Abstract
TGR5 is a G protein-coupled receptor that is activated by bile acids, resulting in an increase in cAMP levels and the subsequent modulation of energy expenditure in brown adipose tissue and muscle. Therefore, the development of a TGR5-specific agonist could lead to the prevention and treatment of various metabolic disorders related to obesity. In the present study, we evaluated the ability of bile alcohols, which are structurally and physiologically similar to bile acids and are produced as the end products of cholesterol catabolism in evolutionarily primitive vertebrates, to act as TGR5 agonists. In a cell-based reporter assay and a cAMP production assay performed in vitro, most bile alcohols with a side chain containing hydroxyl group(s) were highly efficacious agonists for TGR5 comparable to its most potent ligand in the naturally occurring bile acid, lithocholic acid. However, the abilities of the bile alcohols to activate TGR5 varied with the position and number of the hydroxyl substituent in the side chain. Additionally, the conformation of the steroidal nucleus of bile alcohols is also important for its activity as a TGR5 agonist. Thus, we have provided new insights into the structure-activity relationships of bile alcohols as TGR5 agonists.
Keywords: TGR5, structure-activity relationship, FXR, drug development, metabolic syndrome
Bile acids are synthesized from cholesterol in mammalian liver and are excreted into the bile. Bile acids facilitate the formation of micelles, which promote the processing of dietary fat. Recently, bile acids have been shown to act as physiological ligands for farnesoid X receptor (FXR) (1–3). Through the activation of FXR, bile acids regulate the transcription of their own biosynthetic enzymes and of transporter proteins for bile acids. In humans, more than 99% of all bile acids are localized in tissues participating in enterohepatic circulation. However, some bile acids are also present in the systemic circulation at concentrations of 15 μM after a meal or at concentrations of 5 μM even between meals (4), whereas their concentrations are several ten- to several hundred-fold higher in the gallbladder, liver, and intestine (5). In addition, bile acids are known to exhibit immunosuppressive effects on cell-mediated immunity and macrophage functions (6–8). In view of these findings, bile acids might be expected to function not only in the limited tissues involved in enterohepatic circulation, but also in the whole body as signaling molecules.
Recently, a G-protein-coupled plasma membrane receptor responsive to bile acids was discovered using high-throughput screening (9, 10). This receptor, named TGR5, has been shown to stimulate adenylate cyclase, causing a subsequent increase in the intracellular production of cAMP. This signal transduction occurs independently of FXR. Also, the stimulation of TGR5 by bile acids further enhances energy expenditure in adipocytes and myocytes via these cell-specific signal transductions (11). Furthermore, TGR5 expression has been demonstrated in enteroendocrine cells, where bile acids stimulate the secretion of glucagon-like peptide-1 via TGR5 (12). These observations suggest that TGR5 might be an attractive target for the treatment of obesity, diabetes, and metabolic syndrome.
During the course of the development of new reagents with the ability to activate TGR5, a diverse variety of bile acids and chemically modified bile acid analogs were evaluated and their structure-activity relationships were studied in regard to TGR5 activation (13). In a previous study, TGR5 agonist activity and affinity were shown to be increased by neutral compounds obtained from the reduction of the side chain of bile acids. Therefore, we investigated the TGR5 agonist activity and affinity of bile alcohols, which are neutral polyhydroxylated sterols with the same steroid nucleus as bile acids and are abundantly present in nature.
In the present study, we demonstrated that TGR5 activation and cAMP production in response to the bile alcohols varied with the position and number of the hydroxyl groups in the steroid side chain. In addition, we showed that the conformation of the steroidal nucleus of bile alcohols is also important for their activities as TGR5 agonists. Furthermore, we report that the selectivity of bile alcohols toward TGR5 increased as the number of hydroxyl groups in the side chain increased. Thus, we have provided new insights into the structure-activity relationships of bile alcohols as TGR5 agonists.
MATERIALS AND METHODS
Bile acids and bile alcohols
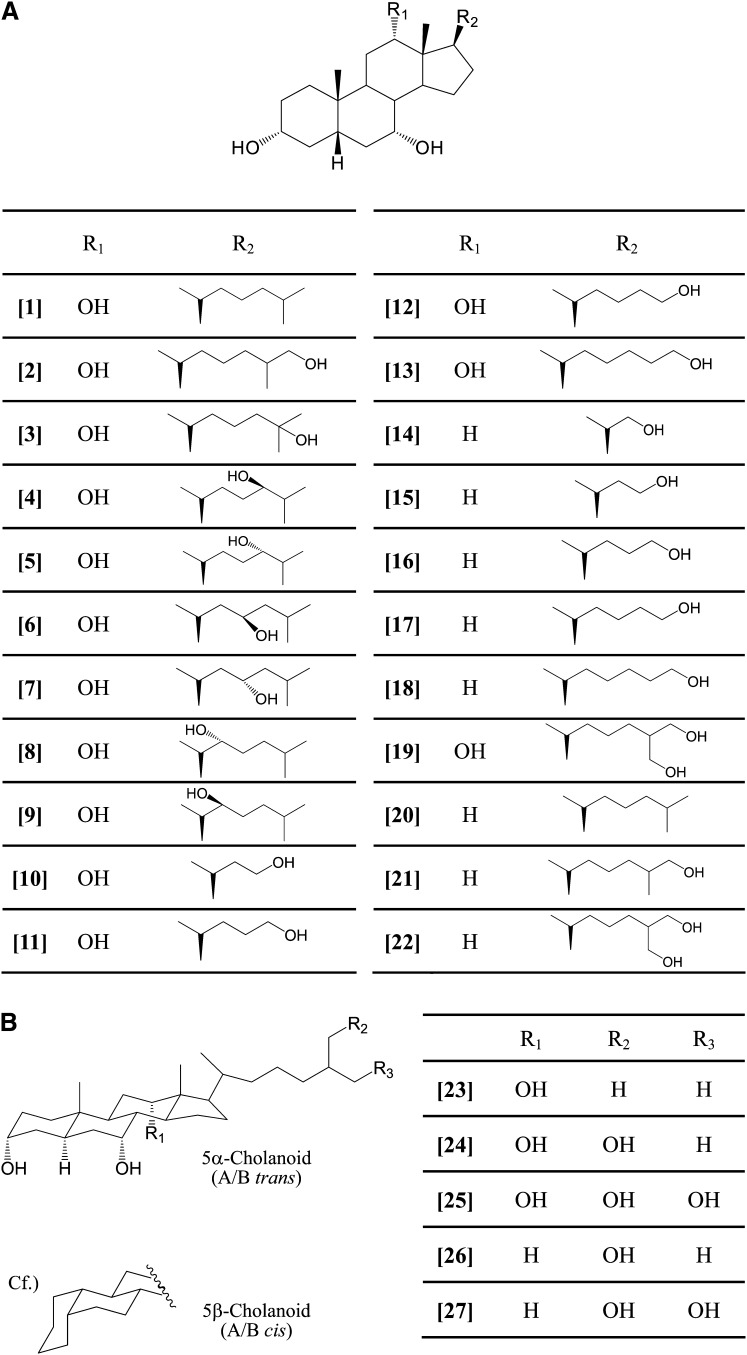
