Abstract
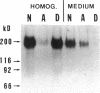
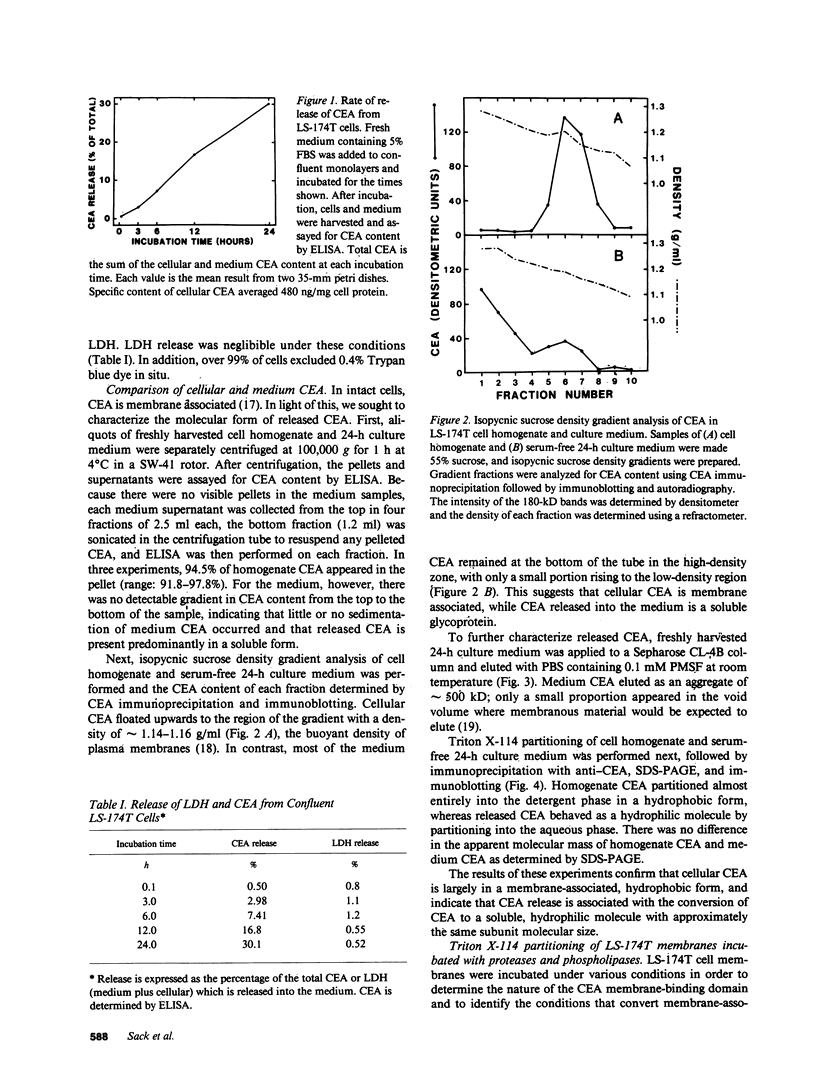
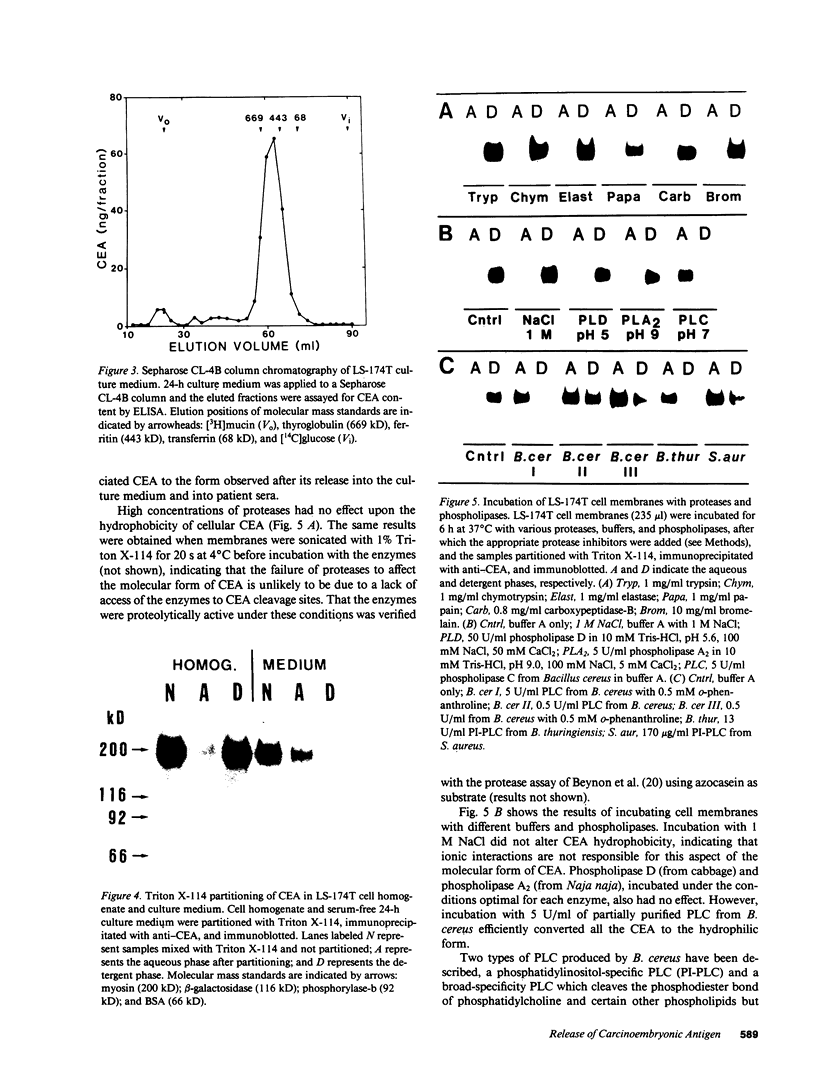
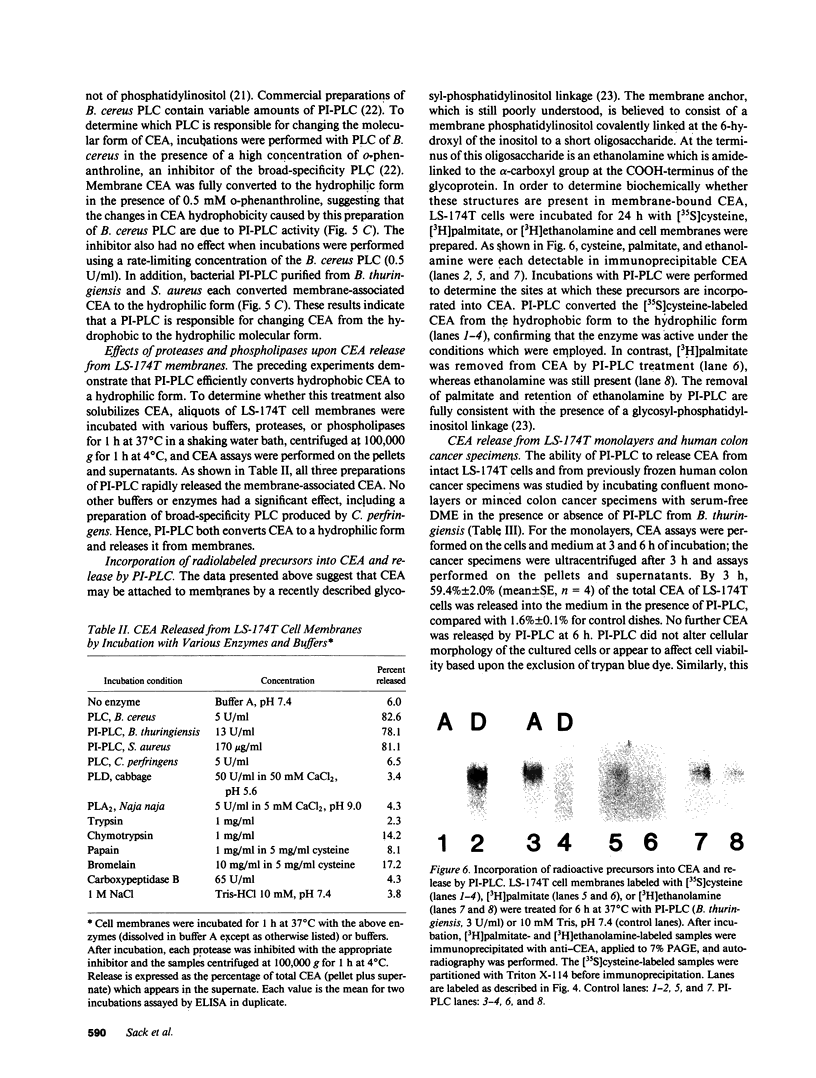
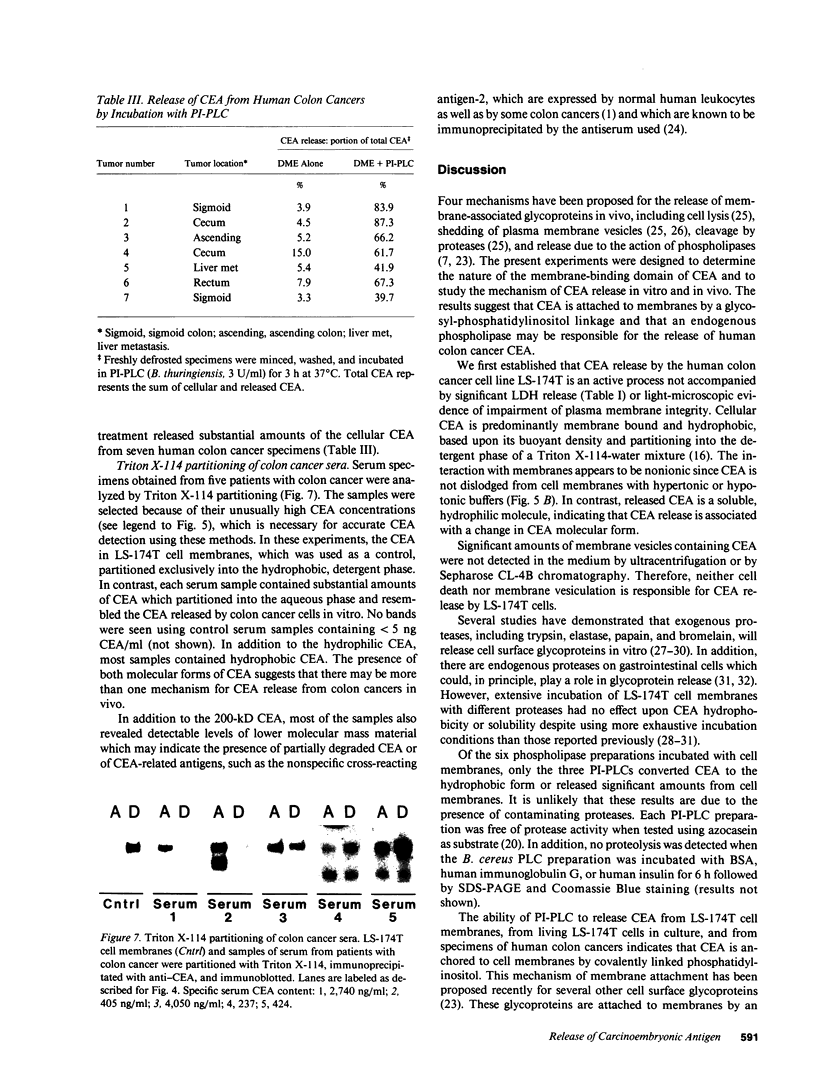
Carcinoembryonic antigen (CEA) is released from colon cancer cells into the circulation where it is monitored clinically as an indicator of the recurrence or progression of cancer. We have studied the mechanism of CEA membrane attachment and release using the human colonic adenocarcinoma cell line LS-174T, specimens of human colon cancers, and serum from colon cancer patients. CEA release by cells in vitro and in vivo is associated with the conversion of CEA from a membrane-bound, hydrophobic molecule to a soluble, hydrophilic form with no apparent decrease in molecular mass. When LS-174T cell membranes were incubated with various buffers, proteases, and phospholipases, the only agents that released CEA and converted it to the hydrophilic form were preparations of phosphatidylinositol-specific phospholipase C (PI-PLC). Both [3H]ethanolamine and [3H]palmitate could be incorporated metabolically into CEA but only palmitate was released by treatment with PI-PLC, consistent with the presence of a glycosyl-phosphatidylinositol linkage. PI-PLC treatment also release significant quantities of CEA from living monolayers and from seven human colon cancer specimens. These experiments suggest that cellular CEA is anchored to membranes by a covalent linkage to a membrane phosphatidylinositol molecule, and that an endogenous