate spray voltage was kept at 1.5 kV; spray current was generally in the range 50–150 nA. For CID-MSn analysis, the following parameters were generally employed: precursor isolation width, m/z 1.0; normalized collision energy, 35 V; activation Q, 0.250; activation time, 30 ms.
1- and 2-D nuclear magnetic resonance spectroscopy
The sample of FMC-7 (∼200 μg) was dissolved in 0.5 ml of DMSO-d6 for NMR analysis. 1-D 1H NMR; 2-D gradient correlation spectroscopy (1H-1H-gCOSY) and total correlation spectroscopy (1H-1H-TOCSY); and 1H-detected, 13C-decoupled, phase-sensitive, gradient-selected 13C-1H heteronuclear single quantum correlation (gsHSQC) spectra were acquired at 800 MHz in pure DMSO-d6 at 300° (27°C). Inverse SQ editing in the gsHSQC program rendered single bond 13C-1H correlations of methylene (CH2) groups into a negative phase (14). Proton chemical shifts are referenced to internal TMS (0.000), and 13C chemical shifts to the centerline of the solvent DMSO signal (set at 40.12 ppm). Spectra were acquired on a Bruker Avance 800 spectrometer equipped with a TXI cryoprobe, located at the Danish Instrument Center for NMR Spectroscopy of Biological Macromolecules, using standard acquisition software available in the Bruker Topspin software package.
Preparation of specific polyclonal rabbit anti-FMC-7 antibody
Polyclonal antibody against FMC-7 was prepared in New Zealand white female rabbits (15, 16). Two rabbits were selected for antibody preparation. Antigen for injections was prepared by chemical acetylation of GalCer (see above). Briefly, 1 mg of the FMC-7 was mixed with 1 ml (2 mg) of keyhole limpet hemocyanin (KLH) adjuvant in PBS, and 1 ml of complete Freund's adjuvant (CFA) was added and emulsified. One ml of the emulsion was injected subcutaneously into the back of a rabbit (4 sites; 0.25 ml/site) followed by a booster immunization [in incomplete Freund's adjuvant (IFA)] after 2 weeks. When the desired titer was obtained, serum was collected by cardiac puncture, and antibody titers were determined (17, 18). The IgG fraction was purified from the collected rabbit serum using a protein A column (Bio-Rad, Hercules, CA), and FMC-specific IgG was prepared using a substrate affinity column. The specificity of the antibody was tested as described previously (11): there was no reactivity with FMC-1 through FMC-4, and there was cross-reactivity with FMC-5 and FMC-6 that is consistent with binding to the tetra-acetyl-galactose moiety common to the FMC-5/-6/-7 trio.
All procedures involving animals were conducted in conformity with Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for Care and Use of Laboratory Animals. Furthermore, these procedures were performed with compliance with the guidelines issued by the Committee on Animal Use for Research and Education at the Medical College of Georgia.
ELISA
Human specimens were screened against endogenous (FMC-7, GalCer, sulfatide) and exogenous microbial (M. fermentans, E. coli J5) glycolipid antigens. Phospholipid-glycoglycerophosphocholine MfGL-II derived from M. fermentans was kindly provided by Dr. U. Zähringer (19), Borstel, Germany, and LPS was extracted and purified from E. coli J5 as described previously (20). The structures of the compounds used in this study are in Fig. 1. Both endogenous and exogenous antigens were coated onto 96-well microtiter plates at a concentration of 500 ng/well in absolute ethanol; nonspecific reactions were blocked by addition of 1% BSA in PBS for 30 min at 37°C. Samples were incubated with CSF diluted 1:10 in blocking buffer overnight at 4°C. After thorough washing with PBS, horseradish peroxidase (HRP)–conjugated sheep anti-human IgG (Amersham-Biosciences, Pittsburgh, PA) diluted in ELISA buffer (1:2000) was incubated for 2 h at 37°C. Plates were again washed thoroughly to remove the unbound antibody, and the color was developed using o-phenylenediamine dihydrochloride (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. The reaction was terminated by adding 3N H2SO4, and the absorbance was read at 492 nm in ELISA plate reader (Bio-Rad) after 15 min. Initial studies using CSF serially diluted 1/10 to 1/100 from three patients showed that all samples had specific absorbance readings of less than 0.10 unit at a 1/10 dilution. Therefore, a 1/10 dilution was used for testing all CSF samples.
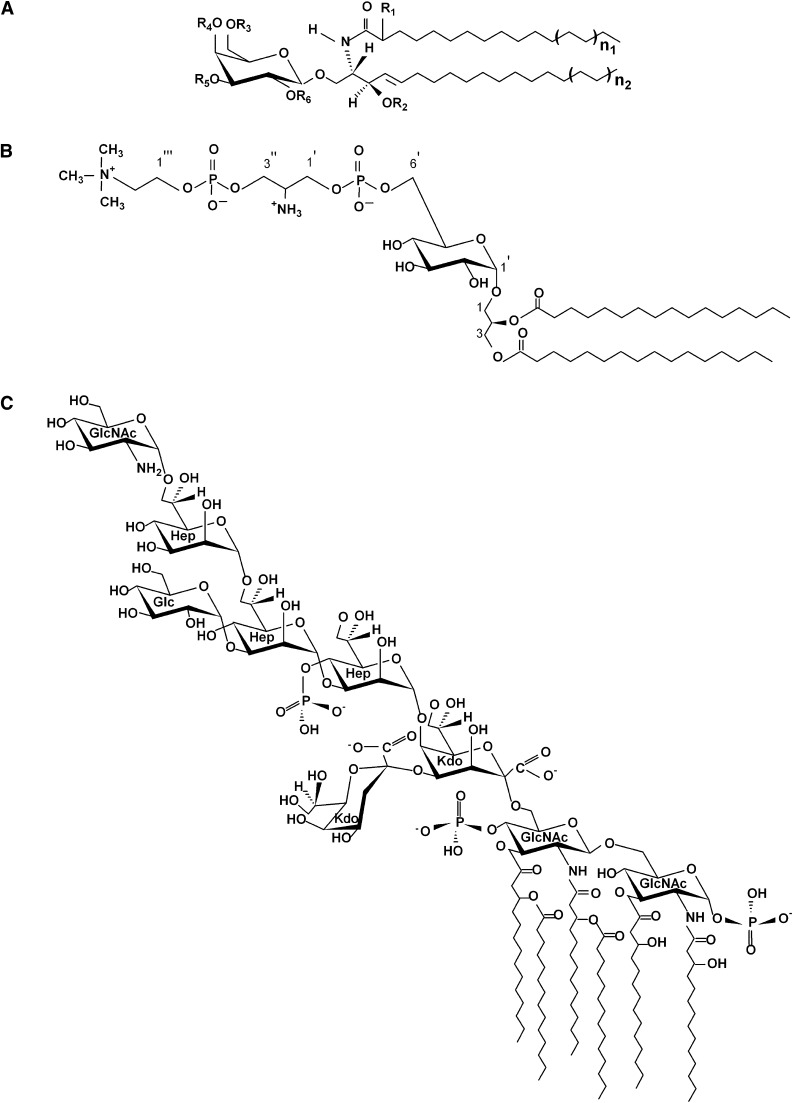
Fig. 1.
Primary structure of different glycolipids examined in this study. A: Diagram represents endogenous antigens, such as GalCer (R1 = H, R2 = H, R3−R6 = H) (1); FMC-7 (R1 = OAc, R2−R6 = Ac) (structure described here); and sulfatide (R1-R4 = H, R5 = HSO−4, R6 = H) (33). R1 and R2 represent hydroxy and nonhydroxy fatty acyl groups, respectively. B: Mycoplasma fermentans glycolipid MfGL-II (19). C: LPS from Escherichia coli J5 (34). Abbreviations: FMC, fast migrating cerebroside; GalCer, galactosylceramide; GlcNAc, N-acetylglucosamine; Hep, L-glycero-D-manno-heptopyranose; Kdo, 3-deoxy-D-manno-octulopyranosonic acid; LPS, lipopolisaccharide.
