Abstract
The relationship between statin-induced increases in HDL cholesterol (HDL-C) concentration and statin-induced decreases in LDL cholesterol (LDL-C) is unknown. The effects of different statins on HDL-C levels, relationships between changes in HDL-C and changes in LDL-C, and predictors of statin-induced increases in HDL-C have been investigated in an individual patient meta-analysis of 32,258 dyslipidemic patients included in 37 randomized studies using rosuvastatin, atorvastatin, and simvastatin. The HDL-C raising ability of rosuvastatin, and simvastatin was comparable, with both being superior to atorvastatin. Increases in HDL-C were positively related to statin dose with rosuvastatin and simvastatin but inversely related to dose with atorvastatin. There was no apparent relationship between reduction in LDL-C and increase in HDL-C, whether analyzed overall for all statins (correlation coefficient = 0.005) or for each statin individually. Percentage increase in apolipoprotein A-I was virtually identical to that of HDL-C at all doses of the three statins. Baseline concentrations of HDL-C and triglyceride (TG) and presence of diabetes were strong, independent predictors of statin-induced elevations of HDL-C. Statins vary in their HDL-C raising ability. The HDL-C increase achieved by all three statins was independent of LDL-C decrease. However, baseline HDL-C and TGs and the presence of diabetes were predictors of statin-induced increases in HDL-C.
Keywords: lipids, predictors of response, atorvastatin, rosuvastatin, simvastatin
The use of statins has revolutionized the management of people at risk of having a cardiovascular event. Several effects of statins have the potential to reduce cardiovascular risk, with compelling evidence that statin-induced reductions in LDL cholesterol (LDL-C) are implicated (1, 2). However, there is mounting circumstantial evidence that the ability of statins to increase HDL cholesterol (HDL-C) may also contribute to the benefit (3, 4). This paper is concerned with the effects of statins on HDL-C.
All statins raise the concentration of HDL-C, although, in contrast to the reduction in LDL-C, the mechanism by which statins increase the concentration of HDL-C is not known. The magnitude of the increase in HDL-C and its relationship to dose varies widely between different statins (5). The reason for these differences is unclear. Furthermore, the HDL-C response to any given statin tends to vary widely from study to study, with little understanding of the factors that may be responsible for such variation.
These issues are addressed in this report that analyzes the results from an individual patient meta-analysis database of the effects of rosuvastatin, atorvastatin, and simvastatin in a total of 32,258 people included in 37 randomized clinical trials.
Several specific questions are asked. i) What are the effects of rosuvastatin, atorvastatin, and simvastatin on the concentration of HDL-C across the recommended dose ranges of each of the statins? ii) What is the relationship between statin-induced changes in HDL-C and those of the major HDL protein, apolipoprotein (apo) A-I? iii) How do statin-induced changes in HDL-C relate to changes in LDL-C? iv) Are there identifiable factors that influence the HDL-C response to statins?
METHODS
Trial selection
This investigation of the effects of statins on the concentration of HDL-C included individual patient results from 37 randomized clinical trials comparing rosuvastatin with either atorvastatin or simvastatin in a total of 32,258 people in the intention-to-treat population (defined as patients with both baseline and end-of-treatment lipid values). A list of the included trials is shown in supplementary Table I. For a trial to be included in the analysis, it had to involve fixed doses of the statins, be of at least 4 weeks duration, have both baseline and on-therapy lipid values recorded for each patient, and have a documented description of the laboratory methods that were used. Studies involving optional dose titration to achieve goals were excluded. Noncomparative and observational studies were also excluded. Eligible trials were identified from comprehensive searches of the Cochrane Controlled Trials Registry (CCTR/CENTRAL), MEDLINE between 1999 (the year of the first comparative randomized study with rosuvastatin) and 2007, EMBASE (1999 to 2007) Citeline Trialtrove™, and PLANET (a comprehensive and up-to-date collection of published literature on AstraZeneca's products). Individual patient data were obtained from the investigators or sponsors and where this was not possible the study was not used.
Laboratory measurements
All lipid parameters were quantified on samples collected in the fasting state. Cholesterol and triglyceride (TG) quantitation was determined by enzymatic assay. LDL-C was calculated using the Friedewald equation for patients with TG ≤ 400 mg/dl and measured by β-quantification for those with TG > 400 mg/dl. HDL-C was quantified following precipitation of apoB containing lipoproteins. Levels of nonHDL-C were calculated by subtraction of HDL-C from total cholesterol. ApoA-I levels were quantified by immunonephalometry.
Diabetic status was determined by two fasting glucose measurements above 126 mg/dl (7.0 mmol/l); glycosylated hemoglobin of 6% or higher; treatment with antidiabetic therapeutic agents (typically metformin or thiazolidinediones); or diabetic retinopathy, nephropathy, or neuropathy.
Statistical analysis
Demographic data were expressed as mean ± SD for continuous variables and percentage for categorical variables. When a parameter was not normally distributed (TG), it was presented as median (interquartile range). In studies where patients were force-titrated to higher doses at predefined intervals, each period was considered an exposure. For each lipid variable, percentage change was calculated from baseline to the end of each fixed dose period.
Percentage change in each lipid parameter for each statin dose was expressed as least-square mean (± SEM) estimated from a mixed effects model that employed fixed effects for trial/periods and statin doses and a random effect for trial/period-by-treatment interaction. The model was formulated in this way so that the contribution of each study was weighted by the inverse of its variance and to provide correct estimates of error terms for calculation of confidence intervals and P-values.