phospholipase may be important for releasing CEA in vitro and in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Singleton J. R., Hoops T. C., Kloppel T. M. Posttranslational processing of secretory component in the rat jejunum by a brush border metalloprotease. J Clin Invest. 1986 Jun;77(6):1841–1848. doi: 10.1172/JCI112510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Hou E., Doyle D. Insertion of biologically active membrane proteins from rat liver into the plasma membrane of mouse fibroblasts. J Biol Chem. 1980 Oct 25;255(20):10001–10012. [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Biosynthesis of the erythrocyte anion transport protein. J Biol Chem. 1981 Nov 10;256(21):11337–11344. [PubMed] [Google Scholar]
- Brower M. S., Levin R. I., Garry K. Human neutrophil elastase modulates platelet function by limited proteolysis of membrane glycoproteins. J Clin Invest. 1985 Feb;75(2):657–666. doi: 10.1172/JCI111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Chung Y. S., Song I. S., Erickson R. H., Sleisenger M. H., Kim Y. S. Effect of growth and sodium butyrate on brush border membrane-associated hydrolases in human colorectal cancer cell lines. Cancer Res. 1985 Jul;45(7):2976–2982. [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Fletcher R. H. Carcinoembryonic antigen. Ann Intern Med. 1986 Jan;104(1):66–73. doi: 10.7326/0003-4819-104-1-66. [DOI] [PubMed] [Google Scholar]