We obtained our general chemicals from Sigma-Aldrich (St. Louis, MO). Naturally occurring bile acids were purchased from Sigma-Aldrich or Steraloids (Newport, RI). 5β-Cholestane-3α,7α,12α-triol (THC) [1] and 5β-cholestane-3α,7α-diol [20] were synthesized as described previously (14). 5β-Cholestane-3α,7α,12α, 26-tetrol [2] (15), 5β-cholestane-3α,7α,12α,25-tetrol [3] (16), (24R)- and (24S)- 5β-cholestane-3α,7α,12α,24-tetrols [4 and 5] (17, 18), (23R)- and (23S)- 5β-cholestane-3α,7α,12α,23-tetrols [6 and 7] (19), (22R)- and (22S)-5β-cholestane-3α,7α,12α,22-tetrols [8 and 9] (19), 24-nor-5β-cholane-3α,7α,12α,23-tetrol [10] (20), 5β-cholane-3α,7α,12α,24-tetrol [11] (20), 26,27-dinor-5β-cholestane-3α,7α,12α,25-tetrol [12], and 27-nor-5β-cholestane-3α,7α,12α,26-tetrol [13] were synthesized from cholic acid, as described previously (21). 23,24-Dinor-5β-cholane-3α,7α,22-triol [14], 26,27-dinor-5β-cholestane-3α,7α,25-triol [17], and 27-nor-5β-cholestane-3α,7α,26-triol [18] were obtained by the reduction of 3α,7α-dihydroxy-23,24-dinor-5β-cholan-22-al, 3α,7α-dihydroxy-26,27-dinor-5β-cholestan-25-oic acid, and 3α,7α-dihydroxy-27-nor-5β-cholestan-26-oic acid using lithium aluminum hydride, respectively (22–24). 24-Nor-5β-cholane-3α,7α,23-triol [15] (20), 5β-cholane-3α,7α,24-triol [16] (25), 5β-cholestane-3α,7α,26-triol [21], and 5β-cholestane-3α,7α,26,27-tetrol [22] were synthesized from chenodeoxycholic acid (CDCA), as described previously (26, 27), and 5α- and 5β-cholestane-3α,7α,12α,26,27-pentols [25 and 19] and 5α-cholestane-3α,7α,12α,26-tetrol [24] were isolated from the bile of carp (28–30). 5α-Cholestane-3α,7α,12α-triol [23] and 5α-cholestane-3α,7α,26-triol [26] were synthesized from 5α-cholestane-3α,7α,12α,26-tetrol [24], as described previously (31–33). 5α-Cholestane-3α,7α,26,27-tetrol [27] was synthesized from 5α-cholestane-3α,7α,12α,26,27-pentol [25], as described previously (34).
Plasmids
The NIH Mammalian Gene Collection clone MGC: 40597 (also named pCMVSPORT6/hTGR5) was obtained from Invitrogen (Carlsbad, CA). cAMP response element (CRE)-driven luciferase reporter construct was obtained from SA Bioscience (Frederick, MD).
Transient transfections and reporter gene assays
The human embryonic kidney cell line 293T (HEK293T cells) was seeded at 50–60% confluence in 96-well plates and was maintained in DMEM containing 10% FBS for 24 h prior to transfection. For transient transfections, the cells were rinsed with PBS and were transfected with 12.5 ng/well of CRE-driven luciferase reporter constructs and 18.75 ng/well of human TGR5 expression plasmids (pCMVSPORT6/hTGR5) using Attractene Transfection Reagent (Qiagen, Chatsworth, CA) in OPTI-MEM I reduced-serum medium (Invitrogen). Six hours after transfection, a volume of DMEM containing 20% fetal calf serum and equivalent to the amount of transfection media that was used was added to the cells. After a further 18 h of incubation, the cells were washed once with PBS and were treated for 5 h with different concentrations of each compound in fresh DMEM containing 0.5% delipidated serum. After treatment, the cells were lysed and luciferase activity was determined using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. The firefly luciferase activity was normalized to the Renilla luciferase activity for each well.
Huh-7 cells were maintained in DMEM containing 10% FBS and were seeded onto 24-well plates at 24 h prior to transfection. The cells were transfected with 188 ng of pFXRE-tk-Luc (35), 63 ng each of the pcDNA3.1 expression vectors for human FXRα and RXRα, and 313 ng of the pSV-β-galactosidase vector (Promega, Madison, WI) using Attractene Transfection Reagent (Qiagen, Chatsworth, CA). Three hours after transfection, the cells were exposed to each compound for 20 h in a medium containing 0.5% delipidated FBS. The cells were then lysed and the luciferase activity level was determined. The firefly luciferase activity was normalized to the β-galactosidase activity for each well.
cAMP production analysis
HEK293T cells, in which the human TGR5 expression plasmid was transiently transfected using the above method, were washed with PBS and the medium was exchanged for cAMP assay medium [DMEM containing 0.1% (w/v) BSA and 0.5 mM 3-isobutyl-1-methylxanthine]. After incubation for 30 min at 37°C, the cells were treated with each compound in fresh cAMP assay medium for 30 min. After treatment, the cAMP amounts were determined using a cAMP screening kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. The Lowry protein assay was used to determine the total protein level (36).
50% Effective concentrations and efficacy determination
Assays were performed in triplicate for each condition. The EC50 values of each compound were calculated using a probit analysis. Efficacy was determined by calculating the percentages of the TGR5 agonist activity of 10 μM of lithocholic acid (LCA) and the FXR agonist activity of 10 μM of CDCA.
Statistical analysis
The statistical analysis was performed using the Student t-test; a value of P < 0.05 was considered statistically significant.
RESULTS
As depicted in Fig. 1, 27 compounds of naturally occurring and chemically modified bile alcohols were evaluated using a transient transfection assay for TGR5 activity. The HEK293T cells were cotransfected with CRE-driven luciferase reporter constructs and expression plasmids for human TGR5. LCA, which has the highest TGR5 agonist activity among the endogenous bile acids produced in humans, was used as a positive control. Because of their toxicity, the maximum concentration of bile alcohols examined in this study was 20 μM. The efficacy of each bile alcohol was expressed as the relative percentage of the TGR5 agonist activity of 10 μM of LCA. The affinity of bile alcohols to TGR5 was represented as the EC50 value.