For identifying factors potentially predicting HDL-C response, univariate and multivariate analyses were carried out using data for all trial/periods. In univariate analyses, results for categorical variables were presented as simple mean percentage responses, and for continuous variables, a regression model with the variable as the only term was used to predict percent change at each of a number of predefined values. For example, predefined values were 50, 60, and 70 years for age; 30, 45, 60, 75, and 90 mg/dl for baseline HDL-C; and 80, 140, 200, 260, and 320 mg/dl for baseline TG. In multivariate analyses, each factor was adjusted for trial, period, and all other factors in a multivariate regression model. Nominal values of 60 years, 50 mg/dl, and 175 mg/dl were used in adjusting for age, baseline HDL-C, and baseline TG, respectively (these are close to actual mean levels).
Relationships between changes in lipid variables were measured by Pearson correlation coefficients.
All statistical analyses were performed using SAS version 9.1 (SAS Inc., Cary, NC). A P-value < 0.05 was considered to be statistically significant, without adjustment for multiple comparisons.
RESULTS
Baseline demographics and lipid levels of the patients included in the VOYAGER database are shown in Table 1.
TABLE 1.
Baseline demographics and lipid levels of the patients included in the VOYAGER database
| Parameter | Value |
|---|---|
| Number of patients | 32,258 |
| Mean (SD) age (years) | 60.0 (11.1) |
| 18–64 (%) | 63.6 |
| 65–69 (%) | 15.2 |
| ≥70 (%) | 21.2 |
| Male (%) | 56.7 |
| Race | |
| Caucasian (%) | 79.9 |
| Black (%) | 5.1 |
| Asian (%) | 8.3 |
| Hispanic of Latino origin (%) | 4.1 |
| Other (%) | 2.6 |
| BMI (kg/m ) | 28.8 (5.5) |
| Percentage with diabetes (%) | 27.5 |
| Percentage with atherosclerotic disease (%) | 48.0 |
| Mean (SD) baseline lipid level (mg/dl) | |
| HDL-C | 48.7 (12.7) |
| LDL-C | 170.9 (38.7) |
| ApoA-I | 148.8 (28.7) |
| TGs (median, interquartile range) | 161.2 (120.4, 215.0) |
BMI, body mass index.
Effects of statins on HDL-C and apoA-I
Rosuvastatin, atorvastatin, and simvastatin all raised the concentrations of both HDL-C and apoA-I across the range of doses used (Table 2). The percentage increase in HDL-C was paralleled by a comparable increase in apoA-I for each of the statins at most doses studied. The magnitude of the increases in HDL-C, however, varied with statin doses and, at any given dose, also between different statins. In the case of rosuvastatin, there was a dose-dependent increase in HDL-C (from 5.5% to 7.9%) over the dose range of 5–40 mg/day. The apoA-I increase with rosuvastatin, while of a similar magnitude to that of HDL-C, appeared, however, not to be dose dependent, being similar (between 6% and 6.5%) across the dose range (Table 2). In the case of atorvastatin, the increase in HDL-C was inversely related to dose, being 4.5% at the 10 mg dose and falling to 2.3% at a dose of 80 mg/day. A similar inverse relationship with atorvastatin dose was also observed for apoA-1 (4.7% increase at 10 mg down to 0.1% increase at 80 mg). For simvastatin, the increase in HDL-C (as was observed with rosuvastatin) was dose dependent, ranging from 4.2% at 10 mg to 5.3% at 80 mg. The effect of simvastatin on apoA-I was similar to that on HDL-C, ranging from an increase of 5.2% at 10 mg to 5.9% at the 80 mg dose (Table 2).
TABLE 2.
Percent change from baseline in LDL-C, HDL-C, and apoA-I across dose range for each statin
| Least-squares mean % change from baseline (SE) |
|||||
|---|---|---|---|---|---|
| Dose (mg) | na | LDL-C | HDL-C | ApoA-I | |
| Rosuvastatin | 5 | 670 | −38.8 (0.9) | 5.5 (0.8) | 6.0 (0.7) |
| 10 | 11,690 | 44.1 (0.6) | 6.1 (0.5) | 6.5 (0.4) | |
| 20 | 3,554 | 49.5 (0.5) | 7.0 (0.4) | 6.0 (0.4) | |
| 40 | 2,983 | 54.7 (0.4) | 7.9 (0.4) | 6.4 (0.5) | |
| Atorvastatin | 10 | 7,837 | 35.5 (0.6) | 4.5 (0.5) | 4.7 (0.4) |
| 20 | 3,908 | 41.4 (0.5) | 3.5 (0.5) | 3.2 (0.4) | |
| 40 | 1,324 | 46.2 (0.5) | 2.4 (0.5) | 2.2 (0.6) | |
| 80 | 2,072 | 50.2 (0.4) | 2.3 (0.4) | 0.1 (0.6) | |
| Simvastatin | 10 | 165 | 27.4 (1.4) | 4.2 (1.3) | 5.2 (1.3) |
| 20 | 2,929 | 33.0 (0.6) | 5.0 (0.6) | 5.2 (0.5) | |
| 40 | 548 | 38.9 (0.9) | 5.0 (0.8) | 6.4 (0.8) | |
| 80 | 479 | 45.0 (1.0) | 5.3 (0.9) | 5.9 (0.9) | |
For approximately 35% patients, apoA-I was not available.