- Goslin R., O'Brien M. J., Steele G., Mayer R., Wilson R., Corson J. M., Zamcheck N. Correlation of Plasma CEA and CEA tissue staining in poorly differentiated colorectal cancer. Am J Med. 1981 Aug;71(2):246–253. doi: 10.1016/0002-9343(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Strobel H. W. Isolation of the membrane-binding peptide of NADPH-cytochrome P-450 reductase. Characterization of the peptide and its role in the interaction of reductase with cytochrome P-450. J Biol Chem. 1981 Jul 25;256(14):7478–7486. [PubMed] [Google Scholar]
- Hamada Y., Yamamura M., Hioki K., Yamamoto M., Nagura H., Watanabe K. Immunohistochemical study of carcinoembryonic antigen in patients with colorectal cancer. Correlation with plasma carcinoembryonic antigen levels. Cancer. 1985 Jan 1;55(1):136–141. doi: 10.1002/1097-0142(19850101)55:1<136::aid-cncr2820550121>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hemperly J. J., Edelman G. M., Cunningham B. A. cDNA clones of the neural cell adhesion molecule (N-CAM) lacking a membrane-spanning region consistent with evidence for membrane attachment via a phosphatidylinositol intermediate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9822–9826. doi: 10.1073/pnas.83.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. I., Sack T. L., Kim Y. S. Effects of cyclic adenosine 3':5'-monophosphate upon glycoprotein and carcinoembryonic antigen synthesis and release by human colon cancer cells. Cancer Res. 1986 Jul;46(7):3371–3374. [PubMed] [Google Scholar]
- Jemmerson R., Millan J. L., Klier F. G., Fishman W. H. Monoclonal antibodies block the bromelain-mediated release of human placental alkaline phosphatase from cultured cancer cells. FEBS Lett. 1985 Jan 7;179(2):316–320. doi: 10.1016/0014-5793(85)80542-1. [DOI] [PubMed] [Google Scholar]