Fig. 1.
Structures of bile alcohols. A: [1], 5β-cholestane-3α,7α,12α-triol (THC); [2], 5β-cholestane-3α,7α,12α,26-tetrol; [3], 5β-cholestane-3α,7α,12α,25-tetrol; [4], (24R)-5β-cholestane-3α,7α,12α,24-tetrol; [5], (24S)-5β-cholestane-3α,7α,12α,24-tetrol; [6], (23R)-5β-cholestane-3α,7α,12α,23-tetrol; [7], (23S)-5β-cholestane-3α,7α,12α,23-tetrol; [8], (22R)-5β-cholestane-3α,7α,12α,22-tetrol; [9], (22S)-5β-cholestane-3α,7α,12α,22-tetrol; [10], 24-nor-5β-cholane-3α,7α,12α,23-tetrol; [11], 5β-cholane-3α,7α,12α,24-tetrol; [12], 26,27-dinor-5β-cholestane-3α,7α,12α,25-tetrol; [13], 27-nor-5β-cholestane-3α,7α,12α,26-tetrol; [14], 23,24-dinor-5β-cholane-3α,7α,22-triol; [15], 24-nor-5β- cholane-3α,7α,23-triol; [16], 5β-cholane-3α,7α,24-triol; [17], 26,27-dinor-5β-cholestane-3α,7α,25-triol; [18], 27-nor-5β-cholestane-3α,7α,26-triol; [19], 5β-cholestane-3α,7α,12α,26,27-pentol; [20], 5β-cholestane-3α,7α-diol; [21], 5β-cholestane-3α,7α,26-triol; [22], 5β-cholestane-3α,7α,26,27-tetrol. B: [23], 5α-cholestane-3α,7α,12α-triol; [24], 5α-cholestane-3α,7α,12α,26-tetrol; [25], 5α-cholestane-3α,7α,12α,26,27-pentol; [26], 5α-cholestane-3α,7α,26-triol; [27], 5α-cholestane-3α,7α,26,27-tetrol. The compounds in the figure are indicated by the bracketed numbers.
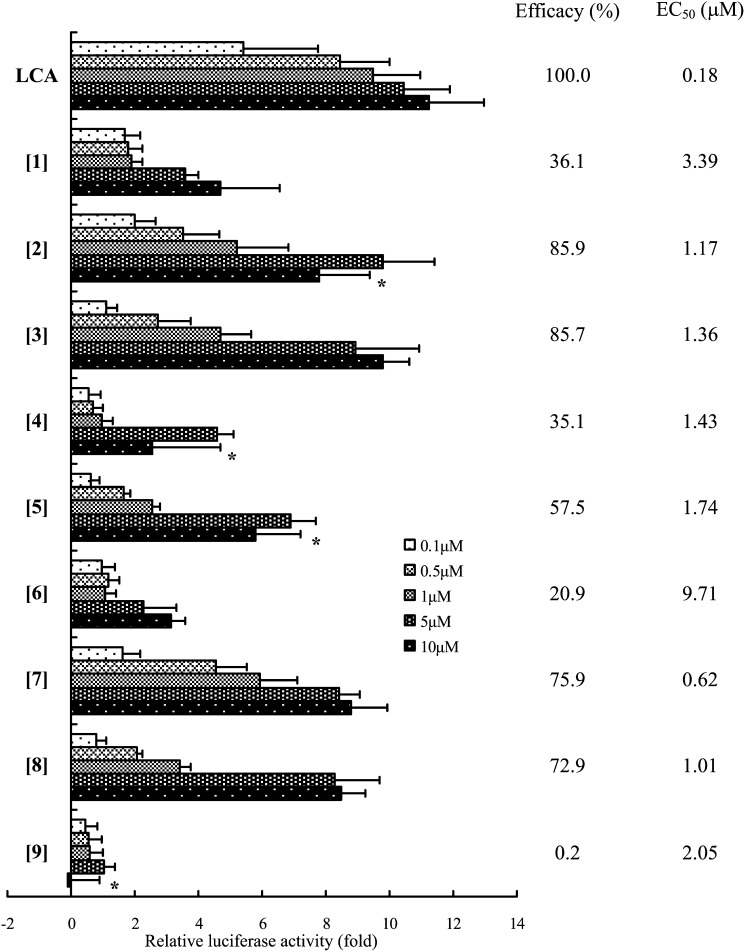
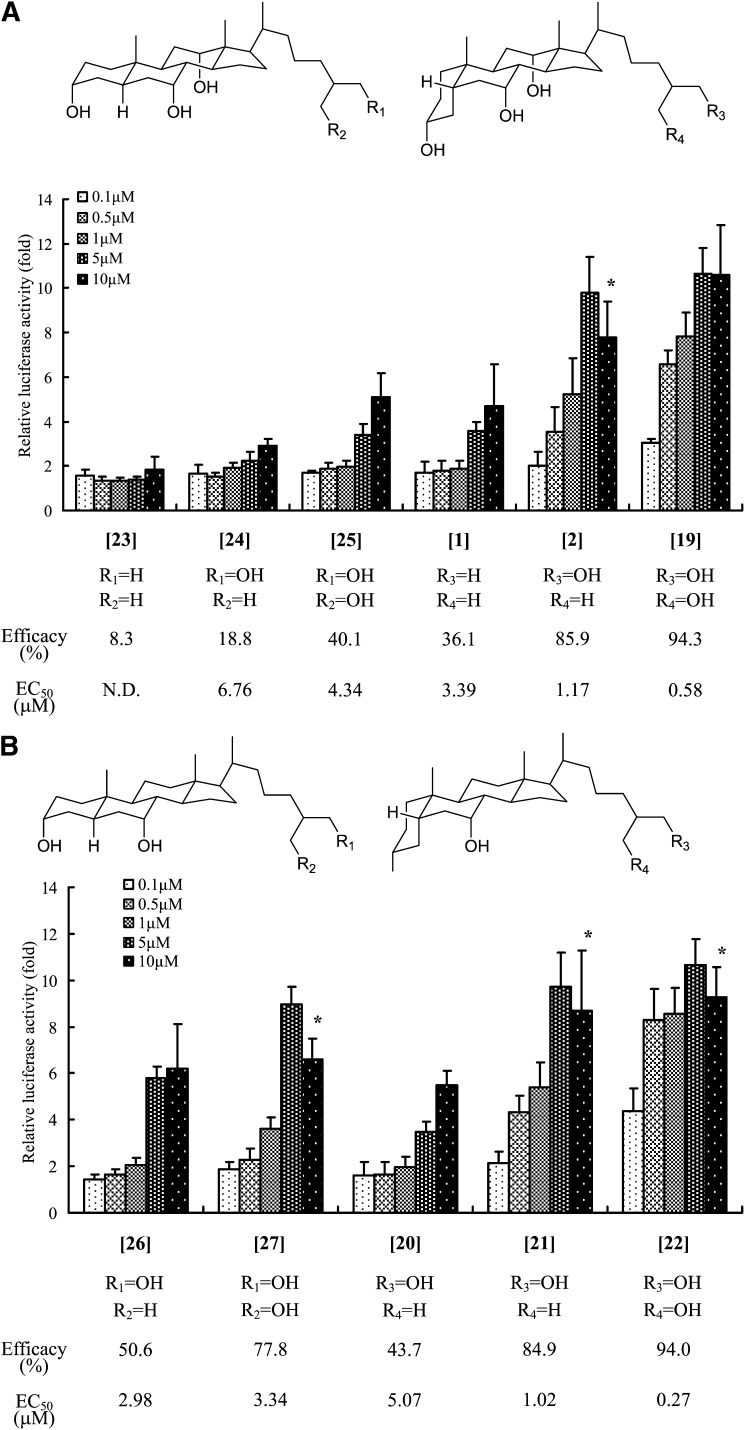
As shown in Fig. 2, THC [1], the side chain of which is the same as that of cholesterol, had a lower TGR5 activity (efficacy = 36.1%) and affinity (EC50 = 3.39 μM) than LCA (efficacy = 100%, EC50 = 0.18 μM). Next, we evaluated whether a series of bile alcohols [2-9] possessing one hydroxyl group at C-22 - C-26 in the side chain of THC were potent TGR5 agonists. The exposure of the cells to 5β-cholestane-3α,7α,12α,26-tetrol [2] or 5β-cholestane-3α,7α,12α,25-tetrol [3] led to