Abstract
To evaluate whether the relative atherogenicity of VLDL and LDL is dependent on the topographic site, atherosclerosis was compared at four topographic sites in homozygous LDL receptor (LDLr)-deficient rabbits fed normal chow and in heterozygous LDLr-deficient rabbits with the same genetic background fed a 0.15% cholesterol diet to match cholesterol levels. VLDL cholesterol was significantly higher and LDL cholesterol significantly lower in LDLr+/− diet rabbits compared with LDLr−/− rabbits. Intimal area in the ascending thoracic aorta and in the abdominal aorta at the level of the renal arteries was 1.4-fold (P < 0.05) and 1.5-fold (P < 0.05) higher, respectively, in LDLr−/− rabbits than in LDLr+/− diet rabbits, whereas no significant difference occurred in the descending thoracic aorta and in the abdominal aorta just above the bifurcation. Differences remained statistically significant after adjustment for plasma cholesterol, triglycerides, and sex. Compared with LDLr+/− diet rabbits, higher intimal lipoprotein lipase (LPL) and apolipoprotein (apo) B levels were observed in LDLr−/− rabbits only at the level of the descending thoracic aorta. Intimal apo E levels in LDLr−/− rabbits were significantly lower in sites with a larger intima than in LDLr+/− diet rabbits. In conclusion, the relative atherogenicity of VLDL and LDL is dependent on the topographic site.
Keywords: atherosclerosis, lipoproteins, topography, rabbit, apolipoprotein E, familial hypercholesterolemia, LDL receptor
Epidemiological, genetic, and intervention studies have identified apolipoprotein (apo) B-containing lipoproteins as a risk factor for atherosclerosis and ischemic vascular diseases. Apo B-containing particles consist of chylomicron remnants, very low-density lipoproteins (VLDLs), intermediate density lipoproteins (IDLs), and low-density lipoproteins (LDLs). Most clinical and epidemiological data indicate a major role for LDL in atherogenesis (1). This is further supported by the development of premature atherosclerosis in patients with familial hypercholesterolemia characterized by very high LDL levels (2). However, correlative and mechanistic data also support a potent proatherogenic role of remnant lipoproteins (3). In a substantial number of patients, especially those with the metabolic syndrome or type 2 diabetes mellitus, hepatic clearance of remnant lipoproteins in the postprandial phase is delayed (3). Transendothelial migration and subendothelial retention of these larger remnant lipoproteins may not be as great as that of smaller LDL particles, but they may deliver up to 40 times more cholesterol per particle to the arterial wall (4, 5). Remnant lipoproteins have been demonstrated in lesion-prone areas of the arterial wall (6, 7). Because many patients have elevated levels of both larger remnants and smaller LDL particles, intrinsic differences in the relative atherogenicity of these particles are difficult to analyze in humans.
A landmark experimental study by Véniant et al. (8) demonstrated that atherosclerosis is more pronounced in LDL receptor (LDLr)-deficient apo B100-only mice than in apo E-deficient apo B100-only mice despite similar plasma cholesterol levels. Because nearly all of plasma cholesterol is packed in smaller LDL particles in LDLr-deficient apo B100-only mice and in larger VLDL in apo E-deficient apo B100-only mice, this suggests that LDL is more proatherogenic than VLDL. One of the limitations of this study is that the presence or absence of apo E may affect atherosclerosis progression independent of lipoprotein levels. Apo E is not only required for the clearance of remnant lipoproteins but is also expressed by macrophages and smooth muscle cells in the vascular wall (9–12). Vascular apo E expression results in pleiotropic antiatherogenic effects such as stimulation of reverse cholesterol transport, antioxidant activity, antiproliferative effects on T-lymphocytes, and inhibition of smooth muscle cell proliferation (9–13).
Clinical observations and pathological data from the Pathobiological Determinants of Atherosclerosis in Youth study strongly suggest that the effect of cardiovascular risk factors on atherogenesis is dependent on the topographic site (14–16). Several murine studies have demonstrated site-specific effects of therapeutic interventions, of different types of immunodeficiency, or of genetic deficiencies (16, 17). Atherosclerosis in patients with type III hyperlipoproteinemia, characterized by the presence of large amounts of chylomicron and VLDL remnants, tends to affect peripheral and cerebral vessels more commonly than in patients with familial hypercholesterolemia (18). Although genetic and other confounding factors may account for this difference, the relative atherogenicity of VLDL and LDL may be dependent on the topographic site. To test this hypothesis, we designed a study comparing atherosclerosis at four different topographic sites in homozygous LDLr-deficient rabbits fed normal chow and heterozygous LDLr-deficient rabbits fed a 0.15% cholesterol diet to match plasma cholesterol levels. The main difference in lipoprotein composition between these rabbits is higher LDL levels in the former and higher β-VLDL levels in the latter. Heterozygous and homozygous littermates born from heterozygous breeding couples were included to minimize variation in genetic background. Our results show that the intimal area at 21 months is higher in homozygous LDLr-deficient rabbits than in heterozygous LDLr-deficient rabbits specifically in those topographic sites where intimal apo E levels were significantly lower compared with heterozygous LDLr-deficient rabbits.
MATERIALS AND METHODS
Animal experiments
New Zealand White rabbits were obtained from the University of Gent (Merelbeke, Belgium). Watanabe Heritable Hyperlipidemic rabbits (19, 20), characterized by homozygous LDLr deficiency, were originally obtained from Charles River (Cléon, France). These rabbits with 100% Japanese White background were crossed with New Zealand White rabbits and the heterozygous female offspring was backcrossed with the homozygous males. The homozygous offspring was further backcrossed with 100% New Zealand White rabbits, until heterozygous LDLr-deficient rabbits in a final background of 62.5% New Zealand White and 37.5% Japanese White were generated. To generate homozygous LDLr-deficient (LDLr−/−) rabbits and heterozygous LDLr-deficient (LDLr+/−) rabbits with the same genetic background, LDLr+/− rabbits with a 62.5% New Zealand White and 37.5% Japanese White background were intercrossed and heterozygous (12 males, 17 females) and homozygous (7 males, 12 females) littermates were included in the current study. To induce hypercholesterolemia in LDLr+/− rabbits at a level that is approximately equivalent compared with LDLr−/− on normal chow, a diet containing 0.15% cholesterol at a daily food amount of 100 g was initiated at 3 months and continued for 18 months. Both homozygous and heterozygous LDLr-deficient rabbits were euthanized for histological analysis at the age of 21 months. All experiments were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Research Advisory Committee of the University of Leuven. Blood sampling and determination of the LDLr genotype were performed as described before (21, 22).