TLC–immuno-overlay
Glycolipids [FMC-1, FMC-7, GalCer, MfGL-II, LPS from Escherichia coli J5, elementary body preparation (EB) of Chlamydia pneumoniae] were separated on TLC plates (Silica gel 60; 5 × 10 cm; E. Merck, Darmstadt, Germany), and developed in n-propanol:methanol:water (50:30:20; v/v/v) for overlay with anti-FMC-7 and in n-propanol:methanol:water (60:20:15; v/v/v) for overlay with the anti-FMC-5/-6/-7 (mixture), respectively. Glycolipids were detected by DPA spray (12). The binding of antibodies on TLC was determined by a variation of the method described by Magnani et al. (21). Briefly, after development, the TLC plate was dipped in n-hexane containing 0.25% polyisobutylmethylacrylate for 30 s, and dried for 30 min. The plates were then overlaid with 1% BSA in PBS for 30 min at 37°C, and with human CSF (1:10 dilution in 1% BSA in PBS) overnight at 4°C, and washed with PBS, overlaid with horseradish peroxidase- (HRP-) conjugated sheep anti-human IgG for 2-4 h at 37°C, and bands developed with diaminobenzidine (DAB) substrate.
Human specimens
CSF specimens from patients with clinically definite MS and OND were obtained from the Human Brain and Spinal Fluid Resource Center (Veterans Affairs West Los Angeles Healthcare Center, Los Angeles, CA). CSF samples were from 29 MS (19 male, 10 female) and 9 OND (7 male, 2 female) controls. In the group of 29 MS patients, 3 were classified as primary progressive MS (PPMS); 10 as secondary progressive MS (SPMS); and 16 as relapsing-remitting MS (RRMS), including both subtypes (5 in relapse, 11 in remission). The nine samples from OND were divided into inflammatory (I-OND) and noninflammatory (NI-OND) and served as controls. The group of five inflammatory OND had the following diagnoses: meningitis (3), meningo-encephalitis (1), and subacute sclerosing panencephalitis (SSPE) (1). The group of 4 patients with NI-OND had diagnoses of neuropathy (3) and cerebellar degeneration (1). Preservation of anonymity, confidentiality, and masking of samples was maintained.
The clinical specimens were gently thawed, centrifuged (12,000 g, 30 min), separated in aliquots to sterile Eppendorf tubes, and stored at −80°C until use. The analyses were carried out as a masked study to protect patient privacy, prevent subject identification, and eliminate technical bias. The study was conducted with Institutional Review Board approval, and informed consent was obtained at UCLA from the human subjects or their representatives.
Statistical analysis
Rank transformation was applied to rank the data from smallest to largest, with the smallest observation having rank 1. Average ranks were assigned in case of ties. The overall effect of each antigen was tested using ANOVA on ranked data and Tukey multiple comparison to compare individual groups. The median for each subject was compared using ANOVA; if P < 0.05, each pair of groups was compared using Tukey's test. Fisher's exact test was used to compare the percentages of subjects with a specific absorbance > 0.10 units (an arbitrary cut-off level). Comparison of pairs of groups was made with Fisher's analysis only if the P < 0.05 for 5 × 2 contingency table values.
RESULTS
Isolation and purification of penta- and hexa-acetyl-cerebrosides of brain
The compounds FMC-5, FMC-6, and FMC-7 purified from rat brain were resolved and appeared homogeneous in two solvent systems (Fig. 2). In addition we prepared chemically synthesized FMC-7 by acetylation of crude GalCer from bovine brain (see “Methods”).
Fig. 2.
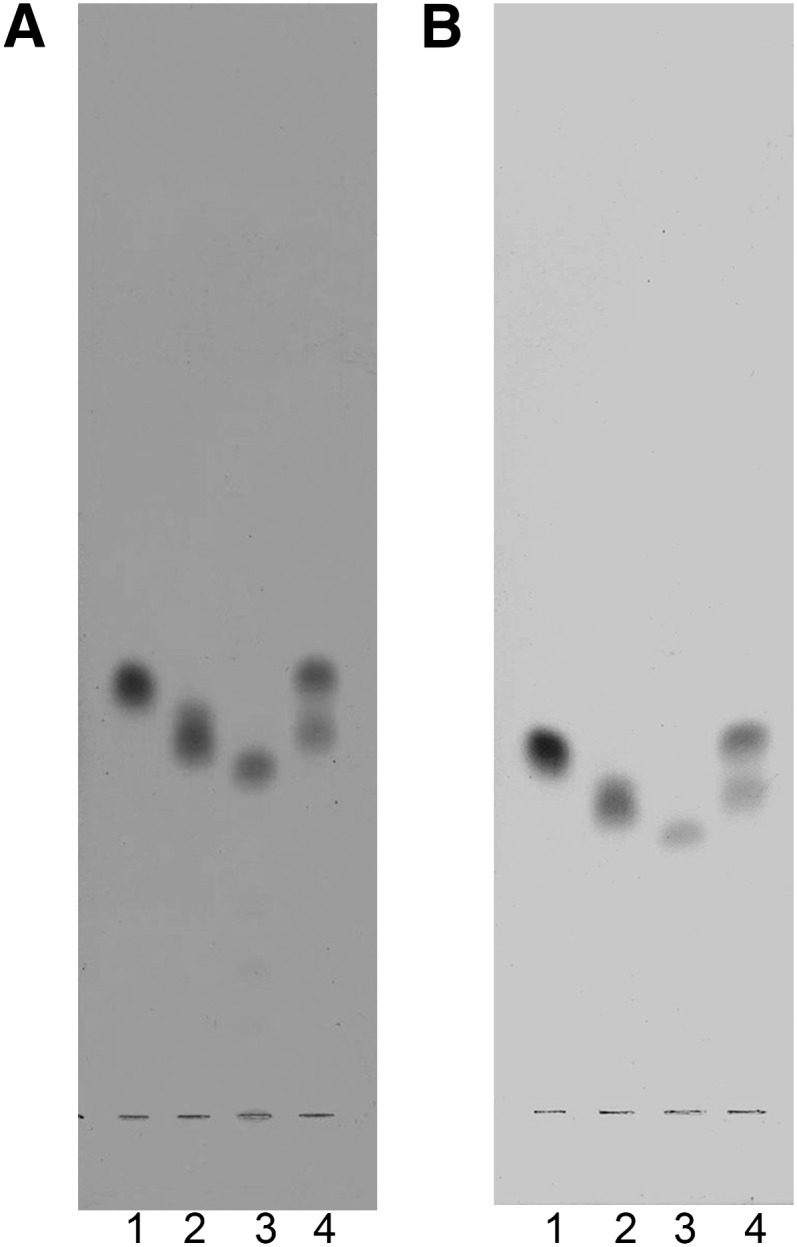
Thin layer chromatogram of the purified fast migrating cerebrosides FMC-5, FMC-6, and FMC-7. Plates (5 × 20 cm) were resolved in A: chloroform:hexane:methanol 75:18:1.2 (v/v/v); B: chloroform:hexane:methanol:glacial acetic acid 75:18:1.2:0.6 (v/v/v/v) and the bands were visualized with DPA-aniline spray. Lane 1 is FMC-7; lane 2, FMC-6; lane 3, FMC-5; and lane 4, a mixture of FMC-5, -6, and -7. Abbreviations: DPA, diphenylamine-aniline; FMC, fast migrating cerebroside.