the induction of luciferase activity at levels comparable to that of LCA (Fig. 2). Bile alcohols possessing a hydroxyl group at C-22, C-23, or C-24 each have two stereoisomers, and the activity of each isomer differed significantly (Fig. 2). In particular, the 23(R)-isomer [6] and the 22(S)-isomer [9] exhibited minimal TGR5 agonist activity.
Fig. 2.
Effect of the presence of a hydroxyl group at different positions in the side chain of THC on TGR5 activation in a cellular transactivation assay. Hek293T cells were transfected with CRE-luciferase reporter constructs and a human TGR5 expression plasmid. The cells were exposed to the vehicle alone or 0.1–10 μM of the indicated bile alcohols. Firefly luciferase activity in the cell extract was normalized to Renilla luciferase activity and expressed as the fold induction relative to vehicle-exposed cells. The values are the means ± SD of three experiments. The compounds in the figure are indicated by the bracketed numbers. *: The parameter was not used for the calculation of EC50 value.
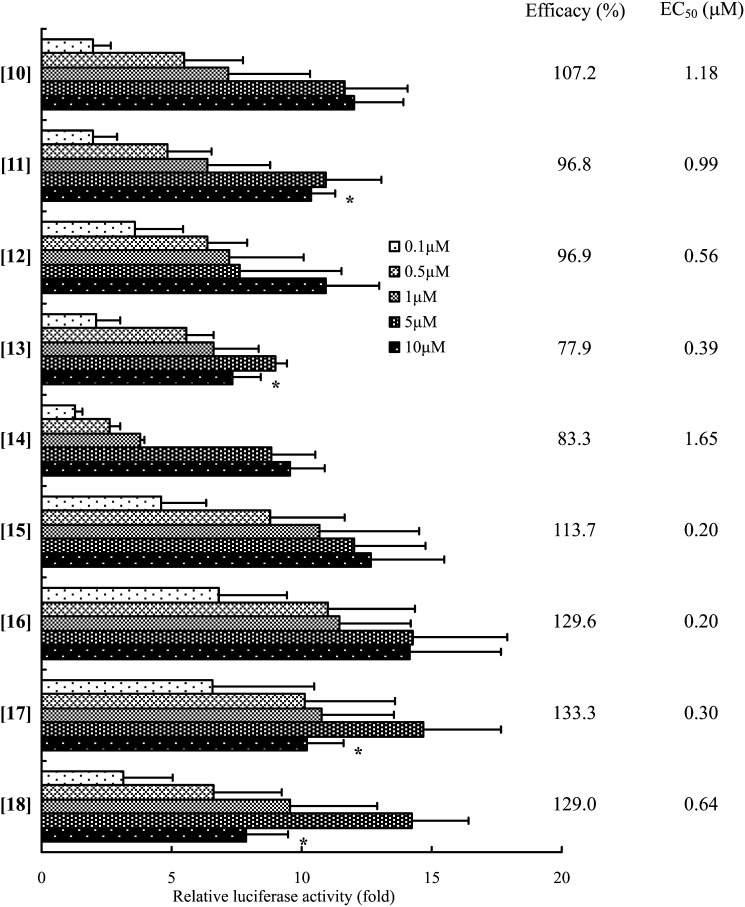
The effects of the length of the side chain in the bile alcohols on TGR5 agonist activity are shown in Fig. 3. Among the bile alcohols with 23 to 26 carbon atoms and a CA-nucleus ([10]-[13]) and with 22 to 26 carbon atoms and a CDCA-nucleus ([14]-[18]), no significant differences in the TGR5-activation ability and affinity were observed (Fig. 3).
Fig. 3.
Effect of length of steroid side chain of bile alcohol on TGR5 activation. TGR5 activation was evaluated using the luciferase reporter assay as described in the legend to Fig. 2. The data represent the mean ± SD of three determinations. The compounds in the figure are indicated by the bracketed numbers. *: The parameter was not used for the calculation of EC50 value.