Biochemical analyses
Lipoprotein ultracentrifugation and plasma lipid analysis were performed as described previously (21). Phospholipids were quantified by the Wako Phospholipids B assay (Wako Chemical GMBH, Neuss, Germany) according to the instructions of the manufacturer. Plasma apo B100, apo B48, and apo E were quantified by Western blot. After separation of 1 μl plasma in SDS-PAGE gels (6% gels for apo B; 12% gels for apo E), proteins were transferred to a nitrocellulose membrane (Amersham Biosciences, New York, NY) by wet blotting. Following overnight incubation with a 1:2,500 dilution of goat anti-human apo B antibody or a 1:1,000 dilution of goat anti-human apo E antibody (both Rockland Inc., Gilbertsville, PA) and subsequent incubation with horseradish peroxidase-conjugated rabbit anti-goat antibody (DAKO, Glostrup, Denmark) in a 1:1,000 dilution, the membrane was developed using ECL detection reagent (Amersham Biosciences). Apo B100, apo B48, and apo E levels (molecular weights 515 kDa, 250 kDa, and 34 kDa, respectively) were quantified on scanned films using Image J software (Wayne Rasband, National Institutes of Health).
Determination of the apo E mass/apo B mass ratio in VLDL, IDL, and LDL lipoprotein fractions by Western blot
See Supplementary Materials and Methods.
Analysis of plasma lipoprotein size by dynamic light scattering
Dynamic light scattering experiments were performed with a Brookhaven 90 Plus nanoparticle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY) at room temperature. Scattered laser light (659 nm, 15 mW) was detected under an angle of 90°. The fluctuations in the scattered laser light intensity were correlated by a digital autocorrelator (200 channels between 0.1 µs and 0.1 s). Correlation functions were analyzed with Igor Pro 6.02A (WaveMetrics Inc., Lake Oswego, OR). The modeling of Decay kinetics with the Clementine package (Maximum Entropy method) resulted in intensity weighted distribution functions versus decay times. By converting the decay times with instrument parameters and Stokes-Einstein law to hydrodynamic diameters, an intensity weighted size distribution was obtained. Peak positions were fitted with a log-normal function to obtain the average diameter of each lipoprotein particle population.
Non-denaturing gel electrophoresis for LDL particle size determination
See Supplementary Materials and Methods.
Histological analyses
At the age of 21 months, cholesterol-fed LDLr+/− rabbits (n = 29) and chow-fed LDLr−/− rabbits (n = 19) were anesthetized with 12.5 mg/kg Ketamine 1000 Ceva® (Ceva Animal Care, Brussels, Belgium) and 5 mg/kg Rompun® (Bayer, Gent, Belgium) subcutaneously and euthanized by intravenous injection of an overdose of sodium pentobarbital together with heparin (100 mg/kg, intravenous) to prevent post mortem clotting. The entire aorta from the heart to the iliac arteries was excised, immersed in Dulbecco's Modified Eagle Medium (Gibco®, Carlsbad, CA), and adventitial fat was removed. At four distinct locations throughout the arterial tree (ascending thoracic aorta, descending thoracic aorta, abdominal aorta at the level of the renal arteries, and the abdominal aorta just above the bifurcation), two adjacent segments were excised. Proximal segments at each location were embedded in OCT compound and snap-frozen in liquid nitrogen. Caudal segments were immersion fixed in 1% paraformaldehyde overnight followed by paraffin embedding. Then 7 µm-thick paraffin-embedded sections were stained with hematoxylin and eosin for quantification of the intimal, medial, and luminal area at 170 µm spaced intervals. Intimal area was quantified as the area between the internal elastical membrane and the endothelial lining. At each location, five sections were analyzed per rabbit and the average value was used to represent that animal for statistical purposes.
To determine the relative collagen content in the intima, paraffin sections stained with Sirius red (Sigma-Aldrich, Steinheim, Germany) were observed under polarized light (23), and the thick, tightly packed red-colored collagen fibers were quantified as percentage of the intimal area. Relative lipid content within the lesions was determined by staining cryosections with Oil Red O (BDH Laboratory Supplies, Poole, UK). For all stainings, computer-assisted image analysis was performed using KS300 software (Zeiss, Zaventem, Belgium).
Paraffin-embedded sections were immunostained with mouse anti-rabbit RAM11 (DAKO; 1:50 dilution), mouse anti-human smooth muscle α-actin (DAKO; 1:500 dilution), goat anti-human apo B (Rockland Inc.; 1:200 dilution), goat anti-human apo E (Rockland Inc.; 1:100 dilution), and the monoclonal anti-LPL antibody 5D2 (MAb 5D2 kindly provided by Dr. J. Brunzell, University of Washington, Seattle, WA; 1:100 dilution). The relative macrophage, smooth muscle cell, apo B, apo E, and LPL content as percentage of intimal area within the red color range of the spectrum were determined by computer assisted image analysis using KS300 software (Zeiss). For all quantifications, five sections were analyzed per rabbit at each location and the average value was used to represent that animal for statistical purposes.
Statistical analysis
Data are expressed as means ± SEM. Areas under the curve were calculated using Prism4 (GraphPad Software, San Diego, CA). Lipid and histological parameters were compared between LDLr+/− diet and LDLr−/− rabbits by a Student's t-test (GraphPad Software). Multiple regression analyses to adjust for plasma cholesterol, plasma triglycerides, and sex were performed by Statistica 7.1 (Statsoft, Tulsa, OK). The assumption of normality was verified by a Normal Probability Plot and Shapiro-Wilk test. Pearson correlation coefficients and semipartial Pearson correlation coefficients were calculated by Statistica 7.1 (Statsoft). A two-sided P-value < 0.05 was considered statistically significant.