Mass spectrometric analysis of FMC-5, FMC-6, and FMC-7
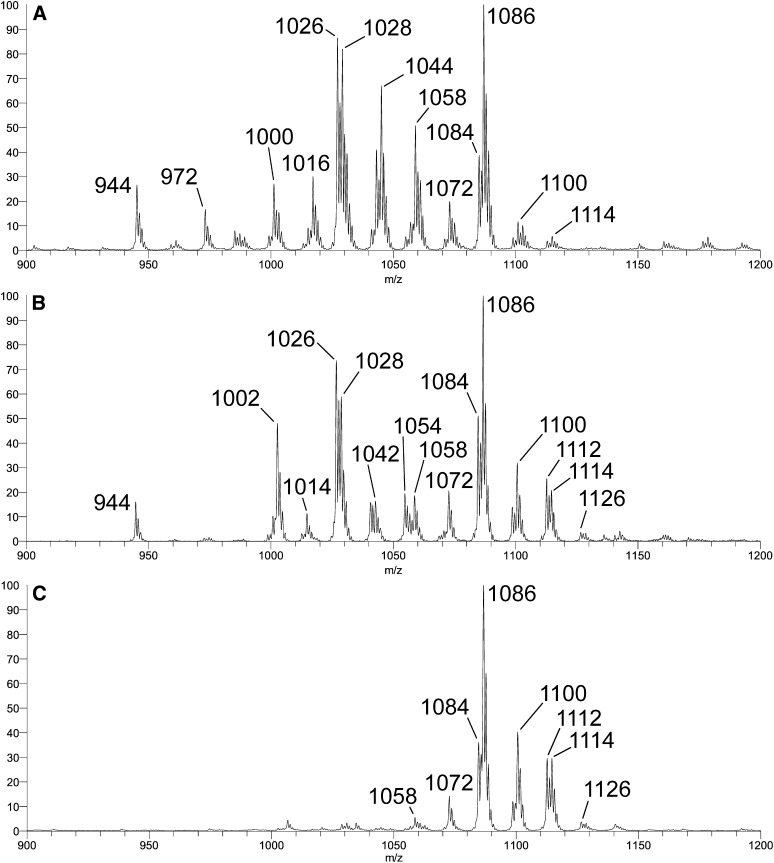
Penta- and hexa-acetyl-cerebrosides of brain were obtained and purified as described above. In Fig. 3, +ESI-LTQ-MS1 molecular ion profiles (as [M + Li]+ adducts) of three FMC fractions are compared. The first was prepared from rat brain (Fig. 3A, previously designated FMC-5/-6/-7) (2); the second was prepared by chemical acetylation of crude GalCer from bovine brain (Fig. 3B); and the third consisted of purified FMC-7 from rat brain (Fig. 3C). The first two profiles clearly differ: a number of molecular components present in Fig. 3A are of much lower abundance or missing from Fig. 3B (e.g., adducts at nominal, monoisotopic m/z 972, 1000, 1016, 1044, 1058). The profile in Fig. 3A is essentially identical to that previously acquired for the rat brain FMC-5/-6/-7 fraction on a different instrument (+ESI-Q/oa-TOF-MS) (20). It was proposed and essentially confirmed that at least three classes of FMC were present in this fraction, all characterized by acetylation of O-3 of the (d18:1) sphingoid moiety and complete O-acetylation of the Gal residue, while differing in functionalization at C-2 of the ceramide fatty-N-acyl moiety (e.g., m/z 944, 972, 1000, 1026, and 1028 representing “Type 2” lipoforms with nonhydroxy fatty acids [18:0, 20:0, 22:0, 24:1, and 24:0, respectively, designated “FMC-5”]; and m/z 1016 and 1044 representing “Type 1” lipoforms with 2-hydroxy fatty acids [h22:0 and h24:0, respectively, designated “FMC-6”]). It was considered likely that the molecular adducts at m/z 1058 and 1086 represent analogous h22:0 and h24:0 Type 1 lipoforms with O-acetylated 2-hydroxy fatty acids (designated “FMC-7”), but as the MS/MS spectra for these components were more complex and difficult to interpret, confirmation of this essential detail was deferred until more extensive analysis could be performed.
Fig. 3.
+ESI-LTQ-MS1 (ThermoFisher LTQ) molecular adduct profiles acquired for [M + Li]+ adducts of A: rat brain FMC-5, -6, -7; B: chemically acetylated mixed GalCer fraction from bovine brain; and C: chromatographically purified rat brain FMC-7. Abbreviations: ESI-LTQ-MS, electrospray ionization-linear ion trap-mass spectrometry; FMC, fast migrating cerebroside; GalCer, galactosylceramide.
Interestingly, the components apparently missing in the profile of Fig. 3B are those representing lipoforms with nonacetylated 2-hydroxy fatty-N-acylation. This is entirely consistent with the artificial nature of the preparation. Under the conditions of the in vitro per-O-acetylation performed, one would not expect to observe significant products with only the 2-hydroxy group of the Type 1 GalCer components left underivatized because this is a feature found only in the natural material. This assessment was confirmed by subsequent analysis of subfractions isolated from the chemically acetylated material by column chromatography (Fig. 4). A detailed mass spectrometric investigation of FMC-7 was carried out chiefly because it was of particular immunological interest as it had been less well characterized than FMC-5 and FMC-6 (2). For this reason, the MSn multistage fragmentation analysis (see supplementary data) focused mainly on characterizing molecular components of the chemically synthesized FMC-7 fraction and included only cursory examination of the other fractions. Note that subsequent MSn fragmentation analysis of the corresponding molecular components of the FMC-7 fraction purified from rat brain (Fig. 3C) produced essentially identical spectra at every stage.
Fig. 4.
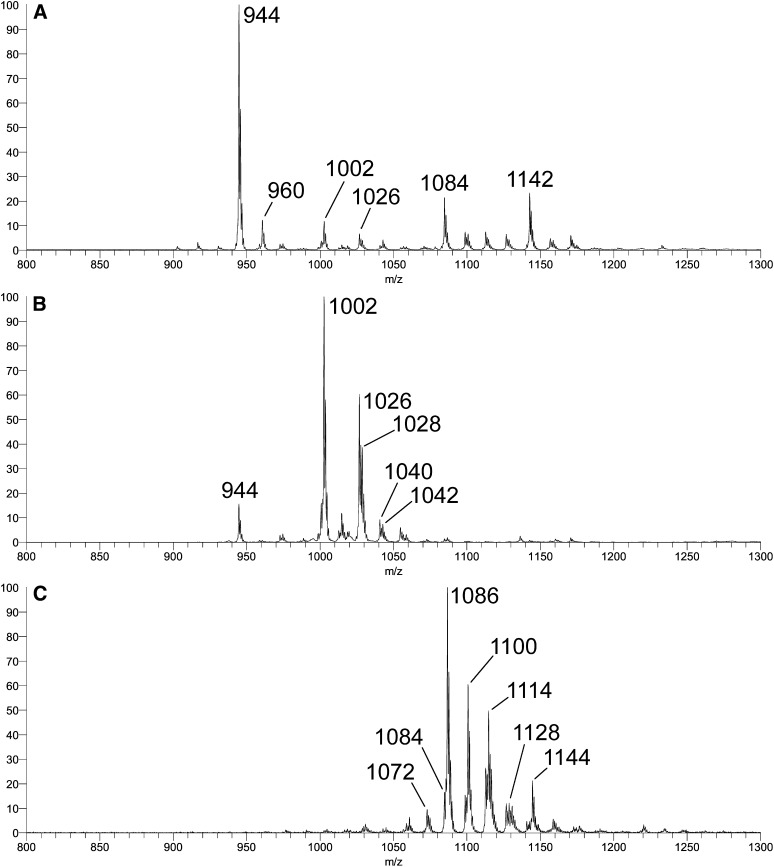
+ESI-LTQ-MS1 (ThermoFisher LTQ) [M + Li]+ adduct profiles acquired for three fractions (Fr.) isolated by silicic acid chromatography from the same chemically acetylated bovine brain GalCer that yielded the profile in Figure 3B. A: Fr. 225-246 (FMC-5); B: Fr. 168-186; and C: Fr. 56-86 (FMC-7). Abbreviations: ESI-LTQ-MS, electrospray ionization-linear ion trap-mass spectrometry; FMC, fast migrating cerebroside; GalCer, galactosylceramide.
As shown in Fig. 4, the three subfractions isolated from per-O-acetylated bovine brain GalCer, via differences in their chromatographic behavior, yielded very different molecular ion profiles upon +ESI-LTQ-MS1 analysis. Multistage MSn fragmentation analysis of individual species observed in the MS1 profiles (see supplementary data) yielded results consistent with the following conclusions: (i) the major species at m/z 944 in panel A is a d18:1/18:0 FMC-5 lipoform; (ii) the major species at m/z 1002 in panel B corresponds to a d18:0/h18:0 lipoform of FMC-7 (i.e., with an O-acetylated fatty-N-acyl 2-hydroxy group), while those at m/z 1026 and 1028 correspond to d18:1/24:1 and d18:1/24:0 lipoforms of FMC-5, respectively; (iii) all of the significant species observed in panel C correspond to FMC-7 lipoforms; and (iv) FMC-6 lipoforms are virtually absent from all three profiles.