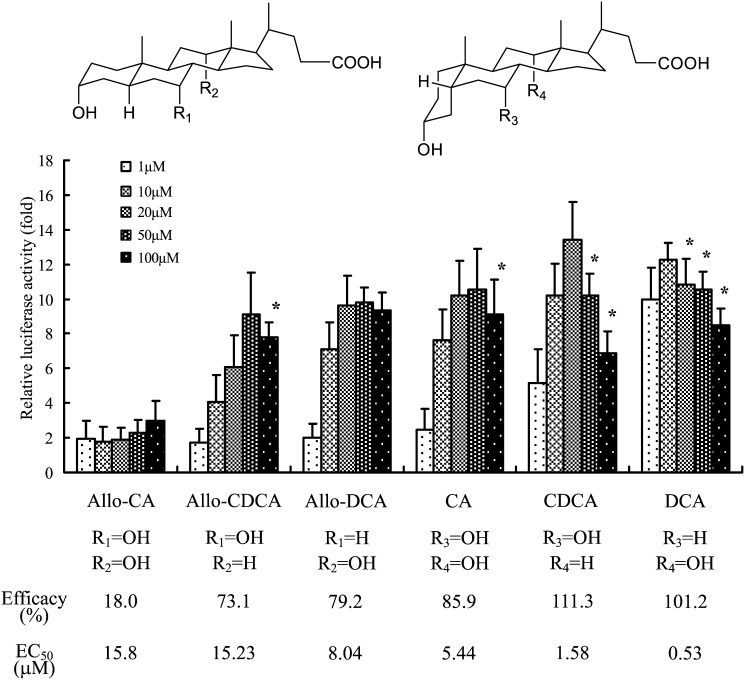
We also evaluated whether the A/B ring juncture conformation in the steroid nucleus of bile acids and bile alcohols affected TGR5 activation. As shown in Fig. 4, 5β-bile acids containing a cis-oriented A/B ring had a higher TGR5 agonist activity and affinity than their 5α-counterparts. Figure 5 shows the effects on TGR5 activation of a series of bile alcohols differing in their conformations at the C-5 position. In a group of bile alcohols with three hydroxyl groups at the 3α, 7α, and 12α positions in the steroid nucleus, the 5β-bile alcohols ([1], [2], [19]) exhibited higher TGR5 agonist activities and affinities than the corresponding 5α-bile alcohols ([23], [24], [25]), as expected (Fig. 5A). Similar results were also obtained in a group of bile alcohols with two hydroxyl groups at the 3α and 7α positions in the nucleus (Fig. 5B). In addition, the abilities of these bile alcohols, especially those of the 5β-bile alcohols, to activate TGR5 increased as the number of hydroxyl groups in their side chain increased (Fig. 5).
Fig. 4.
Effect of A/B ring juncture conformation of bile acids on TGR5 activation. The TGR5 activation ability in a series of both 5α- (allo-) and 5β-bile acids was evaluated using the luciferase reporter assay, as described in the legend to Fig. 2. The data represent the mean ± SD of three determinations. *: The parameter was not used for the calculation of EC50 value.
Fig. 5.
Effect of the number of hydroxyl groups in the side chain of bile alcohols and their A/B ring juncture conformation on TGR5 activation. The TGR5 activation ability of a series of bile alcohols with the hydroxyl group in the 3α-, 7α-, and 12α-positions (A) and in the 3α- and 7α-positions (B) of their steroid nucleus was evaluated using the luciferase reporter assay, as described in the legend to Fig. 2. The data represent the mean ± SD of three determinations. The compounds in the figure are indicated by the bracketed numbers. *: The parameter was not used for the calculation of EC50 value.
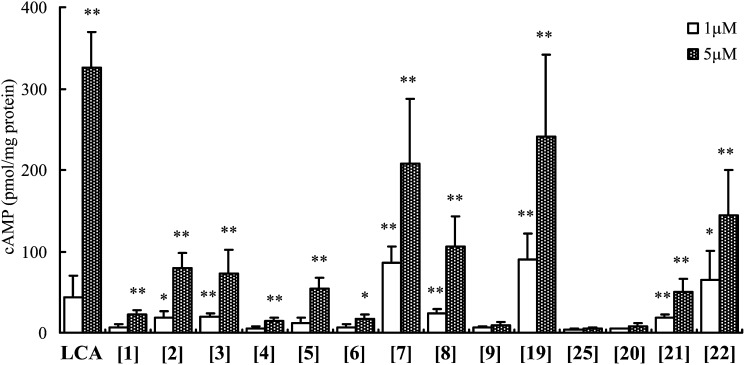
To further confirm the ability of these bile alcohols to modulate intracellular signaling, the intracellular cAMP concentration was measured as a predictive parameter of biological activity. The cAMP-production potencies of the bile alcohols that were tested were strongly correlated with the TGR5 agonist activities evaluated for this set of compounds using the luciferase assay (Fig. 6). In a series of bile alcohols with or without a hydroxyl group in their side chain ([1][9]), a remarkable difference in the cAMP-production levels was noted between each isomer of 5β-cholestane-3α,7α,12α,23-tetrols ([6] and [7]) or 5β-cholestane-3α,7α,12α,22-tetrols ([8] and [9]). Additionally, the cAMP production ability of 5β-cholestane-3α,7α,12α,26,27-pentol [19] was significantly higher than that of its 5α-isomer [25]. Further, we observed a stepwise increase in the cAMP concentration for bile alcohols with an increasing number of hydroxyl groups in their side chains ([20][22]).
Fig. 6.
hTGR5-transfected HEK293T cells produced cAMP after stimulation with LCA or bile alcohols. HEK293T cells were transfected with an hTGR5 expression plasmid. Then, the cells were stimulated with vehicle (DMSO) only or LCA or various bile alcohols at 37°C for 30 min. cAMP production and the protein concentration were then determined. The data represent the mean ± SD of three determinations. The compounds in the figure are indicated by the bracketed numbers. *: P < 0.05; **: P < 0.01 versus vehicle (DMSO)-treated cells.