RESULTS
Feeding a 0.15% cholesterol diet to LDLr+/− rabbits results in similar plasma cholesterol but higher VLDL cholesterol and lower LDL cholesterol levels than in LDLr−/− rabbits fed normal chow
Heterozygous and homozygous littermates born from heterozygous LDLr-deficient breeding couples were included in the study to minimize variation in genetic background. A 0.15% cholesterol diet was initiated in heterozygous LDLr-deficient rabbits at the age of 3 months to match plasma cholesterol levels with chow-fed homozygous LDLr-deficient rabbits. Lipoprotein levels in both LDLr+/− and LDLr−/− rabbits were determined at the age of 3 months, 4 months, 5 months, 6 months, and subsequently every 3 months till the age of 21 months. Average cholesterol concentrations for the 18 month time period in different lipoprotein classes isolated by ultracentrifugation are shown in Table 1. Plasma cholesterol levels were similar in male LDLr+/− diet rabbits compared with male LDLr−/− rabbits (Table 1). In LDLr−/− rabbits, average plasma cholesterol was 1.3-fold (P < 0.0001) higher in females compared with males. Because no sex difference of plasma cholesterol in LDLr+/− diet rabbits was observed, levels were 1.2-fold (P < 0.01) lower in LDLr+/− diet females than in LDLr−/− females. Major differences in lipoprotein class cholesterol were observed for VLDL and LDL particles. VLDL cholesterol in male and female LDLr+/− diet rabbits was 3.0-fold (P < 0.0001) and 1.5-fold (P < 0.0001) higher, respectively, than in their LDLr−/− counterparts. In contrast, LDL cholesterol was 2.4-fold (P < 0.0001) and 2.5-fold (P < 0.0001) lower in male and female LDLr+/− diet rabbits, respectively, than in their LDLr−/− counterparts. No significant differences in HDL cholesterol levels were observed between both genotypes. Average plasma triglycerides were 1.5-fold (P = NS) and 3.7-fold (P < 0.0001) lower in male and female LDLr+/− diet rabbits, respectively, compared with LDLr−/− rabbits (Table 1). This was predominantly due to 2.9-fold (P < 0.05) and 6.5-fold (P < 0.0001) lower levels of triglycerides in IDL in male and female LDLr+/− diet rabbits, respectively, than in LDLr−/− rabbits (Table 1). The phospholipid content of different lipoproteins in LDLr+/− diet rabbits and LDLr−/− rabbits is provided in supplementary Table I. Diameters of VLDL, IDL, and LDL isolated from female LDLr+/− diet and LDLr−/− rabbits were determined by dynamic light scattering and are provided in Table 2. No significant differences in these diameters were observed between both models of hyperlipidemia. To confirm LDL particle size determined by dynamic light scattering, non-denaturing gel electrophoresis was performed on LDL fractions obtained by ultracentrifugation. Size distribution of LDL particles of the four different rabbit groups are shown in supplementary Figure I. Peak diameters ranged from 22.4 nm to 25.8 nm. For both sexes, LDL size distribution was similar in LDLr+/− diet and LDLr−/− rabbits.
TABLE 1.
Average VLDL, IDL, LDL, HDL, and plasma cholesterol and triglyceride levels (mg/dl), and plasma apo B100, apo B48, and apo E levels in heterozygous LDLr-deficient rabbits fed a 0.15% cholesterol diet or in homozygous LDLr-deficient rabbits fed normal chow
| LDLr+/− Diet |
LDLr−/− |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| VLDL cholesterol | 190 ± 8.5 | 170 ± 7.3 | 64 ± 2.8**** | 110 ± 3.0c,**** |
| IDL cholesterol | 210 ± 8.0 | 230 ± 14 | 180 ± 10* | 280 ± 6.2c,**** |
| LDL cholesterol | 82 ± 9.1 | 68 ± 4.0 | 200 ± 10**** | 170 ± 8.8a,**** |
| HDL cholesterol | 24 ± 1.7 | 26 ± 1.0 | 25 ± 2.0 | 23 ± 1.3 |
| Plasma cholesterol | 500 ± 19 | 500 ± 19 | 470 ± 14 | 590 ± 14c |
| VLDL triglycerides | 29 ± 4.9 | 9.0 ± 3.5a | 27 ± 1.2 | 41 ± 3.8a |
| IDL triglycerides | 39 ± 1.6 | 23 ± 4.5a | 120 ± 26* | 150 ± 11**** |
| LDL triglycerides | 38 ± 8.1 | 17 ± 6.5 | 40 ± 12 | 30 ± 4.5 |
| HDL triglycerides | 23 ± 4.1 | 13 ± 3.8 | 10 ± 3.5 | 9.1 ± 4.0 |
| Plasma triglycerides | 130 ± 29 | 62 ± 6.3a | 190 ± 18 | 230 ± 21**** |
| Plasma apo B100 | 1.0 ± 0.049 | 0.83 ± 0.12 | 1.5 ± 0.078**** | 1.9 ± 0.059b,**** |
| Plasma apo B48 | 0.11 ± 0.0089 | 0.090 ± 0.015 | 0.088 ± 0.014 | 0.071 ± 0.020 |
| Plasma apo E | 1.0 ± 0.074 | 1.1 ± 0.061 | 0.89 ± 0.11 | 0.84 ± 0.045*** |
Data are expressed as means ± SEM (n = 7 to 10 for each condition). The atherogenic diet was initiated in the heterozygous rabbits at the age of 3 months. Lipoproteins were isolated by ultracentrifugation of plasma samples obtained at the age of 3 months, 4 months, 5 months, 6 months, and subsequently every 3 months till the age of 21 months. Average values for the 18 months follow-up period were obtained by dividing the area under the curve by time. Plasma apo B100 and apo B48 levels are expressed relative to the apo B100 value in male LDLr+/− rabbits on diet. Plasma apo E levels are expressed relative to the apo E value in male LDLr+/− rabbits on diet. *: P < 0.05, **: P < 0.01, ***: P < 0.001, ****: P < 0.0001 for difference between heterozygous rabbits on diet and homozygous rabbits.
P < 0.05.
P < 0.001.
P < 0.0001 for sex difference.
TABLE 2.
Diameter of VLDL, IDL, and LDL in female rabbits
| LDLr+/− Diet | LDLr−/− | |
|---|---|---|
| VLDL | 61 ± 11 | 60 ± 11 |
| IDL | 33 ± 2.8 | 33 ± 5.3 |
| LDL | 27 ± 0.40 | 25 ± 1.9 |
Data are expressed in nm and represent means ± SEM (n = 4). Diameters were determined by dynamic light scattering using a Brookhaven 90 Plus nanoparticle size analyzer.