The MSn results clearly confirmed the nature of the FMC-7 components, regardless of origin. Thus, essentially identical spectra were produced at every stage from the corresponding molecular precursors in the profile of the mixture of FMC-5/-6/-7 from rat brain (not shown), as well as in the profile of FMC-7 purified from rat brain (not shown, but discussed in the supplementary data). Interestingly, lipoforms with odd-numbered fatty acids were observed at carbon numbers above C24 (e.g., precursors at m/z > 1086 were observed in profiles of both purified FMC-7 fractions shown in Figs. 3C and 4C).
One- and two-dimensional 1H- and 13C-NMR spectroscopy of FMC-7 purified from rat brain
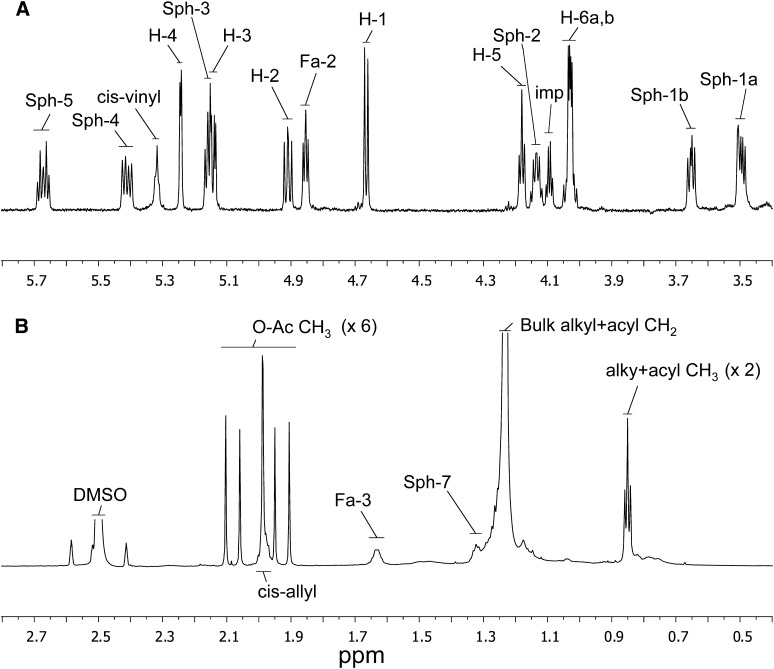
For chemical shift and connectivity assignment of all proton and carbon resonances in FMC-7, high resolution 1-D 1H; 2-D 1H-1H gradient correlation spectroscopy (gCOSY) and total correlation spectroscopy (TOCSY); and 1H-detected, 13C-1H gradient-selected heteronuclear single quantum correlation (gsHSQC) NMR spectra were acquired at 800 MHz in pure DMSO-d6. (Key sections of the 2-D gCOSY, TOCSY, and gsHSQC spectra are shown in supplementary Figs. III, IV and V, respectively.) Spectra were further interpreted by comparison with data acquired previously under similar conditions from standard GalCer preparations from bovine brain having either 2-hydroxy- or nonhydroxy-fatty-N-acylation (GalCer types I and II, respectively) (1). Chemical shift assignments for FMC-7 are summarized in Table 1. The 1H NMR spectrum of FMC-7 (Fig. 5 and Table 1) clearly exhibits all connectivity and coupling features expected for a β-galactopyranosylceramide having 2-hydroxy-fatty-N-acylation (1, 22) (both the Dasgupta and Dabrowski studies specified spectral acquisition at somewhat higher temperatures), but with a number of major differences consistent with modification by per-O-acetylation: (i) a 7-proton, six-carbon spin system with a set of consecutive 3Ji,j proton couplings whose relative magnitudes (large, large, small, small) define a β-galactopyranosyl ring (23); (ii) spin systems consistent with sphing-4-enine and 2-hydroxy-fatty-N-acyl chains of ceramide, including a downfield HN- resonance with 3-bond J-coupling to sphing-4-enine H-2, and very downfield shifted fatty-N-acyl H-2 and C-2 (4.854 and 73.56 ppm, versus 2.024 and 35.85 ppm, respectively, in type II GalCer (but these are also further downfield than observed in type I GalCer, as elaborated below) consistent with 2-hydroxylation of the fatty acid; (iii) the total absence of the exchangeable 1H resonances expected from HO- of a hexose residue, or from sphing-4-enine and 2-hydroxy-fatty-N-acyl chains; (iv) the presence of six 3-proton singlets in the CH3-(-C = O)O- region between 2.10 and 1.90 ppm (correlating with six 13C-resonances between 20.5 and 21.5 ppm), the precise number required for complete O-acetylation of a type I GalCer; and (v) significant shift changes for essentially every 1H and 13C resonance near an acetylatable hydroxyl function in type 1 GalCer.
TABLE 1.
Chemical shift assignments for FMC-7
| 1H (ppm) | J (Hz) | 13C (ppm) | |
|---|---|---|---|
| Hex- | |||
| 1 | 4.665 | 3J1,2 = 8.0 | 100.07 |
| 2 | 4.910 | 3J2,3 = 10.5 | 68.91 |
| 3 | 5.143 | 3J3,4 = 3.6 | 70.66 |
| 4 | 5.244 | 3J4,5 < 1.5 | 67.70 |
| 5 | 4.181 | 3J5,6a = 3J5,6b = 6.5 | 70.28 |
| 6a,b | 4.01–4.05a | 3J6a,6b NFO | 61.57 |
| Sph- | |||
| 1a,b | 3.495, 3.653 | 3J1a,1b = –10.5 | 67.87 |
| 2 | 4.135 | 3J1a,2 = 5.8; 3J1b,2 = 6.8 | 50.51 |
| HN | 7.722 | 3JHN,2 = 7.7 | — |
| 3 | 5.159 | 3J2,3 = 6.3 | 73.36 |
| 4 | 5.411 | 3J3,4 =7.5 | 124.76 |
| 5 | 5.671 | 3J4,5 = 15.4 | 136.09 |
| 6 | 1.987 | 3J5,6 = 6.7 | 32.06 |
| 7 | 1.321 | — | ND |
| Bulk CH2 | 1.230 | — | 29.40 |
| Terminal CH3 | 0.851 | — | 14.45 |
| Fa- | |||
| 2 | 4.854 | — | 73.56 |
| 3 | 1.628 | — | 31.81 |
| cis-vinyl | 5.317 | — | 130.12 |
| cis-allyl | 1.973 | — | 27.06 |
| Bulk CH2 | 1.230 | — | 29.40 |
| Terminal CH3 | 0.851 | — | 14.45 |
| Ac- | 2.103, 2.059, 1.989, 1.987, 1.950, 1.905 | 20.50–21.50 |
1H and 13C chemical shifts (ppm) of hexose, sphingosine, fatty acyl, and acetyl protons of FMC-7 in 100% DMSO-d6 at 27°C. Chemical shift standard, TMS (0.000 ppm). Abbreviations: Ac, acetyl; Fa, fatty acyl; FMC, fast migrating cerebroside; Hex, hexose; ND, not determined. NFO, nonfirst-order coupled; Sph, sphingosine
2H, mutually NFO.
Fig. 5.
One-dimensional proton NMR spectrum of FMC-7 in pure DMSO-d6 at 27°C. A: Downfield region (5.8–3.4 ppm). B: Upfield region (2.8–0.4 ppm). “Sph” and “Fa” refer to protons of the sphingosine and fatty-N-acyl chains, respectively; “cis” refers to vinyl and allyl protons of unsaturated fatty-N-acyl groups; “H” refers to protons of the ring and exocyclic hydroxymethyl groups of the hexose residue; “imp” refers to a signal from a uncharacterized but commonly occurring impurity; “DMSO” refers to the residual proton signal from the deuterated solvent which characteristically appears at 2.50 ppm. Abbreviations: FMC, fast migrating cerebroside; GalCer, galactosylceramide; NMR, nuclear magnetic resonance spectroscopy.