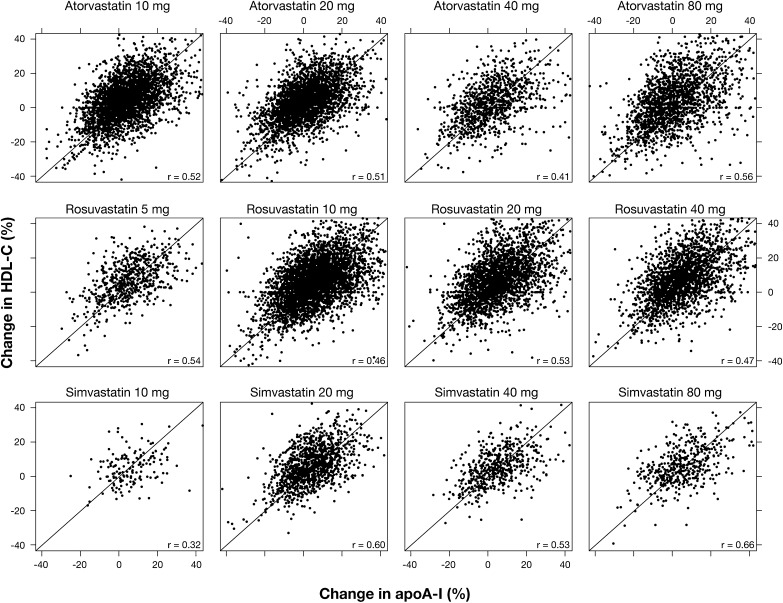
Statin-induced changes in HDL-C correlated positively and significantly with those of apoA-I, with a correlation coefficient of approximately 0.5 for all statins at all doses (Fig. 1).
Fig. 1.
Relationship between changes in concentrations of HDL-C and apoA-I during treatment with atorvastatin at 10, 20, 40, or 80 mg/day, rosuvastatin at 5, 10, 20, or 40 mg/day, or simvastatin at 10, 20, 40, or 80 mg/day. The diagonal lines depict a 1:1 relationship.
Relationship between changes in HDL-C and LDL-C
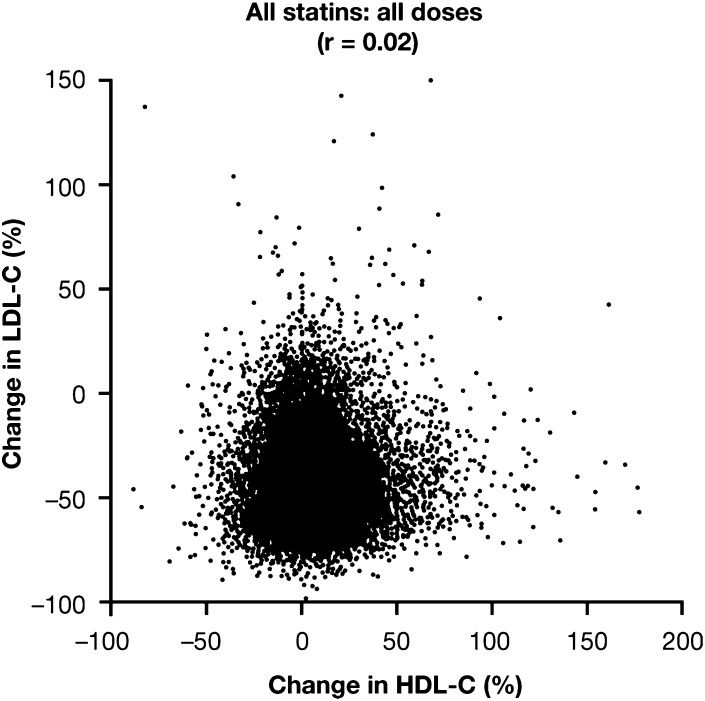
The statin-induced increases in concentration of HDL-C were unrelated to the reduction in LDL-C (Table 2), with a correlation coefficient of 0.02 for the entire database of 38,199 patient exposures that included all statins at all doses studied (Fig. 2). An absence of any significant relationship between changes in HDL-C and LDL-C was also apparent for each of the individual statins at all the doses studied (supplementary Fig. I–III). For rosuvastatin, the correlation coefficients were 0.02, 0.14, 0.02, and 0.02, respectively, for doses of 10 mg, 20 mg, 40 mg, and all doses combined. For atorvastatin, the correlation coefficients were 0.02, 0.05, 0.06, 0.19, and 0.05, respectively, for doses of 10 mg, 20 mg, 40 mg, 80 mg, and all doses combined. For simvastatin, the correlation coefficients were –0.01, 0.01, 0.02, 0.03, and 0.00, respectively, for doses of 10 mg, 20 mg, 40 mg, 80 mg, and all doses combined. These results indicate that statin-induced changes in HDL-C and LDL-C are totally unrelated.
Fig. 2.
Relationship between changes in concentrations of HDL-C and LDL-C. Individual data for all patients (38,199 patient exposures) receiving any dose of rosuvastatin, atorvastatin, or simvastatin are shown (r = 0.02).