Consistent with differences in the relative content of VLDL and LDL, plasma apo B100 levels quantified by Western blot were 1.5-fold (P < 0.0001) and 2.3-fold (P < 0.0001) lower in male and female LDLr+/− diet rabbits, respectively, compared with LDLr−/− rabbits. No significant differences of apo B48 levels were observed (Table 1). Plasma apo E levels were not significantly different between male LDLr+/− diet and male LDLr−/− rabbits and were 1.4-fold (P < 0.001) higher in female LDLr+/− diet rabbits compared with female LDLr−/− rabbits (Table 1). The apo E mass/apo B mass ratio of different lipoproteins (expressed in arbitrary units) is provided in supplementary Table I. These ratios are significantly higher in IDL and LDL of LDLr+/− diet rabbits than in the respective lipoproteins of LDLr−/− rabbits (supplementary Table I).
The relative atherogenicity of VLDL and LDL is dependent on the topographic site
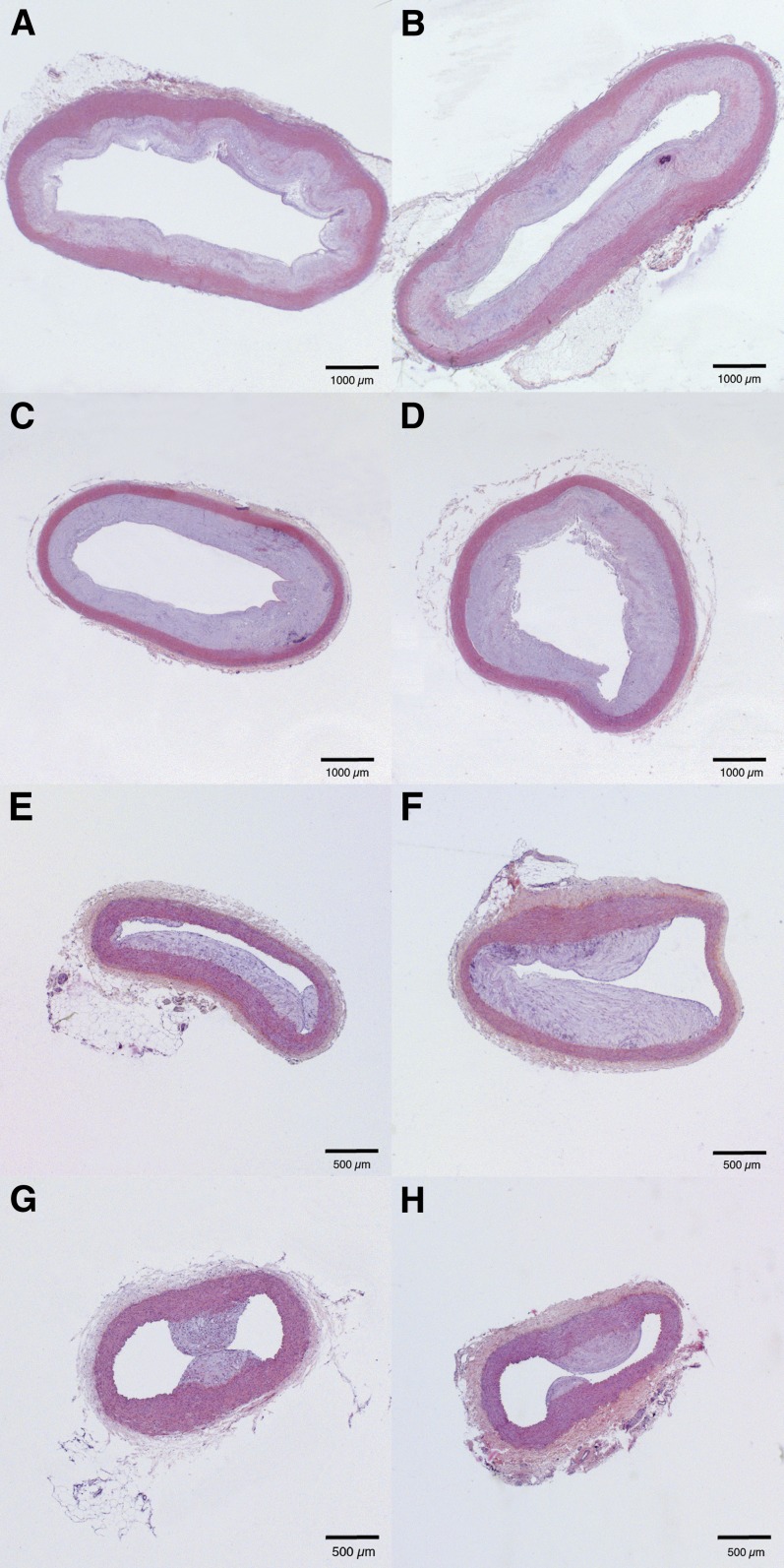
Atherosclerosis was quantified at the age of 21 months in the ascending thoracic aorta, the descending thoracic aorta at the level of the ligamentum arteriosum, the abdominal aorta at the level of the renal arteries, and the abdominal aorta just above the bifurcation. The intimal area is indicated in Table 3 and representative sections are shown in Fig. 1. The intimal area in the ascending thoracic aorta and in the abdominal aorta at the level of the renal arteries was 1.4-fold (P < 0.05) and 1.5-fold (P < 0.05) higher, respectively, in LDLr−/− rabbits than in LDLr+/− diet rabbits. A subgroup analysis performed per sex showed that this difference was significant (P < 0.05) for both sexes in the ascending aorta, whereas at the level of the renal arteries, the difference between LDLr−/− rabbits and LDLr+/− diet rabbits was only statistically significant (P < 0.01) in females. No significant differences in intimal area were observed between both models of hyperlipidemia at the descending thoracic aorta or the abdominal aorta just above the bifurcation. However, a trend for higher intimal area in LDLr−/− rabbits was observed at the latter site.
TABLE 3.