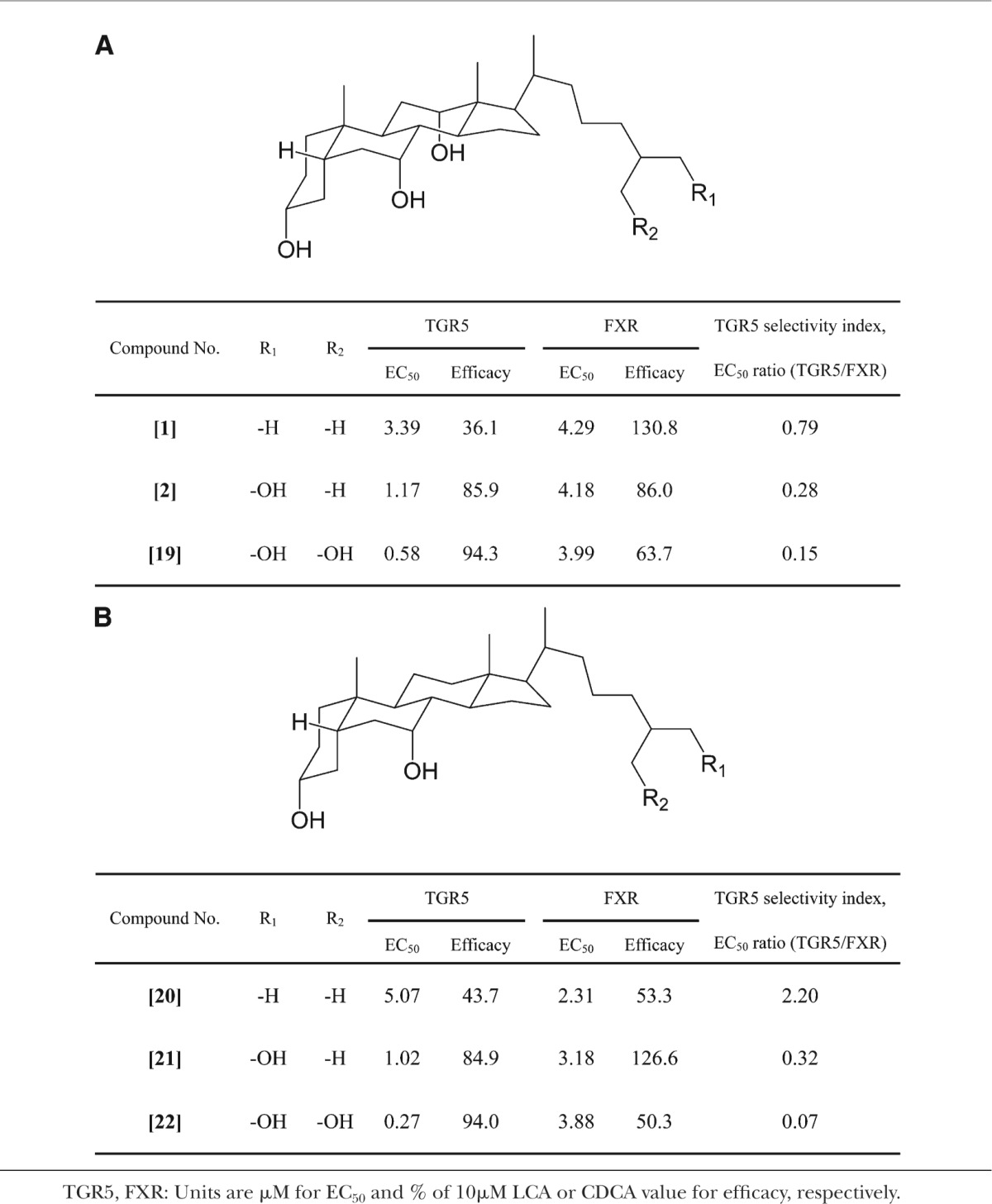
Because bile alcohols were also reported to activate FXR (37–39), we assessed the TGR5 selectivity of bile alcohols using the TGR5/FXR EC50 ratio calculated from the EC50 values for TGR5 and FXR. The EC50 values computed using a probit analysis were both statistically significant (P < 0.01). A selective agonist for TGR5 was not found among the bile alcohols tested in the present study. However, an increasing number of hydroxyl groups in the side chain led to a decrease in the TGR5/FXR EC50 ratio, indicating an increase in TGR5 selectivity (Table 1). 27Among the bile alcohols that were tested in the present study, 5β-cholestane-3α,7α,26,27-tetrol [22], which possess two hydroxyl groups in its side chain and has a CDCA-nucleus, exhibited the highest level of TGR5 selectivity.
TABLE 1.
TGR5 selectivity of bile alcohols
DISCUSSION
TGR5 was recently identified as a metabolic receptor that mediates the nongenomic potency of bile acids (9, 10), whereas FXR is a nuclear receptor that modulates genomic effects on them (40). TGR5 that has been activated by bile acids is thought to be involved in immunomodulation and hepatoprotection via the regulation of nitric oxide production and an increase in energy expenditure followed by the activation of thyroid hormone in brown adipose tissue, subsequently leading to the prevention of obesity (10, 11, 41). Furthermore, TGR5 could play a critical in vivo role in the maintenance of glucose homeostasis (42). Thus, TGR5 is an attractive target for the treatment of metabolic disorders.
Bile alcohols have long been known as the final metabolites in cholesterol elimination and are major constituents of bile in evolutionarily primitive animals, such as fishes and frogs (43, 44). In addition, some bile alcohols are formed as intermediates in the bile acid biosynthesis pathway in mammals, including humans (44). Bile alcohols are present as minor components in bile, feces and urine obtained from healthy controls (44–46). We previously demonstrated that bile alcohols are potent FXR ligands as well as bile acids (37–39). Therefore, investigating whether bile alcohols that are structurally and physiologically related to bile acids are potent TGR5 agonists is of interest. In this study, we comprehensively elucidated the ability of naturally occurring bile alcohols, including intermediates in bile acid biosynthesis as well as chemically modified bile alcohol derivatives, to act as TGR5 agonists.
First, we found that THC, which is an intermediate in the bile acid biosynthesis pathway, exhibited minimal TGR5 agonist activity in a cell-based luciferase assay, whereas 5β-cholestane-3α,7α,12α,26-tetrol, a hydroxylation product at the terminal methyl group of THC, was a potential ligand for TGR5 (Fig. 2). These findings suggested that the presence of a hydroxyl group (or oxygen atom) in the side chain is essential for TGR5 agonist activity. In addition, bile alcohols having a hydroxyl group at the C-22 to C-25 position in the side chain also activated TGR5, though the activity varied with the position and the configuration of the hydroxyl group (Fig. 2). Significant differences were also produced by the configuration of the hydroxyl group at the C-22 and C-23 positions. Four isomers at C-22 and C-23 of 3α,7α-dihydroxy-22,23-methylene-5β-cholan-24-oic acid and two isomers at C-23 of 3α,7α(,12α)-di(tri)hydroxy-23-methyl-5β-cholan-24-oic acid have reportedly exhibited remarkable differences in TGR5 agonist activity (13, 47). These previous results together with the present findings strongly suggest that the stereochemistry of the substituent at the C-22 or C-23 position, irrespective of its hydrophilic (hydroxyl) or hydrophobic (alkyl) properties, substantially affects the ability of bile acids and bile alcohols to activate TGR5.
Second, the shortening of the side chain in bile alcohols did not significantly affect the TGR5 agonist activity (Fig. 3). Even a C22-bile alcohol [14] exhibited some activity (EC50: 1.65 μM, efficacy: 83.3%). This result is consistent with the fact that C21- and C22-steroid hormones are able to activate TGR5 (13). In contrast, progressive shortening of the side chain of bile acids reportedly attenuates TGR5 agonist activity and affinity (13). These contradictory findings between the neutral compounds and bile acids remain to be elucidated.
Third, we examined the effect of the A/B ring junction in bile alcohols on the ability to activate TGR5. A series of 5α-bile alcohols ([23], [24], [25], [26], [27]) exhibited little TGR5 agonist activity compared with their corresponding 5β-isomers ([1], [2], [19], [21], [22]) (Fig. 5). These results were supported by a cAMP production analysis, shown in Fig. 6. These findings suggest that the A/B ring juncture conformation of bile alcohols is an important factor for the expression of TGR5 agonist activity.