Relationship between changes in HDL-C and plasma TG
The statin-induced increases in concentration of HDL-C correlated significantly with the reduction in plasma TG (supplementary Fig. IV). This relationship was apparent for each of the individual statins at all the doses studied (supplementary Fig. IV). For rosuvastatin, the correlation coefficients were –0.29, –0.21, –0.16, and –0.21, respectively, for doses of 5 mg, 10 mg, 20 mg, and 40 mg (P ≤ 0.0001 for all). For atorvastatin, the correlation coefficients were –0.21, –0.24, –0.20, and –0.12, respectively, for doses of 10 mg, 20 mg, 40 mg, and 80 mg (P ≤ 0.0001 for all). For simvastatin, the correlation coefficients were –0.29, –0.22, –0.30, and –0.32, respectively, for doses of 10 mg, 20 mg, 40 mg, and 80 mg (P ≤ 0.0001 for all). These results indicate that while there is a statistically significant relationship between the statin-induced changes in HDL-C and plasma TG, the changes in TG could account for <10% of the changes in HDL-C. When the changes in HDL-C were related to quintiles of change in plasma TG, it was apparent that the greater the decrease in plasma TG, the greater the increase in HDL-C (P < 0.0001 for all except simvastatin 10 mg, where P = 0.003) (supplementary Fig. V). For each statin at each dose, quintiles were constructed with patients having the greatest decrease in TG assigned to the lowest quintile, Q1, and patients with least decrease or an increase in TG assigned to Q5. An inverse relationship was apparent: patients having greatest TG response (i.e., most negative) also had the greatest percent increase in HDL-C, irrespective of statin and dose. Thus, in contrast to the relationships between changes in HDL-C and LDL-C, there was a clear relationship between statin-induced increases in HDL-C and reductions in plasma TG.
Predictors of statin-induced changes in HDL-C
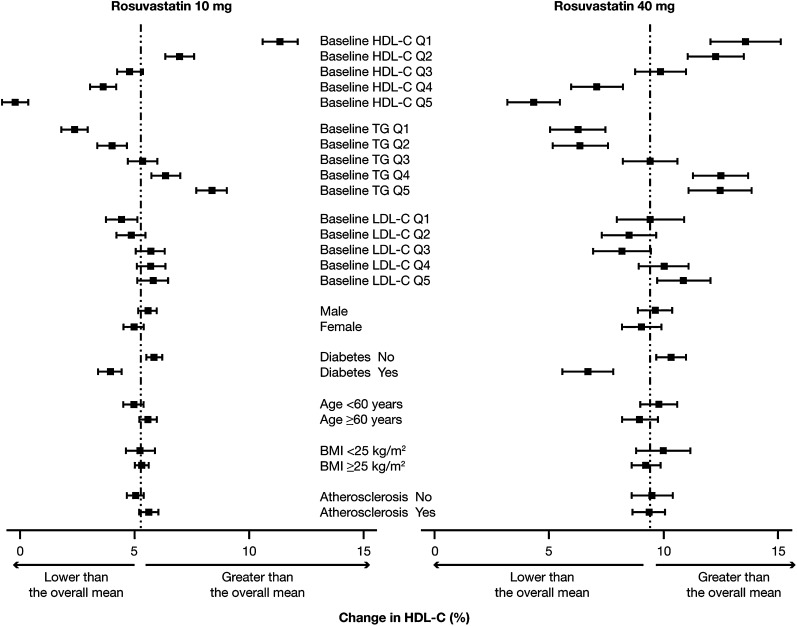
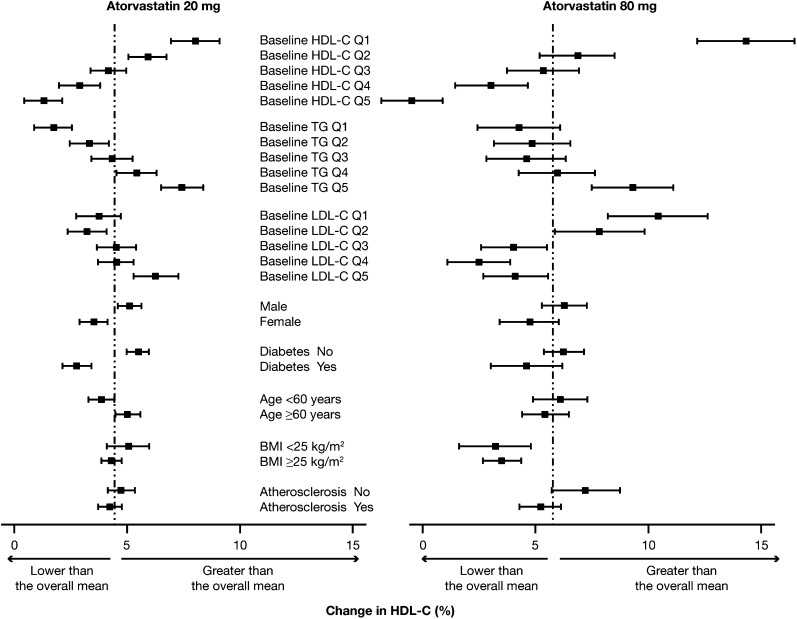
There were several predictors of the HDL-C response to rosuvastatin at its 10 mg and 40 mg doses (Fig. 3) and to atorvastatin at its 20 mg and 80 mg doses (Fig. 4).
Fig. 3.
Impact of baseline concentrations of HDL-C, plasma TG, and LDL-C and patient characteristics on changes in HDL-C during treatment with rosuvastatin at either 10 mg or 40 mg. Results are means (95% CI). BMI, body mass index; Q, quintile (see text for definition).
Fig. 4.