Intimal area at different topographic locations determined at the age of 21 months in heterozygous LDLr -eficient rabbits fed a 0.15% cholesterol diet and in homozygous LDLr-deficient rabbits
| Ascending Thoracic Aorta | Descending Thoracic Aorta | Abdominal Aorta Kidney | Abdominal Aorta Bifurcation | |
|---|---|---|---|---|
| Both sexes | ||||
| LDLr+/− diet (n = 29) | 9.7 ± 0.57 | 6.3 ± 0.45 | 1.2 ± 0.14 | 0.32 ± 0.055 |
| LDLr−/− (n = 19) | 14 ± 1.0** | 5.8 ± 0.49 | 1.9 ± 0.19** | 0.47 ± 0.12 |
| Male rabbits | ||||
| LDLr+/− diet (n = 12) | 11 ± 0.88 | 5.9 ± 0.60 | 1.2 ± 0.28 | 0.21 ± 0.069 |
| LDLr−/− (n = 7) | 14 ± 1.2* | 5.1 ± 0.73 | 1.3 ± 0.33 | 0.44 ± 0.098 |
| Female rabbits | ||||
| LDLr+/− diet (n = 17) | 9.0 ± 0.71 | 6.6 ± 0.65 | 1.3 ± 0.15 | 0.40 ± 0.075 |
| LDLr−/− (n = 12) | 14 ± 1.5** | 6.2 ± 0.63 | 2.2 ± 0.19a | 0.48 ± 0.18 |
Data are expressed in mm2 and represent means ± SEM. The atherogenic diet was initiated in the heterozygous rabbits at the age of 3 months. Both cholesterol-fed LDLr+/− rabbits and chow-fed LDLr−/− rabbits were euthanized for histological analysis at the age of 21 months. *: P < 0.05, **: P < 0.01, ***: P < 0.001 for comparison of LDLr+/− diet versus LDLr−/−.
P < 0.05 for sex difference.
Fig. 1.
Representative hematoxylin and eosin stained sections of the ascending thoracic aorta (A, B), the descending thoracic aorta (C, D), the abdominal aorta at the level of the renal arteries (E, F), and the abdominal aorta just above the bifurcation (G, H) in heterozygous LDLr-deficient rabbits fed a 0.15% cholesterol diet (left panels) and in homozygous LDLr-deficient rabbits (right panels).
Because the main difference between LDLr+/− diet rabbits and LDLr−/− rabbits is the amount of VLDL and LDL cholesterol, the data in Table 3 suggest that the relative atherogenicity of VLDL and LDL is dependent on the topographic site. However, differences of plasma cholesterol, plasma triglycerides, and sex may have contributed to the observed differences in Table 3. Therefore, multiple regression analyses were performed by using intimal area as dependent variable and plasma cholesterol (Table 1), plasma triglycerides (Table 1), LDLr genotype/diet (LDLr−/− chow / LDLr+/− 0.15% diet), and sex as independent variables. At the level of the ascending thoracic aorta, LDLr genotype/diet was an independent determinant (P < 0.05) of the intimal area after adjustment for plasma cholesterol, plasma triglycerides, and sex, whereas the other regressors were not significant predictors. Sex was an independent determinant (P < 0.05) of the intimal area in the abdominal aorta at the level of the renal arteries. Therefore, separate multiple regression analyses were performed in both sexes. Multiple regression analysis in male rabbits did not show an independent predictor of the intimal area. In female rabbits, LDLr genotype/diet was an independent determinant (P < 0.05) of the intimal area in the abdominal aorta at the level of the renal arteries after adjustment for plasma cholesterol (P = NS) and plasma triglycerides (P = NS). In a secondary multivariate analysis, average lipid values reflecting the entire lifespan of the rabbits instead of average lipid values corresponding to month 3 until month 21 (Table 1) were entered in the model to account for the absence of significant hyperlipidemia in LDLr+/− rabbits in the first 3 months of life. The secondary analysis confirmed the results of the primary analysis. Taken together, LDLr genotype/diet is an independent determinant of atherosclerosis in the ascending aorta in both sexes and in the abdominal aorta at the level of the renal arteries in female rabbits after adjustment for plasma cholesterol and plasma triglycerides. Because heterozygous and homozygous LDLr-deficient rabbits are littermates and thus have a similar genetic background, LDLr+/− diet and LDLr−/− rabbits only differ in the relative distribution of their lipoproteins. Thus, LDLr genotype/diet as an independent determinant of the intimal area in two topographic sites but not in the two others strongly suggests that the relative atherogenicity of VLDL and LDL is dependent on the topographic site.
The correlation between plasma cholesterol levels and intimal area was low at most topographic sites (supplementary Table II), which may reflect the absence of major interindividual differences of plasma cholesterol levels in LDLr+/− diet rabbits and in LDLr−/− rabbits of the same sex.
Differences of the histological composition of atherosclerotic lesions between LDLr+/− diet and LDLr−/− rabbits are dependent on the topographic site
To further evaluate differences in atherogenesis between LDLr+/− diet and LDLr−/− rabbits, the histological composition of the lesions was evaluated by quantification of the relative amount of collagen, smooth muscle cells, macrophages, and Oil Red O positive area (Table 4). Quantification of the intimal collagen content by picroSirius red staining and visualization by polarized light showed 1.3-fold (P < 0.001) and 1.2-fold (P < 0.05) higher collagen content in LDLr−/− rabbits compared with LDLr+/− diet rabbits at location of the ascending thoracic aorta and the abdominal aorta at the level of the renal arteries, respectively (Table 4). No significant differences in collagen content were observed at the other two topographic locations. Immunohistochemistry for the detection of smooth muscle α-actin positive smooth muscle cells and RAM11 positive macrophages revealed a 2.6-fold (P < 0.001) higher smooth muscle cell content and a 1.7-fold (P < 0.05) lower macrophage content in the intima of LDLr−/− rabbits compared with LDLr+/− diet rabbits at the level of the descending thoracic aorta, whereas no statistical significant differences were observed for the other locations (Table 4). Oil Red O staining showed that the percentage lipid covered intimal area was 1.2-fold (P < 0.01) lower and 1.2-fold (P < 0.05) lower in LDLr−/− rabbits than in LDLr+/− diet rabbits at the ascending thoracic aorta and at the abdominal aorta just above the bifurcation, respectively (Table 4), but not at the other two sites (Table 4). Immunohistochemical quantification of lipoprotein lipase (LPL) showed that the percentage LPL positive intimal area was 1.7-fold (P < 0.01) higher in LDLr−/− rabbits than in LDLr+/− diet rabbits at the level of the descending thoracic aorta, whereas no statistically significant differences were observed at other sites, similar as for apo B (Table 4). Consistent with a role of LPL in lipoprotein retention, the percentage apo B positive intimal area was 1.8-fold (P < 0.01) higher in LDLr−/− rabbits than in LDLr+/− diet rabbits at the level of the descending thoracic aorta, whereas no statistically significant differences were observed at other sites (Table 4). Intimal apo E levels were 1.8-fold (P < 0.001), 2.0-fold (P < 0.0001), and 1.9-fold (P < 0.001) higher at the level of the ascending thoracic aorta, the abdominal aorta at the level of the kidneys, and the abdominal aorta at the level of the bifurcation, respectively, in LDLr+/− diet rabbits than in LDLr−/− rabbits. No significant difference of intimal apo E was observed at the level of the descending thoracic aorta (Table 4). High magnification multipanel images of the different pathological parameters incorporated in Table 4 are shown in supplementary Figures II, III, IV, and V for LDLr+/− diet rabbits. Extracellular localization of lipids and apolipoproteins and colocalization of apolipoproteins with LPL is illustrated in supplementary Figure VI for LDLr−/− rabbits.