Some of the shift changes are predictable, based on general principles and a comparison with the spectra of FMC-1 and FMC-2, which correspond to type II and type I GalCer, respectively, having 3-O-acetylation of sphing-4-enine O-3 (1). These include the downfield shift of Sph C-3 (a predictable α-effect relative to C-3 of unmodified GalCer), the upfield shift of Sph C-2 (a predictable β-effect), and the major changes induced in Sph C-4 and C-5, which in unmodified GalCer are fairly close in chemical shift (∼131–132 ppm), but move far apart upon 3-O-acetylation (to ∼125–126 and ∼135–136 ppm, respectively). As mentioned above, the fatty-N-acyl C-3 of FMC-7 exhibits a significant downfield shift (Δδ = ∼2.1 ppm) compared with that in type I GalCer, and Fa H-3 relatively more so (Δδ > 1 ppm on the 1H scale). With respect to the Hex residue, all 1H resonances are downfield relative to those in unmodified GalCer as expected, but interestingly, all of the ring 13C resonances shift upfield, with only C-6 experiencing a predictable downfield α-effect shift. The relative shielding β-effects outweighing positive α-effects among the ring carbons; however, the magnitudes of some of the shifts seem anomalously high for β-effects alone, such as the appearance of Gal H-1 at 100.07, rather than ∼104–105 ppm as observed in unmodified type I or II GalCer (and both FMC-1 and FMC-2), which may reflect a longer range shielding effect from the O-Ac at Hex 6-OH or at the Fa 2-OH group, or possibly a slight crowding- induced ring deformation. In any case, all of the data are consistent with the proposed structure of FMC-7, a completely acetylated type I GalCer.
Cross-reactivity of anti–FMC-7 antibody with MfGL-II mycoplasma glycolipid and E. coli J5 LPS
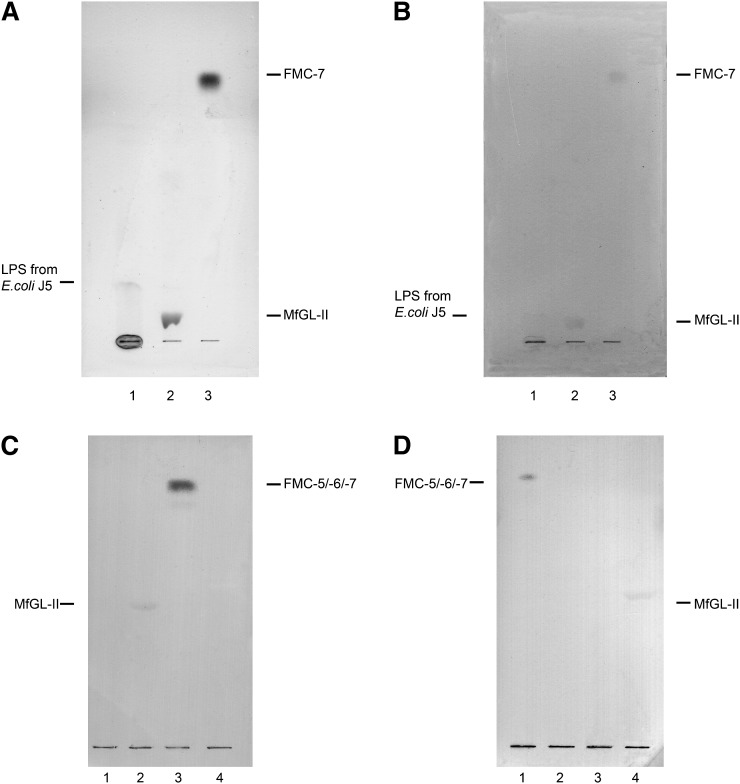
Antibody to FMC-7, the most hydrophobic compound of the series, was successfully raised in rabbits and characterized immunochemically. It bound to FMC-5, FMC-6, and FMC-7 examined individually but not to FMC-1, FMC-2, FMC-3, FMC-4 or other glycolipids, including gangliosides, phospholids, and sterols (not shown). Presumably the reactivity is to the tetra-acetyl-galactosyl moiety that is an epitope common to these three FMCs. Immuno-overlay staining indicated that the anti–FMC-7 antibody binds to purified mycoplasma glycolipid MfGL-II (Fig. 6A), indicating cross-reactivity between myelin and microbial glycolipids and suggesting that there are conformational resemblances between these lipids underlying the observed cross-reactivity. These results echoed those obtained with our first anti-FMC prepared against a mixture of FMC-5/-6/-7 (11) that reacted with the penta- and hexa-acetyl-GalCers and MfGL-II, but not with FMC-1, GalCer, or Chlamydia pneumoniae EBs (Fig. 6C). We also screened anti–FMC-7 antibody reactivity with LPS from another microbe (E. coli, J5 strain) and found cross-reactivity (Fig. 6A), although no binding was observed upon examining a dozen other purified LPS samples from several strains of Escherichia coli, Chlamydia pneumoniae, Campylobacter jejuni and Helicobacter pylori (results not shown).
Fig. 6.
Immuno-TLC of A: anti-FMC-7 antibody with LPS from E. coli J5 strain (lane 1), glycoglycerolipid MfGL-II from Mycoplasma fermentans (lane 2), and FMC-7 (lane 3); B: MS CSF IgG with: LPS from E. coli J5 strain (lane 1), glycoglycerolipid MfGL-II from Mycoplasma fermentans (lane 2), and FMC-7 (lane 3); C: anti-FMC-5/-6/-7 antibody with: EBs Chlamydia pneumoniae (lane 1), MfGL-II (lane 2), mixture FMC-5/-6/-7 (lane 3), and GalCer (lane 4); D: MS CSF IgG with FMC-5/-6/-7 mixture (lane 1), FMC-1 (lane 2), EBs Chlamydia pneumoniae (lane 3), and MfGL-II (lane 4). The bands were resolved on the plate using for 6A and 6B→ n-propanol:methanol:water (50:30:20; v/v/v) and for 6C and 6D→ n-propanol:methanol:water (60:20:15; v/v/v), respectively, and then visualized with DAB staining. Abbreviations: CSF, cerebrospinal fluid; FMC, fast migrating cerebroside; GalCer, galactosylceramide; LPS, lipopolisaccharide; MS, multiple sclerosis.
Because the other E. coli LPSs did not react and E. coli J5 is a rough mutant “aberrant” strain, we think that the reactivity observed is not natural but artifactual; particularly as reactivity was not observed with LPSs from other microbes. We interpret that the reactivity with J5 implicates the GL lipid moiety and probably involves the fatty acid.
Reactivity of immunoglobulins in MS CSF with FMC-7, mycoplasma MfGL-II glycolipid, and E. coli J5 LPS
Cross-reactivity has been observed by immuno-TLC on plates bearing i) purified FMC-7; ii) purified MfGL-II glycolipid of M. fermentans; and iii) LPS from E. coli J5, when MS CSF as the primary antibody was used (Fig. 6B). These results confirmed our initial observation of immunoreactivity of MS CSF IgG with the FMC-5/-6/-7 mixture as well as MfGL-II (Fig. 6D). The observation is intriguing, but specificity has not yet been rigorously established because of the limited amount of purified MfGL-II available.