Impact of baseline concentrations of HDL-C, plasma TG, and LDL-C and patient characteristics on changes in HDL-C during treatment with atorvastatin at either 20 mg or 80 mg. Results are means (95% CI). BMI, body mass index; Q, quintile (see text for definition).
The baseline level of HDL-C was highly predictive of the increase in HDL-C induced by the low and high doses of both rosuvastatin (Fig. 3) and atorvastatin (Fig. 4) (P < 0.0001 for the trend in the case of each dose of each statin). In people whose HDL-C was in the lowest quintile (concentration < 39 mg/dl), the increase in HDL-C with 10 mg rosuvastatin was 11.4% compared with –0.2% in those whose HDL-C was in the top quintile (concentration > 59 mg/dl). A similar result was observed with 40 mg rosuvastatin, in which case the increase in HDL-C was 13.6% in those whose HDL-C at baseline was in the lowest quintile compared with an increase of 4.3% in those whose HDL-C was in the top quintile (Fig. 3). With atorvastatin at the 20 mg dose, the increases in HDL-C in people with the lowest (concentration < 37 mg/dl) and highest (concentration > 56 mg/dl) quintiles of baseline HDL-C were, respectively, 8.1% and 1.4%, while at the 80 mg dose of atorvastatin, the respective increases in HDL-C were 14.3% and –0.5% (Fig. 4).
The baseline level of TG also predicted statin-induced changes in HDL-C. In this case, the higher the plasma TG at baseline, the greater the increase in HDL-C at both the low and high doses of rosuvastatin and atorvastatin (Figs. 3 and 4). The most likely explanation for an impact of TG level on the statin-induced increase in HDL-C is the fact that plasma concentrations of TG and HDL-C are inversely related and that people with high TG tend to have low levels of HDL-C. In contrast to the impact of baseline HDL-C and plasma TG, the baseline level of LDL-C had little effect on the magnitude of the HDL-C increase with either low or high dose rosuvastatin (Fig. 3). There was an apparent effect of baseline LDL-C on the change in HDL-C induced by atorvastatin, although the results with the 20 mg and 80 mg doses were in opposite directions (Fig. 4). The HDL-C increase induced by the 20 mg dose of atorvastatin was greater in those whose baseline LDL-C was in the highest quintile compared with the lowest quintile (trend P < 0.0001). In the case of the 80 mg dose of atorvastatin, however, the HDL-C increased significantly more in those with a baseline LDL-C in the lowest compared with the highest quintile (trend P < 0.0001) (Fig. 4).
Gender had a small influence on the HDL-C response to rosuvastatin at the 10 mg dose (P = 0.04) but not at the 40 mg dose (P = 0.3) (Fig. 3) and for atorvastatin at the 20 mg dose (P = 0.0002) but not at the 80 mg dose (P = 0.07) (Fig. 4). There was no effect of gender on the HDL-C response to 10 mg or 40 mg atorvastatin (results not shown), suggesting that the result with the 20 mg dose was most likely due to chance.
The presence of diabetes was associated with a reduced increase in HDL-C for rosuvastatin at both the 10 mg and 40 mg doses (Fig. 3) and for atorvastatin at the 20 mg and 80 mg doses (Fig. 4) and 10 mg and 40 mg doses (results not shown). All P-values were <0.0001 except P = 0.06 for atorvastatin at 80 mg.
Of the other factors investigated, age, body mass index, and the presence of atherosclerotic disease had little apparent impact on the HDL-C increase induced by either rosuvastatin or atorvastatin (Figs. 3 and 4).
Several potential predictors of the statin-induced increase in HDL-C, including presence of atherosclerotic disease (Y/N), presence of diabetes (Y/N), gender (M/F), age, baseline HDL-C, and baseline TG, were subjected to both univariate and multivariate analysis.
The presence of atherosclerotic disease at baseline (supplementary Table II) had no consistent impact on the HDL-C response to any statin in either univariate or multivariate analysis. In contrast to the absence of a consistent effect of atherosclerotic disease, the presence of diabetes had a substantial impact as a reduced HDL-C increase as assessed in both univariate and multivariate analyses (supplementary Table III). In subjects taking rosuvastatin at the 10 mg and 40 mg doses and atorvastatin at the 80 mg dose, statin-induced increases in HDL-C were significantly greater in females than in males as assessed by multivariate analysis (supplementary Table IV). Age was also a factor, with the HDL-C response increasing with advancing age as assessed by multivariate analysis (supplementary Table V). Of all the variables investigated, the baseline level of HDL-C was the strongest predictor of the HDL-C response to statins in both univariate and multivariate analyses (supplementary Table VI). Baseline TG was also found to be predictive of the HDL-C response to statins in both univariate and multivariate analysis (supplementary Table VII).
DISCUSSION
HDL-C-raising ability of the statins
All statins have the capacity to increase the concentration of HDL-C and apoA-I (3, 5, 6). As has been reported elsewhere (5, 7) and confirmed here, different statins vary in their HDL-C-raising activity, with rosuvastatin and simvastatin having comparable effects and both being more effective than atorvastatin. The dose response of the HDL-C raising also differs markedly between the statins, being positively dose dependent for rosuvastatin and simvastatin, but, as has been reported previously (5), being inversely related to dose for atorvastatin, at least within the dose range of 20 to 80 mg/day. Clearly, all agents must be positively dose-dependent at lower doses. Despite these differing effects on the concentration of HDL-C, all three agents have similar effects on the subpopulation distribution of HDL. All preferentially increase concentrations of large, α 1-migrating HDL (8, 9). Whether or not these differences in HDL-C response affect the cardio-protective properties of the different statins is not known, although there is growing evidence that statin-induced increases in concentration of HDL-C favorably affect coronary atheroma burden (4) and may also contribute to a statin-mediated reduction in cardiovascular events (10).