TABLE 4.
Histological composition of intimal lesions at different topographic locations determined at the age of 21 months in heterozygous LDLr-deficient rabbits fed a 0.15% cholesterol diet initiated at the age of 3 months and in homozygous LDLr-deficient rabbits
| Ascending Thoracic Aorta | Descending Thoracic Aorta | Abdominal Aorta Kidney | Abdominal Aorta Bifurcation | |
|---|---|---|---|---|
| Collagen (% intimal area) | ||||
| LDLr+/− diet | 39 ± 1.5 | 21 ± 1.5 | 26 ± 1.9 | 32 ± 2.3 |
| LDLr−/− | 30 ± 2.0*** | 21 ± 1.9 | 21 ± 1.3* | 26 ± 2.1 |
| Smooth muscle cells (% intimal area) | ||||
| LDLr+/− diet | 0.71 ± 0.092 | 0.61 ± 0.093 | 0.98 ± 0.14 | 1.7 ± 0.22 |
| LDLr−/− | 0.64 ± 0.19 | 1.6 ± 0.21*** | 1.0 ± 0.10 | 1.1 ± 0.17 |
| Macrophages (% intimal area) | ||||
| LDLr+/− diet | 1.0 ± 0.086 | 1.3 ± 0.20 | 1.2 ± 0.20 | 1.3 ± 0.23 |
| LDLr−/− | 0.89 ± 0.10 | 0.76 ± 0.096* | 0.99 ± 0.22 | 1.2 ± 0.40 |
| Oil Red O area (% intimal area) | ||||
| LDLr+/− diet | 11 ± 0.65 | 5.7 ± 0.26 | 7.8 ± 0.42 | 7.3 ± 0.44 |
| LDLr−/− | 9.0 ± 0.52** | 6.2 ± 0.19 | 7.3 ± 0.35 | 6.1 ± 0.41* |
| LPL (% intimal area) | ||||
| LDLr+/− diet | 3.6 ± 0.26 | 3.4 ± 0.40 | 3.6 ± 0.37 | 4.8 ± 0.34 |
| LDLr−/− | 4.2 ± 0.43 | 5.8 ± 0.67** | 3.9 ± 0.41 | 5.1 ± 0.50 |
| Apo B (% intimal area) | ||||
| LDLr+/− diet | 6.3 ± 0.89 | 5.5 ± 0.52 | 4.7 ± 0.45 | 4.8 ± 0.79 |
| LDLr−/− | 6.1 ± 0.84 | 9.7 ± 1.1** | 6.6 ± 0.88 | 5.9 ± 1.2 |
| Apo E (% intimal area) | ||||
| LDLr+/− diet | 11 ± 1.1 | 10 ± 0.51 | 12 ± 1.0 | 9.1 ± 0.88 |
| LDLr−/− | 6.2 ± 0.69*** | 9.9 ± 0.73 | 5.9 ± 0.68*** | 4.9 ± 0.68*** |
Data are pooled for both sexes (n = 29 for LDLr+/− diet and n = 19 for LDLr−/−) and represent means ± SEM. The contribution of male and female rabbits to pooled data is identical as in Table 3. For each parameter, the relative content as percentage of the intimal area was determined within the red/brown color range of the spectrum on high resolution images of complete cross-sections. For the relative collagen content, Sirius red stained slides were observed under polarized light and the red-colored collagen fibers were quantified as percentage of the intimal area. All data were obtained by computer assisted image analysis. *: P < 0.05, **: P < 0.01, ***: P < 0.001 for comparison of LDLr+/− diet rabbits versus LDLr−/− rabbits.
DISCUSSION
In the current study, complex atherosclerotic lesions were compared at four different topographic sites in homozygous LDLr-deficient rabbits and heterozygous LDLr-deficient rabbits fed 0.15% cholesterol to match plasma cholesterol levels with LDLr−/− rabbits. The principal difference between both hyperlipidemia models is the content of cholesterol in VLDL and LDL particles. The main findings of the current study are: 1) LDL is more pro-atherogenic than VLDL in the ascending thoracic aorta and the abdominal aorta at the level of the kidneys but clearly not at the level of the descending thoracic aorta; 2) LPL levels in the intima were higher in LDLr−/− rabbits at the level of the descending thoracic aorta and this coincides with higher intimal apo B levels at this site; and 3) intimal apo E levels are strikingly higher in those sites in LDLr+/− diet rabbits with less atherosclerosis than in LDLr−/− rabbits, whereas no difference of intimal apo E is observed at the level of the descending thoracic aorta. Taken together, these data suggest that intimal apo E levels may play a role in the topographic differences in the relative atherogenicity of VLDL and LDL.