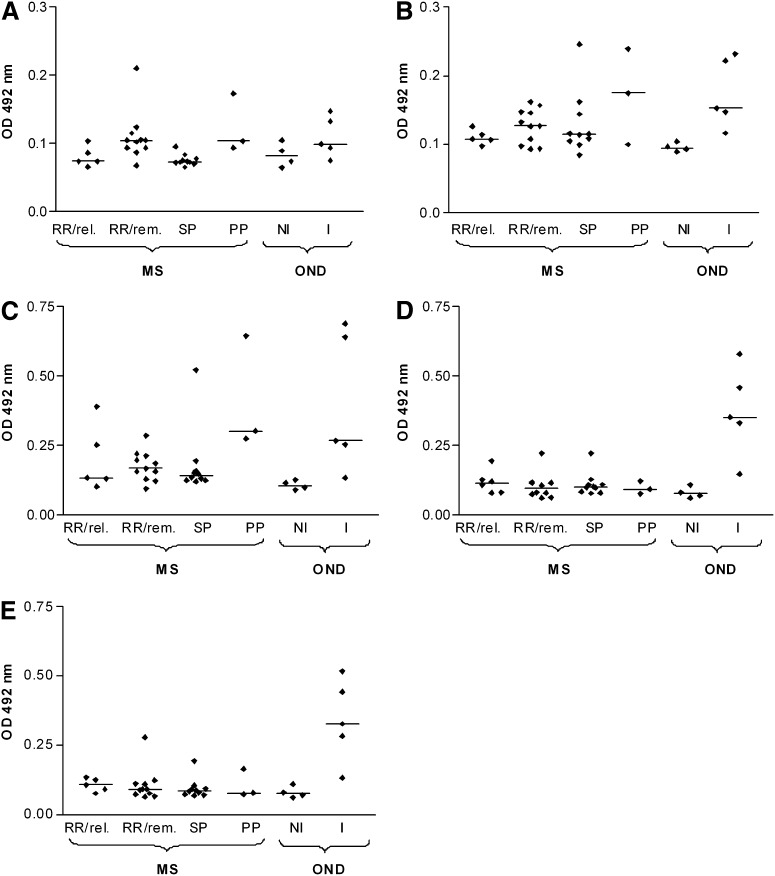
Screening MS CSF specimens by ELISA and statistical analysis
ELISA was used to quantify CSF IgG antibodies against endogenous myelin glycolipid antigens (FMC-7, GalCer, sulfatide), and exogenous complex glycolipid antigens (MfGL-II and E. coli J5 LPS). The extent of reactivity is shown in Fig. 7. Differences in reactivity of immunoglobulins in MS CSF to the glycolipids were observed in some patients. Analysis of the available patient samples was statistically significant for I-OND compared with NI-OND. The range of reactivities observed with some of the MS samples binding to FMC-7, GalCer, sulfatide, MfGL-II, and LPS E. coli J5 suggests that the reactivity is relevant to pathogenesis in some of the MS patients, an interpretation consistent with the recognized heterogeneity of both pathology and clinical features of MS. It is apparent in Fig. 7 that in comparison to the noninflammatory or NI-OND group (the best surrogate for normal) anti-GL (FMC-7, GalCer, sulfatide) titers are increased in some MS patients. We note that the MS CSF samples examined by immuno-TLC (Fig. 6) were from two patients with earlier disease (RRMS-in-remission subtype) and had reactivity greater than the mean (i.e., OD for FMC-7 was 0.1045; for GalCer, 0.1475; for sulfatide, 0.214; for MfGL-II, 0.2225; for E. coli LPS, 0.278) by ELISA assay (Fig. 7). The noteworthy changes are (i) for individual patients, especially in the I-OND group, increased binding to one GL antigen was associated with increased binding to others; (ii) the greatest reactivity was for microbial lipid antigens in the I-OND samples from patients with acute, dramatic bacterial, or viral inflammatory brain diseases; whereas (iii) MS samples displayed lower affinity and reactivity with endogenous GL antigens. The latter is consistent with the less acute and less severe CNS inflammation observed in MS CNS compared with the inflammatory controls (I-OND) who were afflicted by florid CNS inflammatory diseases; meningitis, meningo-encephalitis, and SSPE.
Fig. 7.
XY scatter graphs of reactivity of antigens A: FMC-7, B: GalCer, C: sulfatide, D: MfGL-II, and E: LPS from E. coli J5 in MS patients. RRMS in relapse (n = 5); RRMS in remission (n = 11); SPMS (n = 10); PPMS (n = 3); controls NI-OND (n = 4); I-OND (n = 5). The patients with CNS disease (I-OND) had the following diagnoses: meningitis (3), meningo-encephalitis (1), and subacute sclerosing panencephalitis (SSPE) (1). The group of patients with noninflammatory or NI-OND encompasses neuropathy (3) and cerebellar degeneration (1). Horizontal bars indicate median values. Abbreviations: CNS, central nervous system; FMC, fast migrating cerebroside; GalCer, galactosylceramide; LPS, lipopolisaccharide; MS, multiple sclerosis; OND, other neurological diseases; PPMS, primary progressive MS; RRMS, relapsing remitting MS; SPMS, secondary progressive MS.
ANOVA and Tukey's multiple comparison were used to compare individual groups. For median responses of the six subject groups compared by ANOVA, P < 0.05 was considered significant. Pairs of subject groups were compared using Tukey's test, and there were significant overall group effects for FMC-7, GalCer, sulfatide, LPS from E. coli J5, and MfGL-II, consistent with the notion that glycolipids are relevant antigens for the recognized increased intrathecal immunoglobulin reactivity in MS. For FMC-7, a significantly higher level was found for PPMS compared with the SPMS group (P < 0.05) and for the RRMS-in-remission group compared with SPMS (P < 0.05). These differences may reflect different autoantibody signatures for MS diagnostic subsets as recently proposed by Quintana et al. (24). They found, for example, in examining serum antibodies in MS that RRMS patients (but not SPMS or PPMS patients) had marked reactivity with heat shock proteins, a greater inflammatory response than observed for subtypes associated by temporal linkage to a point in the clinical course or other MS feature with degenerative characteristics. The SPMS median in our study was significantly decreased compared with the I-OND control (P < 0.05), another difference in keeping with a “degenerative” pathogenesis of SPMS contrasting with the inflammatory nature of I-OND. For GalCer, there were no significant differences between the MS subtypes; however, the median of the I-OND patients was significantly higher than that of the NI-OND controls (P < 0.05). For sulfatide, there were significant differences between the PPMS group and the NI-OND control (P < 0.05), and between the I-OND group and NI-OND control (P < 0.05). Again, the differences are consistent with an inflammatory pathology in the I-OND and PPMS subgroups. Screening against exogenous microbial antigens (MfGL-II and E. coli J5 LPS) revealed that antibodies' reactivity with these antigens was significantly less for SPMS compared with I-OND (P < 0.05), and for RRMS-in-remission compared with I-OND (P < 0.05). Elevated anti–MfGL-II and anti-LPS from E. coli J5 reactivity was found in meningitis (three patients), subacute sclerosing panenecephalitis (SSPE), and meningo-encephalitis (1) in the I-OND group versus NI-OND control (P < 0.05)—all in keeping with the highly inflammatory nature of the I-OND samples.
The percentage of individuals in each group with a specific absorbance greater than 0.10 OD was determined and compared by Fisher's exact test. The P values indicated a trend to group difference for FMC-7 (P = 0.0185) and LPS from E. coli J5 (P = 0.0546). There was no significant group difference for MfGL-II (P = 0.1655), GalCer (P = 0.2429), or sulfatide (P = 0.4026). For FMC-7, there were significant differences between PPMS and SPMS (66.67% versus 0%, P = 0.0385) and between RRMS-in-remission and SPMS (63.64% versus 0%, P = 0.0039). For LPS from E. coli J5, the percentage of subjects with an increased response in the I-OND control was significantly higher than in NI-OND (100% versus 25%, P = 0.0476). Moreover I-OND showed a significant difference compared with RRMS-in-remission (100% versus 36.36%, P = 0.0337) and with SPMS (100% versus 20%, P = 0.007). Antibodies from MS CSF samples with elevated activity by ELISA also reacted on immuno-TLC, though the staining was weak (Fig. 6B, D).
DISCUSSION
Altogether, we have now characterized seven novel acetyl derivatives of GalCer (designated as FMCs) in vertebrate brain (see Fig. 1 for structures). They comprise a novel series that all have a unique 3-O-acetylation on the sphingosine long chain-base. Analysis of the chemical composition and primary structures of these compounds has been completed. Four structures of this series (FMC-1, FMC-2, FMC-3, and FMC-4) were previously characterized (1, 2), and in our previous analysis of rat brain FMCs (2), the low-polarity components initially found in a single TLC band designated as FMC-5 (now called “FMC-5” or a mixture) were resolved by modification of two different solvent systems into three components: FMC-5, FMC-6, and FMC-7 (Fig. 2). FMC-5 and FMC-6 were reliably determined to be 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer with nonhydroxy and 2-hydroxy-fatty N-acyl ceramide types, respectively. FMC-7 was proposed to consist of derivatives of FMC-6 having an additional O-acetylation on the 2-hydroxyl of the fatty-N-acyl group. In the current work, the nature of the FMC-7 poly-acetylated components has been confirmed by multistage MSn showing that both chemically and naturally acetylated species of the same molecular mass gave identical spectra at every stage of analysis. In addition, we have confirmed the structure of FMC-7 from rat brain by NMR (Fig. 5, supplementary Figs. III, IV, V, and supplementary Table I).