The relationship between HDL-C-raising with statins and the progression or regression of coronary atheroma has been addressed in an analysis of data from 1,455 patients in four intravascular ultrasound imaging trials. Multivariate analysis showed that both the achieved level of LDL-C and the increase in concentration of HDL-C during statin treatment were significant independent predictors of coronary atheroma progression (4).
Relationship between changes in HDL-C and changes in apoA-I
Statin-induced changes in HDL-C correlated positively and significantly with those of apoA-I, with a correlation coefficient of approximately 0.5 for all three statins at all doses studied (Fig. 1). While this indicates that changes in HDL-C and apoA-I are related, it is apparent that the changes in either one can account for only about 25% of the variance of changes to the other, indicating that there may be differences in the mechanisms by which statins increase HDL-C and apoA-I. It was of interest that in this very large database, the percentage increases in apoA-I were comparable to those of HDL-C with all doses of the three statins. This contrasted with many previous reports that HDL-C-raising therapies tend to increase the concentration of HDL-C to a greater extent than that of apoA-I (3, 5, 6, 11–16).
The mechanism by which statins increase the concentration of HDL-C and apoA-I is not known and is apparently unrelated to the mechanism by which these agents lower the concentration of LDL-C. Statins are known to upregulate hepatic ABC transporter A1 gene expression (17), an effect that may explain at least a portion of the statin-induced increase in HDL-C (17). Statins have also been reported to reduce activity of hepatic lipase (18). In the case of atorvastatin, this effect is positively dose dependent over the 20–80 mg dose range (18). The fact that the increase in HDL-C with atorvastatin is inversely related to dose in this dose range does not therefore support a major involvement of hepatic lipase inhibition in statin-induced increases in HDL-C. Another possible mechanism by which statins increase HDL-C is the known statin-induced inhibition of cholesteryl ester transfer protein (CETP) (19–22). However, the observed 20–25% inhibition of CETP associated with statin treatment would be predicted to increase the concentration of HDL-C by >20% (23). The fact that the increase is much less than this suggests that statins may have other effects that oppose the HDL-C raising resulting from CETP inhibition. It follows that the magnitude of statin-induced elevations of HDL-C may reflect a balance between mechanisms that increase and others that decrease the concentration. Variations in the balance between such opposing effects may be the explanation for the markedly differing patterns of the HDL-C response to treatment with different statins. A possible explanation for the observed loss of the HDL-C-raising effect at higher doses of atorvastatin is the fact that atorvastatin metabolites have potent antioxidant properties (24). It is known that antioxidants such as probucol reduce HDL-C levels (25, 26) and a mixture of vitamins C and E, β carotene, and selenium has been reported to reduce the HDL-C-raising effects achieved by the combination of simvastatin and niacin (27).
Predictors of HDL-C response
Despite the markedly differing HDL-C-raising effects of rosuvastatin and atorvastatin, there were several predictors of response that were common to both low and high doses of each agent. The strongest predictor was the baseline level of HDL-C, an observation that has also been made in people treated with fibrates (28–30). Part of the explanation may be nothing more than the well-recognized statistical phenomenon of regression to the mean. The full explanation is uncertain, but it is clear that mean baseline HDL-C level may explain much of the observed variability in HDL-C response from study to study for a given statin/dose. It is possible that the smaller than expected HDL-C increase achieved with rosuvastatin in the recently reported Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial was a reflection of the high median baseline HDL-C level of 49 mg/dl (31).
The statin-induced elevation of HDL-C was a positive function of the baseline level of plasma TG. The possibility that this was no more than a reflection of the well-documented inverse relationship between the concentrations of TG and HDL-C was excluded by the fact that the relationship remained robust even after adjusting for baseline HDL-C. The mechanism by which a higher concentration of TG may affect a statin-induced increase in HDL-C is not known. It is known, however, that statin-induced decreases in plasma TG are greater in people with elevated levels of plasma TG (32). Given that a reduction in concentration of TG-rich lipoproteins will be accompanied by decrease in the CETP-mediated transfer of cholesteryl esters from HDL-C to TG-rich lipoproteins (33), it is perhaps not surprising that the baseline level of plasma TG is a predictor of the HDL-C response to statins or that the statin-induced reduction in plasma TG correlated significantly with the increase in HDL-C.
People with type II diabetes typically have a lower level of HDL-C than is observed in nondiabetics (34). On this basis, it might have been expected that the HDL-C increase induced by a statin would be greater in diabetics than in nondiabetics. The reality was the opposite, with a substantially smaller HDL-C response in the diabetics that remained statistically significant (with the exception of the 80 mg dose of atorvastatin) after adjusting for a number of potentially confounding variables, including baseline levels of HDL-C and TG. This finding is consistent with previous observations of a smaller increase in HDL-C in diabetics treated with simvastatin in the Heart Protection Study (35) and with gemfibrozil in the Veterans Affairs High-Density Lipoprotein Intervention Trial study (36). The explanation and the clinical implications of a reduced HDL-C response to therapy in people with diabetes warrant further investigation.