The 0.15% cholesterol diet in LDLr+/− rabbits was chosen to match plasma cholesterol levels with male LDLr−/− rabbits. Similarly as described before by Havel et al. (24), female LDLr−/− rabbits have higher plasma cholesterol levels than their male counterparts. Therefore, matching of cholesterol levels between different groups was imperfect. In addition, LDLr+/− diet rabbits and LDLr−/− rabbits differ in plasma triglyceride levels. Hypertriglyceridemia in LDLr−/− rabbits reflects triglyceride accumulation in both VLDL, IDL, and LDL, similarly as previously described (24). The high levels of triglycerides in IDL in LDLr−/− rabbits are related to low hepatic lipase activity (25). In contrast, remnant lipoproteins in cholesterol-fed rabbits are triglyceride poor (26). To adjust for differences in plasma cholesterol, plasma triglycerides, and sex, we performed a multiple regression analysis to evaluate whether the LDLr genotype/diet was an independent determinant of the intimal area. Our data show that LDLr genotype/diet is an independent determinant of the intimal area in the ascending thoracic aorta in both sexes and in the abdominal aorta at the level of the renal arteries in female rabbits. LDLr genotype/diet in a multiple regression model adjusted for plasma cholesterol, plasma triglycerides, and sex essentially reflects differences in lipoprotein classes. First, a critical aspect in the design of the current study was that only heterozygous and homozygous littermates born from heterozygous breeding couples were included so that the genetic background is similar in LDLr+/− and LDLr−/− rabbits. Second, LDLr expression in the vascular wall does not affect atherogenesis (27). Therefore, our data strongly suggest that whether LDL is more proatherogenic than VLDL is dependent on the topographic site.
LPL is produced by macrophages or smooth muscle cells (28, 29) in the vascular wall and promotes lipoprotein retention (5), whereas apo E, lipoprotein derived or produced locally by macrophages (28) or smooth muscle cells (11), has pleiotropic anti-atherogenic effects (9–13). Intimal LPL levels were higher in LDLr−/− rabbits in the thoracic aorta at the level of the ligamentum arteriosum and this coincided with higher intimal apo B levels compared with LDLr+/− diet rabbits. However, the intimal area was not different between both models of hyperlipidemia at this site. In contrast, intimal apo E levels were significantly lower in LDLr−/− rabbits than in LDLr+/− diet rabbits in those topographic sites where LDL is significantly more atherogenic than VLDL (ascending thoracic aorta, abdominal aorta at the level of the kidneys) or where LDL tends to be more atherogenic than VLDL (lower abdominal aorta at the level of the bifurcation). Taken together, the observed differences of intimal apo E levels may play an important role in the topographic differences in the relative atherogenicity of VLDL and LDL.
We euthanized LDLr+/− diet rabbits and LDLr−/− rabbits at the same age of 21 months. Thus, the exposure time to hyperlipidemia was slightly different in LDLr+/− rabbits fed a 0.15% cholesterol diet initiated after weaning at the age of 3 months and LDLr−/− rabbits exposed to genetic hyperlipidemia starting in utero. However, because aging has a major impact on atherosclerosis development in rabbits (30), histological analysis at the same age is more important than an identical cholesterol exposure time. Furthermore, when we accounted for the absence of significant hyperlipidemia in LDLr+/− diet rabbits in the first 3 months of life in a secondary multivariate analysis, the results of the primary multivariate analysis were confirmed.
Whereas the results of the current manuscript suggest that the atherogenicity of VLDL and LDL is dependent on the topographic site, several issues should be considered in the interpretation of the current study. First, the composition of VLDL, IDL, and LDL particles differs between LDLr+/− diet rabbits and LDLr−/− rabbits. Therefore, other factors than quantitative differences in the amount of VLDL cholesterol and LDL cholesterol between both conditions may contribute to the observed topographic differences in atherosclerosis susceptibility. Second, VLDL in cholesterol-fed rabbits represents β-VLDL and differs in composition from classical human VLDL or even human cholesterol-enriched VLDL. Third, differential retention of apo B48 containing lipoproteins versus apo B100 containing lipoproteins may be model dependent as previously shown by Proctor and Mamo (31) and may contribute to the observed differences. Fourth, rabbit LDL contains apo E (32, 33) in contrast to human LDL (34).
In conclusion, the current study using genetically matched rabbits suggests that the relative atherogenicity of VLDL and LDL is dependent on the topographic site. Intimal apo E levels may play an important role in these topographic differences.
Supplementary Material
Footnotes
Abbreviations:
- apo
- apolipoprotein
- IDL
- intermediate density lipoprotein
- LDLr
- LDL receptor
This work was supported by grants G.0212.03 (to B.D.G.), G.0533.08 (to B.D.G.), and G.0338.08 (to J.A.M.) of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. The Center for Molecular and Vascular Biology is supported by the Excellentiefinanciering KU Leuven (EF/05/013). Eline Van Craeyveld is a Research Assistant of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. Frank Jacobs and Yingmei Feng are postdoctoral fellows of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. Leen Thomassen is a Research Assistant of the Belgian Science Policy Ministry (contract SD/HE/02A). The monoclonal anti-LPL antibody 5D2 (MAb 5D2) was kindly provided by Dr. John Brunzell of the University of Washington, Seattle, WA.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of Materials and Methods, two tables, and six figures.