We recently reported that bile alcohols are potent ligands for FXR (39). Therefore, the TGR5/FXR selectivity of bile alcohols was examined by calculating the ratio of the EC50 for TGR5 to that for FXR as the agonist (Table 1). The selectivity seemed to increase as the number of hydroxyl groups in the side chain increased. This result may be attributed to the tertiary protein structure of the ligand binding region in both receptors. Therefore, a site that interacts with the side chain of bile alcohols in the ligand-binding region of TGR5 might contain a larger number of charged amino acids residues compared with that of FXR. Additionally, the site of TGR5 that interacts with the side chain of bile alcohols is likely to be spatially broader than that of FXR. To elucidate these assumptions, a further study, such as crystal structure analysis of TGR5 protein with the ligand, would be required.
We previously demonstrated using 7ξ-methyl-LCA and 7β-methyl-CDCA that when a methyl group is inserted into the C-7 position of LCA and CDCA, respectively, the resulting compound has a higher affinity and selectivity for TGR5 than its parent compound (13). While this finding might indicate that the introduction of the hydrophobic methyl group into the steroid nucleus of bile acids elicits TGR5-selectivity, we could not examine such derivatives in the present study.
We focused on bile alcohols that exist in abundance in nature and discussed the structure-activity relationship between TGR5 and bile alcohols. Consequently, we have shown that some bile alcohols exert a TGR5 agonist activity equal to that of endogenous bile acids, although none of the agonist activities were more robust than that of 7ξ-LCA. Nevertheless, we are firmly convinced that the new insights into the structure-activity relationship obtained from the present study may lead to the development of a new agent for the treatment of metabolic syndrome.
Footnotes
Abbreviations:
- CDCA
- chenodeoxycholic acid
- CRE
- cAMP response element
- LCA
- lithocholic acid
- FXR
- farnesoid X receptor
- THC
- 5β-cholestane-3α,7α,12α-triol
This work was supported in part by a grant from the Japan Health Sciences Foundation.
REFERENCES
- 1.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553. [DOI] [PubMed] [Google Scholar]
- 2.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 4.Everson G. T. 1987. Steady-state kinetics of serum bile acids in healthy human subjects: single and dual isotope techniques using stable isotopes and mass spectrometry. J. Lipid Res. 28: 238–252. [PubMed] [Google Scholar]
- 5.Ho K. J. 1976. Circadian distribution of bile acid in the enterohepatic circulatory system in hamsters. J. Lipid Res. 17: 600–604. [PubMed] [Google Scholar]
- 6.Keane R. M., Gadacz T. R., Munster A. M., Birmingham W., Winchurch R. A. 1984. Impairment of human lymphocyte function by bile salts. Surgery. 95: 439–443. [PubMed] [Google Scholar]
- 7.Kimmings A. N., van Deventer S. J., Obertop H., Rauws E. A., Gouma D. J. 1995. Inflammatory and immunologic effects of obstructive jaundice: pathogenesis and treatment. J. Am. Coll. Surg. 181: 567–581. [PubMed] [Google Scholar]
- 8.Drivas G., James O., Wardle N. 1976. Study of reticuloendothelial phagocytic capacity in patients with cholestasis. BMJ. 1: 1568–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Itadani H., Tanaka K. 2002. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298: 714–719. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., et al. 2003. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278: 9435–9440. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 12.Katsuma S., Hirasawa A., Tsujimoto G. 2005. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329: 386–390. [DOI] [PubMed] [Google Scholar]
- 13.Sato H., Macchiarulo A., Thomas C., Gioiello A., Une M., Hofmann A. F., Saladin R., Schoonjans K., Pellicciari R., Auwerx J. 2008. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 51: 1831–1841. [DOI] [PubMed] [Google Scholar]
- 14.Bergstrom S., Krabisch L. 1957. Preparation of some hydroxycoprostanes: 3α,7α- and 3α,12α-dihydroxycoprostane. Acta Chem. Scand. A. 11: 1067. [Google Scholar]
- 15.Dayal B., Tint G. S., Batta A. K., Shefer S., Salen G. 1981. Synthesis of biological precursors of cholic acid II. Steroids. 37: 205–211. [DOI] [PubMed] [Google Scholar]
- 16.Dayal B., Shefer S., Tint G. S., Salen G., Mosbach E. H. 1976. Synthesis of 5β-cholestane-3α,7α,12α,25-tetrol and 5β-cholestane-3α,7α,12α,24ξ,25-pentol. J. Lipid Res. 17: 74–77. [PubMed] [Google Scholar]
- 17.Masui T., Staple E. 1967. The separation of the stereo-isomers of bile steroids, 5-beta-cholestane-3-alpha, 7-alpha, 12-alpha, 24-alpha-tetrol and 5-beta-cholestane-3-alpha, 7-alpha, 12-alpha, 24-beta-tetrol, by thin layer chromatography. Steroids. 9: 443–450. [DOI] [PubMed] [Google Scholar]
- 18.Dayal B., Batta A. K., Shefer S., Tint G. S., Salen G. 1978. Synthesis of biological precursors of cholic acid. Steroids. 32: 337–344. [DOI] [PubMed] [Google Scholar]
- 19.Kuramoto T., Matsumoto N., Hoshita T. 1978. Syntheses of 22- and 23-hydroxylated bile alcohols. Chem. Pharm. Bull. (Tokyo). 26: 2788–2792. [Google Scholar]
- 20.Kihira K., Mikami T., Ikawa S., Okamoto A., Yoshii M., Miki S., Mosbach E. H., Hoshita T. 1992. Synthesis of sulfonate analogs of bile acids. Steroids. 57: 193–198. [DOI] [PubMed] [Google Scholar]
- 21.Kazuno T., Masui T., Hoshita T. 1961. Stero-bile acids and bile sterols. 36. Isolation of a new bile sterol, 3 alpha, 7 alpha, 12 alpha, 26-tetrahydroxy-delta23-bishomocholene, from bull frog bile. J Biochem. 50: 12–19. [DOI] [PubMed] [Google Scholar]