CONCLUSIONS
The analysis of this large, individual patient database has revealed several robust findings of potential clinical importance. It has confirmed previous observations that statins vary markedly in their ability to raise the level of HDL-C. A novel finding was that the percentage increase in apoA-I was virtually identical to that of HDL-C at all doses of the three statins investigated. The analysis also revealed that the HDL-C raising achieved by all three statins was totally independent of the reduction in LDL-C. And finally, it has been found that baseline concentrations of HDL-C and plasma TG and the presence of diabetes are robust, independent predictors of statin-induced elevations of HDL-C.
Supplementary Material
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CETP
- cholesteryl ester transfer protein
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- TG
- triglycerides
This study was supported by AstraZeneca. Gunnar Brandrup-Wognsen is an employee of AstraZeneca. M. Palmer is a consultant for AstraZeneca, Roche, Prism Ideas, Biota Holdings, Panacea Biotec, and MDL. P. Barter is a consultant for AstraZeneca, CSL, Merck, Pfizer, Roche, and Sanofi-Aventis; has received honoraria from Abbott, AstraZeneca, Merck, Novartis, Pfizer, Resverlogix, Roche, and Sanofi-Aventis; and has participated in clinical trials sponsored by AstraZeneca, Merck, Pfizer, and Roche. S. Nicholls has received honoraria from AstraZeneca, Roche, Pfizer, Merck Schering-Plough, and Takeda; consulting fees from AstraZeneca, Roche, Pfizer, Merck Schering-Plough, and Takeda and research support from AstraZeneca, Novartis, and Resverlogix. Editorial support provided during the later stages of manuscript development was funded by AstraZeneca.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of seven tables and five figures.
REFERENCES
- 1.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. ; Cholesterol Treatment Trialists' (CTT) Collaborators. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 2.Sipahi I., Nicholls S. J., Tuzcu E. M., Nissen S. E. 2006. Coronary atherosclerosis can regress with very intensive statin therapy. Cleve. Clin. J. Med. 73: 937–944. [DOI] [PubMed] [Google Scholar]
- 3.McTaggart F., Jones P. 2008. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc. Drugs Ther. 22: 321–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls S. J., Tuzcu E. M., Sipahi I., Grasso A. W., Schoenhagen P., Hu T., Wolski K., Crowe T., Desai M. Y., Hazen S. L., et al. 2007. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 297: 499–508. [DOI] [PubMed] [Google Scholar]
- 5.Jones P. H., Davidson M. H., Stein E. A., Bays H. E., McKenney J. M., Miller E., Cain V. A., Blasetto J. W. 2003. STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92: 152–160. [DOI] [PubMed] [Google Scholar]
- 6.Jones P. H., Hunninghake D. B., Ferdinand K. C., Stein E. A., Gold A., Caplan R. J., Blasetto J. W. 2004. STELLAR Study Group. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin. Ther. 26: 1388–1399. [DOI] [PubMed] [Google Scholar]
- 7.Faergeman O., Hill L., Windler E., Wiklund O., Asmar R., Duffield E., Sosef F.; ECLIPSE Study Investigators. 2008. Efficacy and tolerability of rosuvastatin and atorvastatin when force-titrated in patients with primary hypercholesterolemia: results from the ECLIPSE study. Cardiology. 111: 219–228. [DOI] [PubMed] [Google Scholar]
- 8.Asztalos B. F., Horvath K. V., McNamara J. R., Roheim P. S., Rubinstein J. J., Schaefer E. J. 2002. Effects of atorvastatin on the HDL subpopulation profile of coronary heart disease patients. J. Lipid Res. 43: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 9.Asztalos B. F., Le Maulf F., Dallal G. E., Stein E. S., Jones P. H., Horvath K. V., Mctaggart F., Schaefer E. J. 2007. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high-density lipoproteins. Am. J. Cardiol. 99: 681–685. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen T. R., Olsson A. G., Faergeman O., Kjekshus J., Wedel H., Berg K., Wilhelmsen L., Haghfelt T., Thorgeirsson G., Pyörälä K., et al. 1998. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation. 97: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 11.Davidson M., McKenney J., Stein E., Schrott H., Bakker-Arkema R., Fayyad R., Black D. 1997. Comparison of one year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. Atorvastatin Study Group I. Am. J. Cardiol. 79: 1475–1481. [DOI] [PubMed] [Google Scholar]
- 12.Davidson M., Ma P., Stein E. A., Gotto A. M., Jr., Raza A., Chitra R., Hutchinson H. 2002. Comparison of effects on low density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am. J. Cardiol. 89: 268–275. [DOI] [PubMed] [Google Scholar]
- 13.Davidson M. H., McGarry T., Bettis R., Melani L., Lipka L. J., LeBeaut A. P., Suresh R., Sun S., Veltri E. P. 2002. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J. Am. Coll. Cardiol. 40: 2125–2134. [DOI] [PubMed] [Google Scholar]
- 14.Bays H. E., Ose L., Fraser N., Tribble D. L., Quinto K., Reyes R., Johnson-Levonas A. O., Sapre A., Donahue S. R.; Ezetimibe Study Group. 2004. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin. Ther. 26: 1758–1773. [DOI] [PubMed] [Google Scholar]