FMCs are myelin constituents, enriched in the CNS and peripheral nervous system (PNS), and concentrated in spinal cord and white matter that are largely composed of myelinated nerve fibers (1). Their developmental appearance in brain parallels that of GalCer, and their enrichment in purified myelin of both CNS and PNS indicate that these glycosphingolipids play a role in myelin structure, and undoubtedly have biological relevance. They might, for example, regulate the final phases of myelin compaction to achieve the metabolic stability that enables it to function as an insulator for nerve fiber impulse conduction. Because of their greater hydrophobicity than the other myelin glycosphingolipids (cerebroside, sulfatide and GM4, and GM1 ganglioside), they may play key roles during critical stages of myelinogenesis. By reducing the polarization of the saccharide domain on the glycolipid, the masking of hydroxyls by acetylation is noteworthy, for it not only mitigates the amphipathic glycolipid nature but also presents an extensive molecular surface differing chemically from other CNS glycolipids and predicting different antigenicity. Their precise location and role in myelin remains to be determined, and the pathways of FMC biosynthesis, degradation, and specific deacetylation have not yet been established (1).
In the first assessment of the myelin acetyl-cerebrosides, we made specific rabbit polyclonal antibody against the acetyl-cerebrosides of highest Rf (11), the most hydrophobic derivatives (mixture of FMC-5/-6/-7), and observed binding (Fig. 6C) to the purified glycoglycerophosphocholine-containing lipid MfGL-II (19) from M. fermentans. This cross-reactivity was confirmed (Fig. 6A) with purified and structurally characterized FMC-7 and may be relevant to an immune triggering of myelin-targeted inflammatory demyelination by the mycoplasma glycolipid. We note, however, that to this point it has not been possible to examine fully affinity and inhibition because purified MfGL-II is not currently available. No cross-reactivity was observed with FMC-1 through FMC-4, other phospholipids and sphingolipids, preparations of chlamydia elementary bodies, or 12 purified LPS samples, though we did find a positive reaction with LPS purified from the E. coli J5 rough mutant “aberrant” strain, a binding believed to reflect binding to a surface-exposed acyl group on J5. An alternative approach examined cross-reactivity to lipid antigens employing the enigmatic-as-to-function antibodies found in CSF of ∼95% of patients with MS. By immuno-TLC overlay, FMC-7, the MfGL-II, and E. coli J5 LPS reacted with IgG of MS cerebrospinal fluid (used as primary antibody) (Fig. 6B). These glycolipid antigens, FMC-7, MfGL-II, and LPS from E. coli J5 strain, bind to immunoglobulins, largely IgG1 and a minor proportion of IgM. They were first reported by Tourtellotte and colleagues (25, 26) to be synthesized intrathecally in CNS. The immunological significance of these reactivities remains unclear and is discussed in greater detail in the with respect to relation of MS to infection and possible mechanisms (see supplementary data).
Seeking a biologically relevant correlation of glycolipids of known structure with clinical status (including inflammatory diseases and MS), we studied CSF by comparing I-ONDs that comprised acute meningitis, meningo–encephalitis and SSPE, NI-ONDs, and MS, including the main clinical subtypes. Analysis of the CSF samples examined yielded several differences by group comparison. Reactivities were greatest for the I-OND samples where there was an evident trend of binding to multiple lipid antigens in individual samples, and greater reactivity with GL antigens from M. fermentans and E. coli as well as myelin GL antigens. The changes were not sufficient to establish unequivocally a significant role for the acetylated cerebrosides as cross-reacting antigens in MS, but suggestions of potentially important immune alterations were noted: (i) several MS CSF samples reacted with FMCs to a greater extent than to noninflammatory disease (NI-OND) controls, and (ii) reactivity was greater in primary progressive MS (PPMS). Thus, continuation of anti-GL screening in MS CSF (and in serum and CNS tissue) to obtain sufficient numbers to achieve the power to discern the relation of infections to the mechanisms of MS-immune disturbance is warranted. Another indication of immune dysregulation involving myelin glycolipids in MS is our observation of hyporesponsiveness (anergy) of peripheral blood lymphocytes to FMC-7 (O'Keeffe, Hogan, unpublished observation) as well as to αGalCer (27).
Molecular mimicry is a hypothesis linking infection with autoimmune disease that might provide insight into the role of infection in the mechanism and generation of auto-immune–damaging inflammatory attack in MS. That molecular mimicry may be implicated in MS pathogenesis has been buttressed by identification of sequence homologies between myelin protein genes and viral and bacterial nucleic acids (28, 29). Such homologies suggest an infectious trigger/immune response transition, which is supported by the relatively frequent occurrence of molecular mimic examples (28–31). The immuno-TLC indication of FMC and MfGL-II cross-reactivity (i.e., of myelin acetyl-cerebrosides and microbial lipids) suggests that lipid conformations could play a similar role in triggering auto-immune reactions in MS. Clinical and immunological aspects of this are considered further in the supplementary data.
Mycoplasma infection may induce demyelination in the central nervous system during the course of MS. Mycoplasmas, in particular M. pneumoniae, have been implicated as plausible candidates to trigger CNS inflammation by the occurrence of mycoplasma-associated neurological conditions. Mycoplasmas can cause both acute and postinfectious neurological disease, which further justifies exploring an association of mycoplasma with MS (see the supplementary data for additional clinical analysis, description, and references). Although detection of mycoplasma DNA in serum and CSF of MS and other diseases using sensitive nucleic-acid–based diagnostic techniques is difficult and has not been possible thus far (32), CNS infection has not yet been completely excluded. The cross-reactivity of myelin and mycoplasma lipid antigens raises the possibility of indirect or immune-mediated CNS involvement in MS, including endogenous cerebrosides of the 3-O-acetyl-sphingosine-GalCer series whose structure has now been characterized.
This report completes the characterization of a series of myelin glycolipids that are human immuno-reactive antigens. Our initial studies suggest that myelin O-acetyl-cerebrosides, infection, and neurological disease may be linked.
Supplementary Material
Acknowledgments
The authors thank Dr. A. Chiu and Dr. K. Krishnamurthy for assistance in handling and immunizing the rabbits. We gratefully acknowledge the use of the Bruker Avance 800 MHz spectrometer of the Danish Instrument Center for NMR Spectroscopy of Biological Macromolecules and the expert assistance of Dr. Bent Petersen for acquisition of all NMR spectra. We also thank Ms. Maribeth Johnson and Ms. Li Fang Zhang, Department of Biostatistics, Medical College of Georgia, for statistical consultations.