- Kapeller M., Gal-Oz R., Grover N. B., Doljanski F. Natural shedding of carbohydrate-containing macromolecules from cell surfaces. Exp Eye Res. 1973 Apr;79(1):152–158. [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Phosphatidylinositol-specific phospholipase C from Staphylococcus aureus. Methods Enzymol. 1981;71(Pt 100):741–746. doi: 10.1016/0076-6879(81)71087-5. [DOI] [PubMed] [Google Scholar]
- Low M. G., Prasad A. R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):980–984. doi: 10.1073/pnas.85.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Malik A. S., Low M. G. Conversion of human placental alkaline phosphatase from a high Mr form to a low Mr form during butanol extraction. An investigation of the role of endogenous phosphoinositide-specific phospholipases. Biochem J. 1986 Dec 1;240(2):519–527. doi: 10.1042/bj2400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A. Shedding eucaryotic cells. Biomembranes. 1983;11:21–52. [PubMed] [Google Scholar]
- Pihl E., McNaughtan J., Ward H. A., Nairn R. C. Immunohistological patterns of carcinoembryonic antigen in colorectal carcinoma. Correlation with staging and blood levels. Pathology. 1980 Jan;12(1):7–13. doi: 10.3109/00313028009060048. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Kim B. H., Rosenberry T. L. Differences in the glycolipid membrane anchors of bovine and human erythrocyte acetylcholinesterases. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7817–7821. doi: 10.1073/pnas.84.22.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. T. Carcinoembryonic antigens and related glycoproteins. Molecular aspects and specificity. Biochim Biophys Acta. 1983 Dec 29;695(3-4):227–249. doi: 10.1016/0304-419x(83)90013-6. [DOI] [PubMed] [Google Scholar]
- Seetharam B., Tiruppathi C., Alpers D. H. Hydrophobic interactions of brush border alkaline phosphatases: the role of phosphatidyl inositol. Arch Biochem Biophys. 1987 Feb 15;253(1):189–198. doi: 10.1016/0003-9861(87)90651-5. [DOI] [PubMed] [Google Scholar]
- Thomas P., Zamcheck N. Role of the liver in clearance and excretion of circulating carcinoembryonic antigen (CEA). Dig Dis Sci. 1983 Mar;28(3):216–224. doi: 10.1007/BF01295116. [DOI] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J., Salem N., Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981 Jul 6;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- Tsao D., Kim Y. S. Glycoproteins from human colonic adenocarcinoma. Isolation and characterization of cell surface carcinoembryonic antigen from a cultured tumor cell line. J Biol Chem. 1978 Apr 10;253(7):2271–2278. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y., Nagura H., Watanabe K. Immunohistochemical observations of carcinoembryonic antigen (CEA) and CEA-related substances in normal and neoplastic pancreas. Pitfalls and caveats in CEA immunohistochemistry. Am J Clin Pathol. 1984 Nov;82(5):535–542. doi: 10.1093/ajcp/82.5.535. [DOI] [PubMed] [Google Scholar]