REFERENCES
- 1.Lusis A. J. 2000. Atherosclerosis. Nature. 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutter C. M., Austin M. A., Humphries S. E. 2004. Familial hypercholesterolemia, peripheral arterial disease, and stroke: a HuGE minireview. Am. J. Epidemiol. 160: 430–435. [DOI] [PubMed] [Google Scholar]
- 3.Twickler T., Dallinga-Thie G. M., Chapman M. J., Cohn J. S. 2005. Remnant lipoproteins and atherosclerosis. Curr. Atheroscler. Rep. 7: 140–147. [DOI] [PubMed] [Google Scholar]
- 4.Proctor S. D., Vine D. F., Mamo J. C. 2002. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr. Opin. Lipidol. 13: 461–470. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I., Williams K. J., Boren J. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116: 1832–1844. [DOI] [PubMed] [Google Scholar]
- 6.Rapp J. H., Lespine A., Hamilton R. L., Colyvas N., Chaumeton A. H., Tweedie-Hardman J., Kotite L., Kunitake S. T., Havel R. J., Kane J. P. 1994. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler. Thromb. 14: 1767–1774. [DOI] [PubMed] [Google Scholar]
- 7.Chung B. H., Tallis G., Yalamoori V., Anantharamaiah G. M., Segrest J. P. 1994. Liposome-like particles isolated from human atherosclerotic plaques are structurally and compositionally similar to surface remnants of triglyceride-rich lipoproteins. Arterioscler. Thromb. 14: 622–635. [DOI] [PubMed] [Google Scholar]
- 8.Veniant M. M., Sullivan M. A., Kim S. K., Ambroziak P., Chu A., Wilson M. D., Hellerstein M. K., Rudel L. L., Walzem R. L., Young S. G. 2000. Defining the atherogenicity of large and small lipoproteins containing apolipoprotein B100. J. Clin. Invest. 106: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtiss L. K., Boisvert W. A. 2000. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 11: 243–251. [DOI] [PubMed] [Google Scholar]
- 10.Greenow K., Pearce N. J., Ramji D. P. 2005. The key role of apolipoprotein E in atherosclerosis. J. Mol. Med. 83: 329–342. [DOI] [PubMed] [Google Scholar]
- 11.Majack R. A., Castle C. K., Goodman L. V., Weisgraber K. H., Mahley R. W., Shooter E. M., Gebicke-Haerter P. J. 1988. Expression of apolipoprotein E by cultured vascular smooth muscle cells is controlled by growth state. J. Cell Biol. 107: 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui D. Y. 2004. Apolipoprotein E-induced cell signaling in the vessel wall. Rev. Endocr. Metab. Disord. 5: 335–341. [DOI] [PubMed] [Google Scholar]
- 13.Shimano H., Ohsuga J., Shimada M., Namba Y., Gotoda T., Harada K., Katsuki M., Yazaki Y., Yamada N. 1995. Inhibition of diet-induced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J. Clin. Invest. 95: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGill H. C., Jr., McMahan C. A. 1998. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am. J. Cardiol. 82: 30T–36T. [DOI] [PubMed] [Google Scholar]
- 15.McGill H. C., Jr., McMahan C. A., Gidding S. S. 2008. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 117: 1216–1227. [DOI] [PubMed] [Google Scholar]
- 16.VanderLaan P. A., Reardon C. A., Getz G. S. 2004. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 24: 12–22. [DOI] [PubMed] [Google Scholar]
- 17.Methia N., Andre P., Denis C. V., Economopoulos M., Wagner D. D. 2001. Localized reduction of atherosclerosis in von Willebrand factor-deficient mice. Blood. 98: 1424–1428. [DOI] [PubMed] [Google Scholar]
- 18.Feussner G., Wagner A., Kohl B., Ziegler R. 1993. Clinical features of type III hyperlipoproteinemia: analysis of 64 patients. Clin. Investig. 71: 362–366. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T., Watanabe Y. 1975. [A heritable hyperlipemic rabbit (author's transl)]. Jikken Dobutsu. 24: 89–94. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe Y. 1980. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL- rabbit). Atherosclerosis. 36: 261–268. [DOI] [PubMed] [Google Scholar]
- 21.Van Craeyveld E., Lievens J., Jacobs F., Feng Y., Snoeys J., De Geest B. 2009. Apolipoprotein A-I and lecithin:cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 16: 757–765. [DOI] [PubMed] [Google Scholar]
- 22.Sun H., Usui S., Shiomi M., Watanabe T., Fan J. 2002. A rapid PCR method of genotyping LDL receptor mutations in WHHL rabbits. J. Atheroscler. Thromb. 9: 145–148. [DOI] [PubMed] [Google Scholar]
- 23.Junqueira L. C., Bignolas G., Brentani R. R. 1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11: 447–455. [DOI] [PubMed] [Google Scholar]
- 24.Havel R. J., Kita T., Kotite L., Kane J. P., Hamilton R. L., Goldstein J. L., Brown M. S. 1982. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 2: 467–474. [DOI] [PubMed] [Google Scholar]
- 25.Warren R. J., Ebert D. L., Mitchell A., Barter P. J. 1991. Rabbit hepatic lipase cDNA sequence: low activity is associated with low messenger RNA levels. J. Lipid Res. 32: 1333–1339. [PubMed] [Google Scholar]
- 26.Kovanen P. T., Brown M. S., Basu S. K., Bilheimer D. W., Goldstein J. L. 1981. Saturation and suppression of hepatic lipoprotein receptors: a mechanism for the hypercholesterolemia of cholesterol-fed rabbits. Proc. Natl. Acad. Sci. USA. 78: 1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herijgers N., Van Eck M., Groot P. H., Hoogerbrugge P. M., Van Berkel T. J. 1997. Effect of bone marrow transplantation on lipoprotein metabolism and atherosclerosis in LDL receptor-knockout mice. Arterioscler. Thromb. Vasc. Biol. 17: 1995–2003. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien K. D., Deeb S. S., Ferguson M., McDonald T. O., Allen M. D., Alpers C. E., Chait A. 1994. Apolipoprotein E localization in human coronary atherosclerotic plaques by in situ hybridization and immunohistochemistry and comparison with lipoprotein lipase. Am. J. Pathol. 144: 538–548. [PMC free article] [PubMed] [Google Scholar]
- 29.Yla-Herttuala S., Lipton B. A., Rosenfeld M. E., Goldberg I. J., Steinberg D., Witztum J. L. 1991. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 88: 10143–10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnoli L. G., Orlandi A., Mauriello A., Santeusanio G., de Angelis C., Lucreziotti R., Ramacci M. T. 1991. Aging and atherosclerosis in the rabbit. 1. Distribution, prevalence and morphology of atherosclerotic lesions. Atherosclerosis. 89: 11–24. [DOI] [PubMed] [Google Scholar]
- 31.Proctor S. D., Mamo J. C. 2003. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler. Thromb. Vasc. Biol. 23: 1595–1600. [DOI] [PubMed] [Google Scholar]
- 32.Yamada N., Shames D. M., Stoudemire J. B., Havel R. J. 1986. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: heterogeneity related to the presence of apolipoprotein E. Proc. Natl. Acad. Sci. USA. 83: 3479–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon T. H., Mori N., Kitamura K., Ishibashi S., Shimano H., Mokuno H., Gotoda T., Takaku F., Yamada N. 1991. Characterization of monoclonal anti-rabbit apolipoprotein E antibodies and chemical composition of lipoproteins separated by anti-apolipoprotein E immuno-affinity chromatography. J. Biochem. 109: 204–210. [PubMed] [Google Scholar]
- 34.Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. 1980. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J. Clin. Invest. 66: 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.