- 15.Schuster H., Barter P.J., Stender S., Cheung R.C., Bonnet J., Morrell J.M., Watkins C., Kallend D., Raza A.; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. 2004. Effects of switching statins on achievement of lipid goals: measuring effective reductions in cholesterol using rosuvastatin therapy (MERCURY I) study. Am. Heart J. 147: 705–712. [DOI] [PubMed] [Google Scholar]
- 16.Cheung R. C., Morrell J. M., Kallend D., Watkins C., Schuster H. 2005. Effects of switching statins on lipid and apolipoprotein ratios in the MERCURY I study. Int. J. Cardiol. 100: 309–316. [DOI] [PubMed] [Google Scholar]
- 17.Tamehiro N., Shigemoto-Mogami Y., Kakeya T., Okuhira K., Suzuki K., Sato R., Nagao T., Nishimaki-Mogami T. 2007. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282: 21090–21099. [DOI] [PubMed] [Google Scholar]
- 18.Berk-Planken I. I., Hoogerbrugge N., Stolk R. P., Bootsma A. H., Jansen H.; DALI Study Group. 2003. Atorvastatin dose-dependently decreases hepatic lipase activity in type 2 diabetes: effect of sex and the LIPC promoter variant. Diabetes Care. 26: 427–432. [DOI] [PubMed] [Google Scholar]
- 19.Homma Y., Ozawa H., Kobayashi T., Yamaguchi H., Sakane H., Nakamura H. 1995. Effects of simvastatin on plasma lipoprotein subfractions; cholesterol esterification rate, and cholesteryl ester transfer protein in type II hyperlipoproteinemia. Atherosclerosis. 114: 223–234. [DOI] [PubMed] [Google Scholar]
- 20.McPherson R. 1999. Comparative effects of simvastatin and cholestyramine on plasma lipoproteins and CETP in humans. Can. J. Clin. Pharmacol. 6: 85–90. [PubMed] [Google Scholar]
- 21.Guerin M., Lassel T. S., Le Goff W., Farnier M., Chapman M. J. 2000. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 20: 189–197. [DOI] [PubMed] [Google Scholar]
- 22.Kassai A., Illyés L., Mirdamadi H. Z., Seres I., Kalmár T., Audikovszky M., Paragh G. 2007. The effect of atorvastatin therapy on lecithin:cholesterol acyltransferase, cholesteryl ester transfer protein and the antioxidant paraoxonase. Clin. Biochem. 40: 1–5. [DOI] [PubMed] [Google Scholar]
- 23.de Grooth G. J., Kuivenhoven J. A., Stalenhoef A. F., de Graaf J., Zwinderman A. H., Posma J. L., van Tol A., Kastelein J. J. 2002. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 105: 2159–2165. [DOI] [PubMed] [Google Scholar]
- 24.Aviram M., Rosenblat M., Bisgaier C. L., Newton R. S. 1998. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 138: 271–280. [DOI] [PubMed] [Google Scholar]
- 25.Johansson J., Olsson A. G., Bergstrand L., Elinder L. S., Nilsson S., Erikson U., Molgaard J., Holme I., Walldius G. 1995. Lowering of HDL sub 2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia: a probucol quantitative regression Swedish trial (PQRST) report. Arterioscler. Thromb. Vasc. Biol. 15: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 26.Yashiro A., Nakashima Y., Segawa J., Kawashima T., Kuroiwa A. 1990. Factors predictive of marked decrease in HDL-C by probucol treatment. Artery. 18: 16–31. [PubMed] [Google Scholar]
- 27.Cheung M. C., Zhao X. Q., Chait A., Albers J. J., Brown B. G. 2001. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler. Thromb. Vasc. Biol. 21: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 28.Manttari M., Tenkanen L., Maenpaa H., Manninen V., Huttunen J. K. 1993. High density lipoprotein cholesterol elevation with gemfibrozil: effects of baseline level and modifying factors. Clin. Pharmacol. Ther. 54: 437–447. [DOI] [PubMed] [Google Scholar]
- 29.le Roux C. W., Murphy E., Seed M. 2002. A retrospective assessment of the effectiveness of fenofibrate 267 mg on high-density lipoprotein cholesterol levels in patients attending a lipid clinic. Clin. Ther. 24: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 30.Després J. P., Lemieux I., Salomon H., Delaval D. 2002. Effects of micronized fenofibrate versus atorvastatin in the treatment of dyslipidaemic patients with low plasma HDL-cholesterol levels: a 12-week randomized trial. J. Intern. Med. 251: 490–499. [DOI] [PubMed] [Google Scholar]
- 31.Ridker P. M., Danielson E., Fonseca F. A., Genest J., Gotto A. M., Jr., Kastelein J. J., Koenig W., Libby P., Lorenzatti A. J., MacFadyen J. G., et al. ; JUPITER Study Group. 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359: 2195–2207. [DOI] [PubMed] [Google Scholar]
- 32.Stein E. A., Lane M., Laskarzewski P. 1998. Comparison of statins in hypertriglyceridemia. Am. J. Cardiol. 81: 66B–69B. [DOI] [PubMed] [Google Scholar]
- 33.Chapman M. J., Le Goff W., Guerin M., Kontush A. 2010. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 31: 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyörälä K., Laakso M., Uusitupa M. 1987. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab. Rev. 3: 463–524. [DOI] [PubMed] [Google Scholar]
- 35.Collins R., Armitage J., Parish S., Sleigh P., Peto R. 2003. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 361: 2005–2016. [DOI] [PubMed] [Google Scholar]
- 36.Rubins H. B., Robins S. J., Collins D., Nelson D. B., Elam M. B., Schaefer E. J., Faas F. H., Anderson J. W. 2002. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch. Intern. Med. 162: 2597–2604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.