- 22.Kihira K., Morioka Y., Hoshita T. 1981. Synthesis of (22R and 22S)-3 alpha, 7 alpha, 22-trihydroxy-5 beta-cholan-24-oic acids and structure of haemulcholic acid, a unique bile acid isolated from fish bile. J. Lipid Res. 22: 1181–1187. [PubMed] [Google Scholar]
- 23.Cohen B. I., Tint G. S., Kuramoto T., Mosbach E. H. 1975. New bile alcohols–synthesis of 5beta-cholestane-3alpha, 7alpha, 25-triol and 5beta-cholestane-3alpha, 7alpha, 25–24 (14C)-triol. Steroids. 25: 365–378. [DOI] [PubMed] [Google Scholar]
- 24.Mikami T., Mosbach E. H., Cohen B. I., Ayyad N., Yoshii M., Kihira K., Hoshita T. 1995. Synthesis and metabolism of sodium 3 alpha,7 alpha-dihydroxy-25,26-bishomo-5 beta-cholane-26-sulfonate in the hamster. Lipids. 30: 71–78. [DOI] [PubMed] [Google Scholar]
- 25.Kihira K., Yoshii M., Okamoto A., Ikawa S., Ishii H., Hoshita T. 1990. Synthesis of new bile salt analogues, sodium 3 alpha, 7 alpha-dihydroxy-5 beta-cholane-24-sulfonate and sodium 3 alpha, 7 beta-dihydroxy-5 beta-cholane-24-sulfonate. J. Lipid Res. 31: 1323–1326. [PubMed] [Google Scholar]
- 26.Dayal B., Batta A. K., Shefer S., Tint G. S., Salen G., Mosbach E. H. 1978. Preparation of 24(R)- and 24(S)-5beta-cholestane-3alpha,7alpha,24-triols and 25(R)- and 25(S)-5beta-cholestane-3alpha,7alpha,26-triols by a hydroboration procedure. J. Lipid Res. 19: 191–196. [PubMed] [Google Scholar]
- 27.Une M., Shinonaga Y., Matoba N., Kuroki S., Kihira K., Hoshita T. 1986. Identification of new bile alcohols, 5 beta-cholestane-3 alpha,7 alpha,24,26-tetrol, 5 beta-cholestane-3 alpha,7 alpha,25,26-tetrol, and 5 beta-cholestane-3 alpha,7 alpha,26,27-tetrol in human gallbladder bile. J. Lipid Res. 27: 1318–1323. [PubMed] [Google Scholar]
- 28.Hoshita T., Sasaki T., Tanaka Y., Betsuki S., Kazuno T. 1965. Stero-bile acids and bile sterols. LXXIV. Biosynthesis of bile acids and bile alcohols in toad. J Biochem. 57: 751–757. [DOI] [PubMed] [Google Scholar]
- 29.Une M., Matsumoto N., Kihira K., Yasuhara M., Kuramoto T., Hoshita T. 1980. Bile salts of frogs: a new higher bile acid, 3 alpha, 7 alpha, 12 alpha, 26-tetrahydroxy-5 beta-cholestanoic acid from the bile Rana plancyi. J. Lipid Res. 21: 269–276. [PubMed] [Google Scholar]
- 30.Hoshita T., Sasaki T., Kazuno T. 1965. Isolation of a bile alcohol, 5alpha-cholestane-3alpha,7alpha,12alpha,26-tetrol from carp bile. Steroids. 5: 241–247. [Google Scholar]
- 31.Hoshita T., Hirofuji S., Nakagawa T., Kazuno T. 1967. Studies on the bile salts of the newt and synthesis of 5alpha-cholestane-3alpha,7alpha,12alpha,25,26-pentol (5alpha-bufol). J Biochem. 62: 62–66. [Google Scholar]
- 32.Hoshita T., Shefer S., Mosbach E. H. 1968. Conversion of 7-alpha,12-alpha-dihydroxycholest-4-en-3-one to 5-alpha-cholestane-3-alpha, 7-alpha,12-alpha-triol by iguana liver microsomes. J. Lipid Res. 9: 237–243. [PubMed] [Google Scholar]
- 33.Une M., Kihira K., Kuramoto T., Hoshita T. 1978. Two new bile alcohols, 3-epimyxinol and 3-epi-16-deoxymyxinol from the hagfish, Heptatretus burgeri. Tetrahedron Lett. ▪▪▪: 2527–2530. [Google Scholar]
- 34.Kuramoto T., Kikuchi H., Sanemori H., Hoshita T. 1973. Bile salts of anura. Chem. Pharm. Bull. (Tokyo). 21: 952–959. [DOI] [PubMed] [Google Scholar]
- 35.Fujino T., Une M., Imanaka T., Inoue K., Nishimaki-Mogami T. 2004. Structure-activity relationship of bile acids and bile acid analogs in regard to FXR activation. J. Lipid Res. 45: 132–138. [DOI] [PubMed] [Google Scholar]
- 36.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 37.Nishimaki-Mogami T., Une M., Fujino T., Sato Y., Tamehiro N., Kawahara Y., Shudo K., Inoue K. 2004. Identification of intermediates in the bile acid synthetic pathway as ligands for the farnesoid X receptor. J. Lipid Res. 45: 1538–1545. [DOI] [PubMed] [Google Scholar]
- 38.Nishimaki-Mogami T., Kawahara Y., Tamehiro N., Yoshida T., Inoue K., Ohno Y., Nagao T., Une M. 2006. 5Alpha-bile alcohols function as farnesoid X receptor antagonists. Biochem. Biophys. Res. Commun. 339: 386–391. [DOI] [PubMed] [Google Scholar]
- 39.Iguchi Y., Kihira K., Nishimaki-Mogami T., Une M. 2010. Structure-activity relationship of bile alcohols as human farnesoid X receptor agonist. Steroids. 75: 95–100. [DOI] [PubMed] [Google Scholar]
- 40.Makishima M. 2005. Nuclear receptors as targets for drug development: regulation of cholesterol and bile acid metabolism by nuclear receptors. J. Pharmacol. Sci. 97: 177–183. [DOI] [PubMed] [Google Scholar]
- 41.Keitel V., Reinehr R., Gatsios P., Rupprecht C., Gorg B., Selbach O., Haussinger D., Kubitz R. 2007. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 45: 695–704. [DOI] [PubMed] [Google Scholar]
- 42.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshita T., Kazuno T. 1968. Chemistry and metabolism of bile alcohols and higher bile acids. Adv. Lipid Res. 6: 207–254. [DOI] [PubMed] [Google Scholar]
- 44.Karlaganis G., Sjovall J. 1984. Formation and metabolism of bile alcohols in man. Hepatology. 4: 966–973. [DOI] [PubMed] [Google Scholar]
- 45.Kuroki S., Shimazu K., Kuwabara M., Une M., Kihira K., Kuramoto T., Hoshita T. 1985. Identification of bile alcohols in human bile. J. Lipid Res. 26: 230–240. [PubMed] [Google Scholar]
- 46.Ludwig-Kohn H., Henning H. V., Sziedat A., Matthaei D., Spiteller G., Reiner J., Egger H. J. 1983. The identification of urinary bile alcohols by gas chromatography-mass spectrometry in patients with liver disease and in healthy individuals. Eur. J. Clin. Invest. 13: 91–98. [DOI] [PubMed] [Google Scholar]
- 47.Pellicciari R., Sato H., Gioiello A., Costantino G., Macchiarulo A., Sadeghpour B. M., Giorgi G., Schoonjans K., Auwerx J. 2007. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J. Med. Chem. 50: 4265–4268. [DOI] [PubMed] [Google Scholar]