Footnotes
Abbreviations:
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- DPA
- diphenylamine-aniline
- EAE
- experimental autoimmune encephalitis
- ESI-LTQ-MS
- electrospray ionization-linear ion trap-mass spectrometry
- FMC
- fast migrating cerebroside
- GalCer
- galactosylceramide
- GC-MS
- gas chromatography mass spectrometry
- I-OND
- inflammatory other neurological diseases
- LPS
- lipopolisaccharide
- MS
- multiple sclerosis
- MS/CID-MS
- mass spectrometry/collision-induced dissociation-mass spectrometry
- MSn
- multistage ion trap mass spectrometry
- NI-OND
- non-inflammatory other neurological diseases
- NMR
- nuclear magnetic resonance spectroscopy
- OD
- optical density
- OND
- other neurological diseases
- PNS
- peripheral nervous system
- PPMS
- primary progressive MS
- RRMS
- relapsing remitting MS
- SPMS
- secondary progressive MS
- SSPE
- subacute sclerosing panencephalitis
This study was supported by Award RG3473 from the National Multiple Sclerosis Society (NY); by National Institutes of Health Grant NS-115666; by the Irish Health Research Board; and by the Copenhagen Center for Glycomics. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Dasgupta S., Levery S. B., Hogan E. L. 2002. 3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide. J. Lipid Res. 43: 751–761. [PubMed] [Google Scholar]
- 2.Bennion B., Dasgupta S., Hogan E. L., Levery S. B. 2007. Characterization of novel myelin components 3-O-acetyl-sphingosine galactosylceramides by electrospray ionization Q-TOF MS and MS/CID-MS of Li+ adducts. J. Mass Spectrom. 42: 598–620. [DOI] [PubMed] [Google Scholar]
- 3.Katzenellenbogen E., Kocharova N. A., Korzeniowska-Kowal A., Gamian A., Bogulska M., Szostko B., Shashkov A. S., Knirel Y. A. 2008. Immunochemical studies of the lipopolysaccharides of Hafnia alvei PCM 1219 and other strains with the O-antigens containing D-glucose 1-phosphate and 2-deoxy-2-[(R)-3-hydroxybutyramido]-D-glucose. Arch. Immunol. Ther. Exp. (Warsz.). 56: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parolis H., Parolis L. A., Olivieri G. 1997. Structural studies on the Shigella-like Escherichia coli O121 O-specific polysaccharide. Carbohydr. Res. 303: 319–325. [DOI] [PubMed] [Google Scholar]
- 5.Ali T., Weintraub A., Widmalm G. 2006. Structural determination of the O-antigenic polysaccharide from the Shiga toxin-producing Escherichia coli O171. Carbohydr. Res. 341: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 6.Sidorczyk Z., Toukach F. V., Zych K., Drzewiecka D., Arbatsky N. P., Shashkov A. S., Knirel Y. A. 2002. Structural and serological relatedness of the O-antigens of Proteus penneri 1 and 4 from a novel Proteus serogroup O72. European J. Biochem. 269: 358–363. [DOI] [PubMed] [Google Scholar]
- 7.Kubler-Kielb J., Vinogradov E., Chu C., Schneerson R. 2007. O-Acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr. Res. 342: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder N. W., Schombel U., Heine H., Gobel U. B., Zahringer U., Schumann R. R. 2003. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J. Biol. Chem. 278: 33645–33653. [DOI] [PubMed] [Google Scholar]
- 9.Yildirim H. H., Li J., Richards J. C., Hood D. W., Moxon E. R., Schweda E. K. 2005. Complex O-acetylation in non-typeable Haemophilus influenzae lipopolysaccharide: evidence for a novel site of O-acetylation. Carbohydr. Res. 340: 2598–2611. [DOI] [PubMed] [Google Scholar]
- 10.MacLean L. L., Webb A. C., Perry M. B. 2006. Structural elucidation of the O-antigenic polysaccharide from enterohemorrhagic (EHEC) Escherichia coli O48:H21. Carbohydr. Res. 341: 2543–2549. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta S., Bhat N. R., Spicer S. S., Hogan E. L., Furuya S., Hirabayashi Y. 2007. Cell-specific expression of neutral glycosphingolipids in vertebrate brain: immunochemical localization of 3-O-acetyl-sphingosine-series glycolipid(s) in myelin and oligodendrocytes. J. Neurosci. Res. 85: 2856–2862. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta S., Everhart M. B., Bhat N. R., Hogan E. L. 1997. Neutral monoglycosylceramides in rat brain: occurrence, molecular expression and developmental variation. Dev. Neurosci. 19: 152–161. [DOI] [PubMed] [Google Scholar]
- 13.Saito T., Hakomori S. I. 1971. Quantitative isolation of total glycosphingolipids from animal cells. J. Lipid Res. 12: 257–259. [PubMed] [Google Scholar]
- 14.Willker W., Leibfritz D., Kerssebaum R., Bermel W. 1993. Gradient selection in inverse heteronuclear correlation spectroscopy. Magn. Reson. Chem. 31: 287–292. [Google Scholar]
- 15.Kohriyama T., Ariga T., Yu R. K. 1988. Preparation and characterization of antibodies against a sulfated glucuronic acid-containing glycosphingolipid. J. Neurochem. 51: 869–877. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta S., Hogan E. L., Spicer S. S. 1996. Stage-specific expression of fuco-neolacto- (Lewis X) and ganglio- series neutral glycosphingolipids during brain development: characterization of Lewis X and related glycosphingolipids in bovine, human and rat brain. Glycoconj. J. 13: 367–375. [DOI] [PubMed] [Google Scholar]
- 17.Sanai Y., Ogawa J., Nagai Y. 1981. Enzyme-linked immunosorbent assay (ELISA) for detection of antibodies against glycosphingolipids and its application to patients with systemic lupus erythematosus. Jpn. J. Exp. Med. 51: 309–316. [PubMed] [Google Scholar]
- 18.Kusunoki S., Yu R. K., Kim J. H. 1988. Induction of experimental autoimmune encephalomyelitis in guinea pigs using myelin basic protein and myelin glycolipids. J. Neuroimmunol. 18: 303–314. [DOI] [PubMed] [Google Scholar]
- 19.Zahringer U., Wagner F., Rietschel E. T., Ben-Menachem G., Deutsch J., Rottem S. 1997. Primary structure of a new phosphocholine-containing glycoglycerolipid of Mycoplasma fermentans. J. Biol. Chem. 272: 26262–26270. [DOI] [PubMed] [Google Scholar]
- 20.Moran A. P., Rietschel E. T., Kosunen T. U., Zahringer U. 1991. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J. Bacteriol. 173: 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V. 1982. Detection of glycolipid ligands by direct binding of carbohydrate-binding proteins to thin-layer chromatograms. Methods Enzymol. 83: 235–241. [DOI] [PubMed] [Google Scholar]
- 22.Dabrowski J., Egge H., Hanfland P. 1980. High resolution nuclear magnetic resonance spectroscopy of glycosphingolipids. I: 360 MHz 1H and 90.5 MHz 13C NMR analysis of galactosylceramides. Chem. Phys. Lipids. 26: 187–196. [DOI] [PubMed] [Google Scholar]
- 23.Koerner T. A., Prestegard J. H., Yu R. K. 1987. Oligosaccharide structure by two-dimensional proton nuclear magnetic resonance spectroscopy. Methods Enzymol. 138: 38–59. [DOI] [PubMed] [Google Scholar]
- 24.Quintana F. J., Farez M. F., Viglietta V., Iglesias A. H., Merbl Y., Izquierdo G., Lucas M., Basso A. S., Khoury S. J., Lucchinetti C. F., et al. 2008. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 105: 18889–18894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tourtellotte W. W., Walsh M. J., Baumhefner R. W., Staugaitis S. M., Shapshak P. 1984. The current status of multiple sclerosis intra-blood-brain-barrier IgG synthesis. Ann. N. Y. Acad. Sci. 436: 52–67. [DOI] [PubMed] [Google Scholar]
- 26.Walsh M. J., Tourtellotte W. W. 1986. Temporal invariance and clonal uniformity of brain and cerebrospinal IgG, IgA, and IgM in multiple sclerosis. J. Exp. Med. 163: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Keeffe J., Gately C. M., Counihan T., Hennessy M., Leahy T., Moran A. P., Hogan E. L. 2008. T-cells expressing natural killer (NK) receptors are altered in multiple sclerosis and responses to alpha-galactosylceramide are impaired. J. Neurol. Sci. 275: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldstone M. B. 2005. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr. Top. Microbiol. Immunol. 296: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett L. A., Fujinami R. S. 1992. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. 6: 840–844. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone M. B. 1998. Molecular mimicry and immune-mediated diseases. FASEB J. 12: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Herrath M. G., Fujinami R. S., Whitton J. L. 2003. Microorganisms and autoimmunity: making the barren field fertile? Nat. Rev. Microbiol. 1: 151–157. [DOI] [PubMed] [Google Scholar]
- 32.Casserly G., Barry T., Tourtellotte W. W., Hogan E. L. 2007. Absence of Mycoplasma-specific DNA sequence in brain, blood and CSF of patients with multiple sclerosis (MS): a study by PCR and real-time PCR. J. Neurol. Sci. 253: 48–52. [DOI] [PubMed] [Google Scholar]
- 33.Hsu F. F., Bohrer A., Turk J. 1998. Electrospray ionization tandem mass spectrometric analysis of sulfatide. Determination of fragmentation patterns and characterization of molecular species expressed in brain and in pancreatic islets. Biochim. Biophys. Acta. 1392: 202–216. [DOI] [PubMed] [Google Scholar]
- 34.Muller-Loennies S., Holst O., Lindner B., Brade H. 1999. Isolation and structural analysis of phosphorylated oligosaccharides obtained from Escherichia coli J-5 lipopolysaccharide. European J. Biochem. 260: 235–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.